Abstract
Blue light–filtering (BLF) intraocular lenses (IOLs) are designed to mimic the healthy natural adult crystalline lens. Studies that evaluated the relative merit of ultraviolet-only IOL design (ie, blocking wavelengths <400 nm) versus BLF IOL design (ie, filtering wavelengths ~400–475 nm in addition to blocking wavelengths <400 nm) on protection and function of the visual system suggest that neither design had a deleterious impact on visual acuity or contrast sensitivity. A BLF design may reduce some aspects of glare, such as veiling and photostress. BLF has been shown in many contexts to improve visual performance under conditions that are stressed by blue light, such as distance vision impaired by short-wave dominant haze. Furthermore, some data (mostly inferential) support the notion that BLF IOLs reduce actinic stress. Biomimetic BLF IOLs represent a conservative approach to IOL design that provides no harm for visual acuity, contrast sensitivity, or color vision while improving vision under certain circumstances (eg, glare).
Introduction
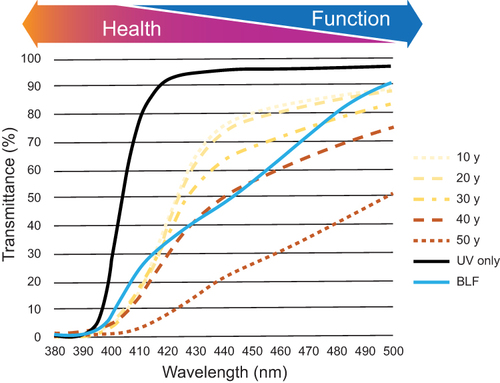
The visible spectrum is a relatively narrow portion of the electromagnetic spectra ranging from 380 to 740 nm. Energy and wavelength are inversely related; shorter-wavelength light is inherently more damaging to surface tissues such as skin and eyes,Citation1,Citation2 with longer wavelengths playing a more important role in visual function (). The native crystalline lens filters ultraviolet (UV) light and reduces transmission of short-wave or “blue” light.Citation1,Citation2 Although the crystalline lens is highly transparent in children, light transmission gradually decreases with age, especially at shorter wavelengths, as shown by the dotted lines in . Transmission of 480-nm light, for instance, is expected to decrease by 72% between the ages of 10 and 80 years.Citation3 In addition to lens absorption, the pigments within the inner layer of the macula (ie, meso-zeaxanthin, lutein, and zeaxanthin) absorb wavelengths between 380 and 520 nm.Citation4,Citation5
Figure 1 Transmittance on the UV-violet-blue spectral zone of the human crystalline lens at different ages (dotted and dashed).
After cataract extraction, the cloudy native lens is replaced with an artificial intraocular lens (IOL). According to their transmittance properties, IOLs can be classified as UV-only lenses designed to block UV light or blue light–filtering (BLF) lenses developed to mimic the naturally aged human lens (). As occurs with the natural crystalline lens, filtering light between 380 and 500 nm is designed to provide protection and visual benefits while causing “no harm” with respect to visual function; multiple types of BLF IOLs are currently available ().
Table 1 Summary of Blue Light–Filtering Intraocular Lensesa
The aim of this review is to evaluate recent literature and assess effects of BLF on visual and nonvisual health and function while maintaining a clinical perspective. The impact of BLF on visual function (ie, visual acuity, contrast sensitivity, and color vision) and ocular and systemic health (ie, retinal protection and regulation of circadian rhythms) is also reviewed.
Methods
The initial reference list for this narrative review was provided by the authors. An additional literature search of PubMed was performed (search terms included age-related macular degeneration, blue light filtration, circadian rhythm, color vision, contrast sensitivity, glaucoma, visual acuity), and search results were screened for relevance. The reference lists from relevant publications were reviewed for additional articles of interest. Included were publications in English from peer-reviewed journals.
Blue Light Filtration and Visual Function
Visual Acuity and Contrast Sensitivity
The first goal of any IOL is to maintain and improve spatial vision,Citation7 classically measured as visual acuity and contrast sensitivity. A recent meta-analysis suggests that there are no clinically meaningful differences in corrected distance visual acuity (CDVA) or contrast sensitivity in patients with BLF versus UV-only IOLs.Citation8 A recent prospective randomized clinical study in 60 patients with bilateral cataract surgery reported no significant differences in uncorrected distance visual acuity or contrast sensitivity in eyes with BLF versus UV-only IOLs 60 days after surgery. Contrast sensitivity was significantly lower under glare conditions for BLF versus UV-only IOLs (P<0.05) at some spatial frequencies (12 and 3 cycles per degree).Citation9 However, a consecutive case-control study in 60 patients who received BLF or UV-only IOLs found no significant differences in CDVA or scotopic contrast sensitivity with or without glare.Citation10 An intraindividual comparison in 47 patients found no significant differences in uncorrected visual acuity, best corrected visual acuity, or contrast vision in eyes with BLF versus UV-only IOLs at 11 to 13 months after surgery.Citation11 A similar conclusion was reached in a 5-year longitudinal study that reported no significant differences for contrast sensitivity under photopic or scotopic conditions with BLF versus UV-only IOLs.Citation12 The results of these studies suggest that BLF IOLs appear to have no effect on visual acuity compared with UV-only IOLs and support the “no harm” hypothesis.
Color Vision
Multiple studies assessed the effects of BLF IOLs on color vision and found no differences when using common clinical tests (eg, Farnsworth-Munsell 100-hue test) or clinical devices (eg, anomaloscope).Citation13–16 However, most of these gross clinical measures (eg, pseudoisochromatic plates) were designed to identify major color defectsCitation17 and may miss subtle differences in actual visual perception. Furthermore, recognizing that two colors match or detecting a hidden figure is different than measuring whether blue actually looks the same to an individual with a perfectly clear or BLF IOL. On the other hand, the white point method using a tricolorimeter to evaluate perception of white light may be a better assessment of color vision and defects.Citation18
Previous reports and reviews suggested no long-term effect of BLF IOLs on chromatic discrimination.Citation4,Citation13,Citation14,Citation19 Several studies, however, have indicated a transient effect of BLF IOLs on tritan-based discrimination soon after cataract surgery. In one study, chromatic discrimination assessed in 44 eyes using the Farnsworth-Munsell 100-hue test was significantly better for UV-only IOLs compared with orange-tinted BLF IOLs at 3 months postimplantation.Citation20 A prospective case-series study in 55 patients reported cyanopsia was significantly more common in patients receiving BLF IOLs versus violet-filtering IOLs at 1 week. There was no significant difference in cyanopsia between groups 1 month after surgery.Citation21 This finding reflects the general observation that effects of BLF on color matching for blue hues is transient, and the visual system recalibrates within a relatively short time.Citation22
Color, like many aspects of vision, is perceptual and dynamic, and the visual system will adapt over time. A long-term follow-up in 30 patients who underwent bilateral cataract surgery and received either BLF or UV-only IOLs reported no significant differences between groups on the Farnsworth-Munsell 100-hue test after 5 years.Citation12 More recently, chromatic discrimination in 60 patients was tested using the anomaloscope for the Moreland blue-green test. There was an anticipated shift in chromatic discrimination in the blue part of the spectrum in patients with BLF IOLs; however, no significant differences were observed for blue light discrimination for BLF versus UV-only IOLs, although a slight shift was observed toward green and yellow.Citation10
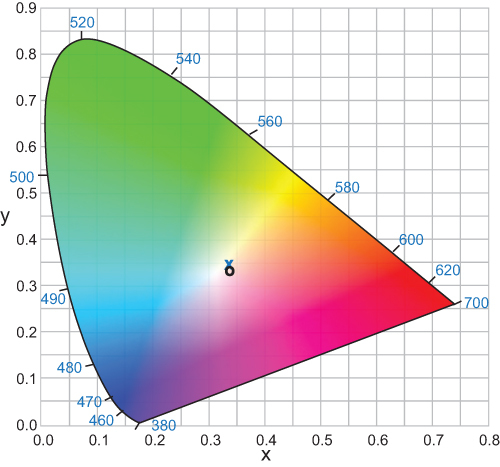
To better assess color appearance in patients with BLF IOLs, a recent study used the achromatic (white point) method. This method is based on patient response to the appearance of light; the white point is achieved when the chromatic systems are balanced.Citation18 A custom-built tricolorimeter was used to test white light perception by mixing three primary colors. Additive trichromatic colorimetry was used to measure color appearance with BLF IOLs, UV-only IOLs, and natural lenses. Patients were asked to identify “pure white”, and color appearance was expressed as chromaticity coordinates. No significant differences in color appearance were observed in eyes with BLF IOLs compared with UV-only IOLs ().Citation18 Similar outcomes were observed in participants using BLF versus clear contact lenses.Citation23 When comparing different age groups, a small shift in the white point was observed, suggesting that color perception in younger adults was different compared with older adults with BLF and UV-only IOLs.Citation18 Based on these results, the BLF would have minimal effect on long-term color appearance in patients who received artificial IOLs after cataract surgery.
Figure 2 Between-group comparison of the chromaticity coordinates of the BLF (x) and clear (o) IOL groups.
Glare
Glare is a general phenomenon that takes many deleterious forms for a typical patient with cataracts.Citation24 Increased intraocular scatter and the associated higher-order ocular aberrations from ocular inhomogeneities and bright light can cause veiling (glare disability), halos, and spokes that form around an intense point source (positive dysphotopsia) or increase pain and discomfort (glare discomfort). Even when not manifested as overt disability and discomfort (and at lower light intensity), scatter and aberrations degrade many of the fine aspects of vision, such as reduced contrast sensitivity across frequencies. Implanting an IOL often ameliorates many of these issues, but some visual complaints can remain. Light scattering by the nasal edge of the IOL can cause a perceived shadow over the temporal peripheral region (a form of negative dysphotopsia).Citation25
Several studies reviewed by Hammond in 2019Citation4 reported that BLF reduced such symptoms.Citation26–29 In a recent study, effects of BLF on light scatter were assessed in 52 participants with different levels of iris pigmentation. Eyes with BLF IOLs could perceive 2 point sources of light with significantly less distance between them compared with eyes implanted with UV-only IOLs under both broadband and short-wave experimental conditions; these outcomes were further improved in participants with darker iris color.Citation30 These results suggest that filtering blue light (a strategy often employed in nature)Citation31 improved some of the detrimental effects of broadband light and decreased the 2-point light threshold.
However, some reports suggest that BLF IOLs can increase the perception of glare while driving at night. A recent study found that 9/80 (11%) patients with BLF IOLs complained of glare issues while driving at night compared with 0/83 patients with UV-only IOLs.Citation32 Such results are hard to interpret, and it is not clear, mechanistically, how a filtering lens would increase the perception of glare; previous studies that measured driving safety found that BLF IOLs improved driving under glare conditions.Citation29,Citation33 The perception of glare might be due to a negative effect of BLF on scotopic functioning.Citation32 This interpretation, however, seems unlikely because of a minimal overlap between BLF IOL optical density and scotopic sensitivity. The reduction of broadband light by a BLF IOL versus UV-only IOL is equivalent to an optical density of ~0.07 log units, which is inconsequential when compared with scotopic sensitivity range over 4 log units.Citation34 Thus, BLF IOLs represent significant increase in blue light reception compared with a lens with cataracts (although not as much blue light as would be transmitted through a completely clear IOL). The system is dynamic, so sensitivity adjusts to offset normal changes on preretinal filtering.
Enhanced Long-Distance Vision: Visibility Hypothesis
Young healthy eyes, even with the same refractive condition, differ in how far they can see (visual range).Citation35 Walls and Judd suggested that distance vision was a common challenge across species that was aided by intraocular BLF (eg, pigmented oil droplets in the eyes of birds).Citation36 More recently, these effects were modeled for humans and their natural macular pigments (MP) that block high-energy blue light, leading to the proposed “visibility hypothesis” ().Citation37 Based on this analysis, an individual with high levels of MP would be able to see ~30% farther than an individual with low levels of MP.Citation37 This prediction was confirmed in a study that used a variable path-length filter with carotenoid solution to mimic the absorption spectrum of MP. When contrast thresholds were assessed under blue haze conditions, simulated MP caused an average improvement of 25%.Citation38 A cross-sectional study measuring contrast sensitivity function (CSF) under blue haze conditions found a similar effect: individuals with higher MP detected the grating through denser haze than those with lower MP levels.Citation39 Furthermore, an effect of lutein and zeaxanthin supplements (increasing retinal BLF) on improving contrast sensitivity is a relatively consistent finding.Citation40–45
Figure 3 Relative energy and percentage of transmission for blue haze, clear (UV-only) IOL, and BLF IOL.
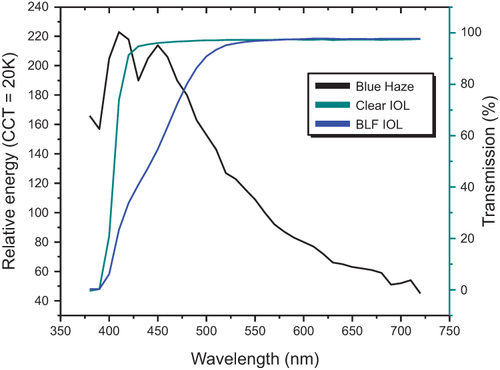
The ultimate effect of BLF on the CSF is likely situation-specific. Filtering may improve the CSF under some conditions (eg, blue haze) and be a null factor in other situations (eg, stimuli with minimal short-wave energy or dim light conditions). Both the photopic and scotopic systems have large dynamic ranges (eg, BLF filtering would be analogous to a few seconds of dark adaptation); therefore, mechanistically, we do not expect BLF filtering to be detrimental under most conditions.
Blue Light Filtration, Ocular Health, and Systemic Health
Age-Related Macular Degeneration and Blue Light
Age-related macular degeneration (AMD) is a late-onset, multifactorial disease caused by multiple genetic variants that leads to irreversible vision loss. Increased risk of late AMD is linked to increasing age, smoking, cataract surgery, and family history.Citation46 Drusen (acellular polymorphous debris) and pigmentary abnormalities are characteristic signs of AMD; together with geographic atrophy and neovascularization, they can be used to classify AMD severity. The two types of macular degeneration are dry AMD, characterized by geographic atrophy at the advanced stage, and wet AMD, characterized by choroidal neovascularization and pigment epithelial detachment associated with rapid progression of vision loss.Citation47
High-energy visible blue light in the 400- to 500-nm wavelength range can cause tissue damage, including photochemical damage to the retina and retinal pigment epithelium (RPE) cells, and may contribute to AMD.Citation48–52 In a case-control study with 3701 participants from the European Genetic Database, AMD was significantly associated with both past sunlight exposure and outside work.Citation49 However, there was no evidence that environmental light exposure resulted in AMD, and although acute exposure to phototoxicity would be damaging to the macula, it would not simulate lifelong environmental light exposure.Citation53 It would be difficult, if not impossible, to do the absence-of-proof studies that would provide the evidence that is absent.Citation53,Citation54 Because chronic interventions and longitudinal data are not available, most scientists do acute laboratory studiesCitation55,Citation56 and make inferences; these inferences are based on a fairly voluminous body of evidence.Citation57,Citation58
Because BLF IOLs mimic a younger lens, patients would receive significantly more blue light after cataract surgery compared with presurgical levels (but not all blue light as would occur with a clear IOL or in the eyes of an infant).
Protective Role of Blue Light Filters: In Vitro Studies
Retinal pigment epithelium cells support photoreceptor cells, and damage to and loss of RPE cells are characteristic of AMD.Citation59 Recent in vitro studies suggested possible protective effects of BLF, including reversal of blue light effects on cell viability, metabolic activity, and cell shape in spontaneously arising retinal pigment epithelial 19 (ARPE-19) and primary RPE cells and improved survival under blue light–emitting diode (LED) irradiation in porcine primary RPE cells.Citation60–62 Another study in ARPE-19 cells, however, reported that BLF IOLs did not reduce the expression of heme oxygenase-1 or have a protective effect on mitochondrial membrane potential in these experiments, although blue LED light exposure led to increased oxidative stress (measured by heme oxygenase-1 levels).Citation63
The results of these in vitro studies confirm that blue light can cause damage to RPE cells. Some of the studies supported the protective function of BLF IOLs,Citation60–62 but others did not report significant effects with BLF,Citation63 possibly because of variations in study design and assessments. All these studies support the “no harm” hypothesis.
Furthermore, in vitro studies reported a higher proliferation rate of human uveal melanoma cells exposed to blue light compared with control samples shielded from blue light.Citation64,Citation65 Although rare, uveal melanoma is associated with high mortality because of its high propensity to metastasize to the liverCitation66 and limited treatment options. Although current evidence suggests blue light may be potentially harmful and BLF IOLs could prevent the increase in proliferation of uveal melanoma cells,Citation65 additional studies are needed to assess whether BLF IOLs may reduce the risk of uveal melanoma.
Protective Role of Blue Light Filters: Clinical Studies
Clinical studies provide conflicting evidence about the role of BLF IOLs in protection against AMD. AMD develops over a lifetime; although damage to disease tissue cascades (an argument for increased protection later in life), for many, the disease may have progressed too far. A meta-analysis of clinical trials reported no clinically meaningful difference in the development of AMD in patients receiving BLF IOLs versus those with UV-only IOLs and suggested that the benefits of BLF IOLs remained inconclusive.Citation8 However, the variability in methodologies and the use of AMD diagnosis as an endpoint to evaluate the effects of BLF IOLs may also complicate interpretation of the outcomes.
A registry-based, real-world cohort study in Finland assessed effects of BLF IOLs on prevention of wet AMD. One year after cataract surgery (n = 164 eyes), wet AMD rates were not significantly different in the BLF and UV-only IOL groups. A Cox regression analysis that controlled for age, sex, and a documented diagnosis of macular degeneration found that the use of BLF IOLs did not predict AMD development.Citation67 The incidence, progression, and severity of wet AMD were similar in BLF and UV-only IOL groups, suggesting there were no apparent advantages or disadvantages associated with BLF IOLs.
A retrospective case-control study (196 patients with AMD, 196 matching control patients) assessed whether BLF IOLs prevented onset of wet AMD. Similar proportions of eyes with BLF IOLs were reported in patients with wet AMD and in the control group, suggesting that there was no association between BLF IOLs and the likelihood of AMD during the 6-year follow-up.Citation68
Most recently, a retrospective cohort study in Taiwan assessed the incidence of AMD in patients with BLF (n = 21,126) and UV-only IOLs (n = 165,465) throughout a 10-year follow-up. After propensity score matching, AMD incidence was similar in BLF and UV-only groups (1.22 and 1.26 events per 1000 person-years, respectively, for wet AMD; 9.95 and 10.37 events per 1000 person-years, respectively, for dry AMD).Citation69 Based on these results, no advantage and no harm were associated with one type of lens over the other. However, the allocation to IOL groups in this retrospective study was not randomized, and there were differences in age, sex, and socioeconomic status between the groups. Although propensity score matching was used to control for significantly different variables, other confounders (smoking, diet, sunlight exposure) may complicate the interpretation of results.
An AMD diagnosis may not be the optimal endpoint to assess benefits and harm of BLF IOLs; instead, continuous quantifiable endpoints, such as geographic atrophy area or fundus autofluorescence, may be more appropriate. However, there are limited studies assessing these endpoints. A retrospective analysis of 66 eyes with AMD reported that mean geographic atrophy progression was significantly less in eyes with BLF versus UV-only IOLs (P=0.0002).Citation70 A prospective comparative observational study in patients with BLF IOLs (n = 52) and UV-only IOLs (n = 79) reported that abnormal fundus autofluorescence (which is predictive of AMD) did not develop or increase in size or density in eyes with BLF IOLs. In the UV-only group, 15% of eyes developed or progressed in abnormal fundus autofluorescence. Furthermore, a significantly higher incidence of AMD was observed in patients who received UV-only IOLs (11%) versus those receiving BLF IOLs (2%) 2 years after implantation (P=0.042).Citation71 The outcomes of these studies support the role of the BLF IOLs in providing protection against AMD progression.
Age-related macular degeneration is a disease marked by visual loss. Although there is a lack of consensus from the currently available clinical studies, we suggest that if BLF is palliative (ie, improves chromatic contrast) and decreases blue light exposure of a retina filled with photosensitizers maximally sensitive to short-wave activation, then including BLF as an option for cataract replacement is reasonable.
BLF IOLs may also provide an advantage in patients without pre-existing glaucoma by extending glaucoma-free survival. Glaucoma, characterized by a progressive degeneration of retinal ganglion cells, may result in irreversible blindness. The two major classifications of glaucoma are open-angle and angle-closure glaucoma, and both may be primary diseases or caused by trauma or medication (eg, corticosteroids).Citation72 A retrospective cohort study assessed the risk of developing glaucoma in patients with BLF IOLs (n = 5188) and UV-only IOLs (n = 5840). During the mean follow-up of 55 ± 34 months, a significantly better glaucoma-free survival rate was reported in patients with BLF IOLs compared with patients with UV-only IOLs (P=0.036). After 7 years, 9.6% of patients with UV-only IOLs were diagnosed with glaucoma, compared with 6.7% of patients with BLF IOLs, a 43% improvement.Citation73 Future studies will need to confirm the protective role of BLF IOLs and glaucoma risks in cataract patients.
Circadian Rhythm and Sleep
Circadian rhythm, which plays a role in regulating sleep, is controlled by light input and mediated through retinal photoreceptors. These cells absorb blue light in the peak absorption range of 446–477 nm, which regulates melatonin secretion.Citation74,Citation75 Although concerns have been raised that BLF IOLs provide less melatonin suppression compared with clear IOLs, affecting circadian clock and sleep,Citation76 results from numerous studies suggest that BLF does not interfere with basic functions, such as circadian rhythms and sleep, supporting the “no harm” hypothesis on this topic. A small retrospective study in patients who underwent cataract surgery (n = 49) reported no significant differences in any Pittsburgh Sleep Quality Index component or global score between BLF and UV-only IOL groups.Citation77 A randomized controlled study (n = 204) assessed sleep in patients with BLF and UV-only IOLs and found no significant differences in total sleep time variation or sleep quality between groups 8 weeks after surgery.Citation78 Furthermore, a single-center, double-masked, block-randomized clinical trial (n = 76) found that IOL type had no significant effect on sleep efficiency or subjective sleep quality.Citation79 A 1-year follow-up of a randomized controlled trial (n = 67) reported no difference between BLF and UV-only IOL groups for sleep-specific and circadian-specific parameters assessed by actigraphy. Interestingly, the BLF versus UV-only IOL group had better sleep efficiency and a lower melatonin concentration.Citation80 A large cohort study conducted in Taiwan (19,604 participants with BLF IOLs; 151,811 participants with UV-only IOLs) found no significant difference in incidence rate of insomnia in BLF versus the UV-only IOL group after propensity score matching.Citation81
Based on results from a prospective cohort study (n = 152), cataract surgery and BLF IOL implantation significantly improved sleep quality at 1 month after implantation (P<0.01) compared with preoperative assessment; this effect was maintained at 12 months after implantation.Citation82 Improvement of sleep quality at 1 month after implantation was significantly greater with UV-only versus BLF IOLs (P<0.01); however, there were no significant differences between groups at 1 year.Citation82 Similarly, a recent meta-analysis reported that UV-only IOLs were associated with improvements in subjective sleep quality compared with BLF IOLs at 3 to 8 weeks after implantation, but there were no significant differences between groups for sleep quality at 7 to 12 months after implantation.Citation83 This could be because of greater blue light transmittance with UV-only IOLs; the visual system can dynamically adjust sensitivity over a range of several log units (certainly, well over the amount of light absorbed by a typical BLF filter). After patients adapt, there appeared to be no long-term detrimental effect on quality of sleep. Overall, these findings suggest that there were no long-term negative effects on sleep or circadian rhythms associated with BLF IOLs.
Mood and Cognition
Recent research has shown that blue light is one of the more important synchronizing agents for circadian rhythms and mood regulation.Citation84 This has raised the question of whether BLF IOLs, despite somewhat minimal filtering, might negatively influence mood by decreasing retinal exposure to blue light.Citation78,Citation85 Conversely, a different set of studies suggest that decreased blue light exposure at night might improve mood (eg, decreasing depression).Citation86,Citation87 The disparity arises from the observation that it is not just the quantity of exposure that matters but also the timing. To mimic the natural phases of sunlight under which our chronobiology has evolved, it is optimal to increase exposure to short-wave light in the morningCitation88 but decrease exposure at night.Citation89 Because BLF IOLs are a constant (ie, do not affect the timing of exposure), it seems unlikely that BLF IOLs could influence mood, especially over time.
However, IOLs in general can improve many other aspects of brain function. A recent study has shown, for instance, that cataract surgery is related to a 29% lower rate of dementia.Citation90 Cognition is an endpoint that is based on sensory input; improving the input may lead to improvement in subsequent processing.Citation91 In a study that assessed visual comfort and mental effort during light exposure (n = 29), patients with BLF IOLs had significantly lower levels of perceived ambient light glare (P=0.01) and visual tension (P=0.042) compared with patients with UV-only IOLs or the age-adjusted control group.Citation92
Discussion
There is a consensus that short-wave “blue” light can be damaging to the retina.Citation93 The change in transparency of the natural crystalline lens with age occurs as the retina and RPE become increasingly sensitive to short-wave energy as a result of accumulation of blue-sensitive photosensitizers like Bis-retinoid N-retinyl-N-retinylidene ethanolamine (A2E).Citation94 The yellow pigments in the inner layers of the retina provide additional protection to the vulnerable cones within the macula.Citation95
The fact that the visual system so readily compensates for dense crystalline lenses and high levels of MP suggests that heavy screening is a natural phenomenon, balancing photoprotection and photoreception.Citation22,Citation96 It is unlikely that the visual system evolved mechanisms to protect the retina from diseases of old age (past reproductive fecundity). Although effects of light damage may be seen relatively early, a more likely explanation is that BLFs have an acute influence on visual function, which may potentially influence the mechanisms of natural selection. Screening the retina from the shortest wavelengths of light improves glare disability and discomfort as well as chromatic contrast while speeding photostress recovery by both BLF IOL and MP.Citation4,Citation97
The longer life expectancy and the desire of some cataract patients for clear IOLs in order to maximize visible light reaching the retina highlight the importance of patient care that continues after the IOL implantation. Healthcare professionals should advise patients on wearing sunglasses outdoors to block blue light to reduce the risks of retinal damage. Furthermore, because the effects of light damage are lifelong and begin early, a related question is whether a pediatric population would benefit from increased blue-light protection. MPs lutein and zeaxanthin are present in the rapidly developing fovea in the retina of the eye.Citation98 Lutein and zeaxanthin are present in breastmilk, are currently used in infant formula, and may play a protective role against the harmful effects of both sunlight and artificial light from electronic devices.Citation99,Citation100 Potential protection provided by BLF IOLs in pediatric patients should be evaluated in future studies.
Disclosure
Prof. Kohnen has served as a consultant and received research funding from Alcon Vision LLC, Carl Zeiss Meditec AG, Johnson & Johnson, Oculentis, Oculus Optikgeräte GmbH, Presbia, Schwind eye-tech-solutions, and Zeiss; served as a consultant for Allergan, Inc., Avedro, Inc., Bausch & Lomb, Inc., Dompé, Geuder, LensGen, Med Update, Nevarkar, Santen, Staar, Tear Lab, Thieme, and Ziemer. Dr. Hammond has received research funding from Alcon Vision LLC in the last three years.
Acknowledgments
Medical writing assistance was provided by Natalia Zhukovskaya, PhD, of ICON (Blue Bell, PA) and was funded by Alcon.
Additional information
Funding
References
- Di Carlo E, Augustin AJ. Prevention of the onset of age-related macular degeneration. J Clin Med. 2021;10(15):3297. doi:10.3390/jcm10153297
- Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006;84(1):4–15. doi:10.1111/j.1600-0420.2005.00627.x
- Kessel L, Lundeman JH, Herbst K, Andersen TV, Larsen M. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg. 2010;36(2):308–312. doi:10.1016/j.jcrs.2009.08.035
- Hammond BR, Sreenivasan V, Suryakumar R. The effects of blue light-filtering intraocular lenses on the protection and function of the visual system. Clin Ophthalmol. 2019;13:2427–2438. doi:10.2147/OPTH.S213280
- Werner JS. Development of scotopic sensitivity and the absorption spectrum of the human ocular media. J Opt Soc Am. 1982;72(2):247–258. doi:10.1364/JOSA.72.000247
- Hartzer MK, Akinay A, Ong M, et al. Light transmission characteristics of the human lens as a function of age. Invest Ophthalmol Vis Sci. 2008;49(13):3789.
- Kohnen T, Baumeister M, Kook D, Klaproth OK, Ohrloff C. Cataract surgery with implantation of an artificial lens. Dtsch Arztebl Int. 2009;106(43):695–702. doi:10.3238/arztebl.2009.0695
- Downie LE, Busija L, Keller PR. Blue-light filtering intraocular lenses (IOLs) for protecting macular health. Cochrane Database Syst Rev. 2018;5:CD011977.
- Tzamalis A, Kynigopoulos M, Pallas G, Tsinopoulos I, Ziakas N. Influence of intraocular lens asphericity and blue light filtering on visual outcome, contrast sensitivity, and aberrometry after uneventful cataract extraction. J Ophthalmic Vis Res. 2020;15(3):308–317. doi:10.18502/jovr.v15i3.7449
- Popov I, Jurenova D, Valaskova J, et al. Effect of blue light filtering intraocular lenses on visual perception. Medicina. 2021;57(6):559. doi:10.3390/medicina57060559
- Mester U, Holz F, Kohnen T, Lohmann C, Tetz M. Intraindividual comparison of a blue-light filter on visual function: AF-1 (UY) versus AF-1 (UV) intraocular lens. J Cataract Refract Surg. 2008;34(4):608–615. doi:10.1016/j.jcrs.2007.11.049
- Kara-Junior N, Espindola RF, Gomes BA, et al. Effects of blue light-filtering intraocular lenses on the macula, contrast sensitivity, and color vision after a long-term follow-up. J Cataract Refract Surg. 2011;37(12):2115–2119. doi:10.1016/j.jcrs.2011.06.024
- Cionni RJ, Tsai JH. Color perception with AcrySof natural and AcrySof single-piece intraocular lenses under photopic and mesopic conditions. J Cataract Refract Surg. 2006;32(2):236–242. doi:10.1016/j.jcrs.2005.12.129
- Khokhar SK, Jindal A, Agarwal T, Panda A. Comparison of color perception after tinted blue light-filtering and clear ultraviolet-filtering intraocular lens implantation. J Cataract Refract Surg. 2011;37(9):1598–1604. doi:10.1016/j.jcrs.2011.03.044
- Hayashi K, Hayashi H. Visual function in patients with yellow tinted intraocular lenses compared with vision in patients with non-tinted intraocular lenses. Br J Ophthalmol. 2006;90(8):1019–1023. doi:10.1136/bjo.2006.090712
- Zhu XF, Zou HD, Yu YF, Sun Q, Zhao NQ. Comparison of blue light-filtering IOLs and UV light-filtering IOLs for cataract surgery: a meta-analysis. PLoS One. 2012;7(3): e33013.
- Cao D. Chapter 10 - Color vision and night vision. Ryan SJ, editor. Ryan’s Retina. Vol. 1. 5th ed. Elsevier Inc.; 2012:285–299.
- Hammond BR, Wooten BR, Saint SE, Renzi-Hammond L. The effects of a blue-light filtering versus clear intraocular implant on color appearance. Transl Vis Sci Technol. 2021;10(12):25. doi:10.1167/tvst.10.12.25
- Downes SM. Ultraviolet or blue-filtering intraocular lenses: what is the evidence? Eye. 2016;30(2):215–221. doi:10.1038/eye.2015.267
- Schmack I, Schimpf M, Stolzenberg A, et al. Visual quality assessment in patients with orange-tinted blue light-filtering and clear ultraviolet light-filtering intraocular lenses. J Cataract Refract Surg. 2012;38(5):823–832. doi:10.1016/j.jcrs.2011.12.028
- Nakano S, Miyata A, Kizawa J, et al. Blue light-filtering and violet light-filtering hydrophobic acrylic foldable intraocular lenses: intraindividual comparison. J Cataract Refract Surg. 2019;45(10):1393–1397. doi:10.1016/j.jcrs.2019.05.027
- Delahunt PB, Webster MA, Ma L, Werner JS. Long-term renormalization of chromatic mechanisms following cataract surgery. Vis Neurosci. 2004;21(3):301–307. doi:10.1017/S0952523804213025
- Hammond BR, Buch J, Renzi-Hammond LM, Bosten JM, Nankivil D. The effect of a short-wave filtering contact lens on color appearance. J Vis. 2023;23(1):2. doi:10.1167/jov.23.1.2
- Hammond BR, Fletcher LM, Elliott JG. Glare disability, photostress recovery, and chromatic contrast: relation to macular pigment and serum lutein and zeaxanthin. Invest Ophthalmol Vis Sci. 2013;54(1):476–481. doi:10.1167/iovs.12-10411
- Bhalla JS, Gupta S. Dysphotopsia - unraveling the enigma. Delhi J Ophthalmol. 2016;27(2):97–101. doi:10.7869/djo.217
- Hammond BR. Attenuating photostress and glare disability in pseudophakic patients through the addition of a short-wave absorbing filter. J Ophthalmol. 2015;2015:607635. doi:10.1155/2015/607635
- Hammond BR, Renzi LM, Sachak S, Brint SF. Contralateral comparison of blue-filtering and non–blue-filtering intraocular lenses: glare disability, heterochromatic contrast, and photostress recovery. Clin Ophthalmol. 2010;4:1465–1473. doi:10.2147/OPTH.S15102
- Hammond BR, Bernstein B, Dong J. The effect of the AcrySof natural lens on glare disability and photostress. Am J Ophthalmol. 2009;148(2):272–276.e2. doi:10.1016/j.ajo.2009.03.014
- Gray R, Hill W, Neuman B, Houtman D, Potvin R. Effects of a blue light-filtering intraocular lens on driving safety in glare conditions. J Cataract Refract Surg. 2012;38(5):816–822. doi:10.1016/j.jcrs.2011.11.047
- Renzi-Hammond LM, Hammond BR. Blue-light filtering intraocular implants and darker irises reduce the behavioral effects of higher-order ocular aberrations. Curr Eye Res. 2022;47(5):753–758. doi:10.1080/02713683.2022.2025844
- Hammond BR. The visual effects of intraocular colored filters. Scientifica. 2012;2012:424965. doi:10.6064/2012/424965
- Kanclerz P, Hecht I, Cunha M, et al. Association of blue light-filtering intraocular lenses with all-cause and traffic accident-related injuries among patients undergoing bilateral cataract surgery in Finland. JAMA Network Open. 2022;5(8):e2227232. doi:10.1001/jamanetworkopen.2022.27232
- Gray R, Perkins SA, Suryakumar R, Neuman B, Maxwell WA. Reduced effect of glare disability on driving performance in patients with blue light-filtering intraocular lenses. J Cataract Refract Surg. 2011;37(1):38–44. doi:10.1016/j.jcrs.2010.07.034
- Werner JS. Night vision in the elderly: consequences for seeing through a “blue filtering” intraocular lens. Br J Ophthalmol. 2005;89(11):1518–1521. doi:10.1136/bjo.2005.073734
- Hammond BR, Buch J. Individual differences in visual function. Exp Eye Res. 2020;199:108186. doi:10.1016/j.exer.2020.108186
- Walls GL, Judd HD. The intra-ocular colour-filters of vertebrates. Br J Ophthalmol. 1933;17(11):641–675. doi:10.1136/bjo.17.11.641
- Wooten BR, Hammond BR. Macular pigment: influences on visual acuity and visibility. Prog Retin Eye Res. 2002;21(2):225–240. doi:10.1016/S1350-9462(02)00003-4
- Hammond BR, Wooten BR, Engles M, Wong JC. The influence of filtering by the macular carotenoids on contrast sensitivity measured under simulated blue haze conditions. Vision Res. 2012;63:58–62. doi:10.1016/j.visres.2012.04.019
- Fletcher LM, Engles M, Hammond BR. Visibility through atmospheric haze and its relation to macular pigment. Optom Vis Sci. 2014;91(9):1089–1096. doi:10.1097/OPX.0000000000000355
- Lawler T, Liu Z, Nalbandyan M, et al. Lutein and zeaxanthin supplement use is associated with increased macular pigment density over 15 years and greater contrast sensitivity in the Carotenoids in Age-Related Eye Disease Study of older-adult women. Invest Ophthalmol Vis Sci. 2021;62(8):2950.
- Nolan JM, Power R, Stringham J, et al. Enrichment of macular pigment enhances contrast sensitivity in subjects free of retinal disease: Central Retinal Enrichment Supplementation trials - report 1. Invest Ophthalmol Vis Sci. 2016;57(7):3429–3439. doi:10.1167/iovs.16-19520
- Wolf-Schnurrbusch UE, Zinkernagel MS, Munk MR, Ebneter A, Wolf S. Oral lutein supplementation enhances macular pigment density and contrast sensitivity but not in combination with polyunsaturated fatty acids. Invest Ophthalmol Vis Sci. 2015;56(13):8069–8074. doi:10.1167/iovs.15-17586
- Sasamoto Y, Gomi F, Sawa M, Tsujikawa M, Nishida K. Effect of 1-year lutein supplementation on macular pigment optical density and visual function. Graefes Arch Clin Exp Ophthalmol. 2011;249(12):1847–1854. doi:10.1007/s00417-011-1780-z
- Loughman J, Nolan JM, Howard AN, et al. The impact of macular pigment augmentation on visual performance using different carotenoid formulations. Invest Ophthalmol Vis Sci. 2012;53(12):7871–7880. doi:10.1167/iovs.12-10690
- Machida N, Kosehira M, Kitaichi N. Clinical effects of dietary supplementation of lutein with high bio-accessibility on macular pigment optical density and contrast sensitivity: a randomized double-blind placebo-controlled parallel-group comparison trial. Nutrients. 2020;12(10):2966. doi:10.3390/nu12102966
- Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. doi:10.1186/1471-2415-10-31
- Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: a review. Clin Interv Aging. 2017;12:1313–1330. doi:10.2147/CIA.S143508
- Klein BE, Howard KP, Iyengar SK, et al. Sunlight exposure, pigmentation, and incident age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(9):5855–5861. doi:10.1167/iovs.14-14602
- Schick T, Ersoy L, Lechanteur YT, et al. History of sunlight exposure is a risk factor for age-related macular degeneration. Retina. 2016;36(4):787–790. doi:10.1097/IAE.0000000000000756
- Ham WT, Ruffolo JJ, Mueller HA, Clarke AM, Moon ME. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Invest Ophthalmol Vis Sci. 1978;17(10):1029–1035.
- Wang L, Yu X, Zhang D, et al. Long-term blue light exposure impairs mitochondrial dynamics in the retina in light-induced retinal degeneration in vivo and in vitro. J Photochem Photobiol B. 2023;240:112654. doi:10.1016/j.jphotobiol.2023.112654
- Li X, Zhu S, Qi F. Blue light pollution causes retinal damage and degeneration by inducing ferroptosis. J Photochem Photobiol B. 2023;238:112617. doi:10.1016/j.jphotobiol.2022.112617
- Mainster MA, Findl O, Dick HB, et al. The blue light hazard versus blue light hype. Am J Ophthalmol. 2022;240:51–57. doi:10.1016/j.ajo.2022.02.016
- Hammond BR, Renzi-Hammond L. Comment on: the blue light hazard versus blue light hype. Am J Ophthalmol. 2022;241:282–283. doi:10.1016/j.ajo.2022.03.032
- Chen P, Lai Z, Wu Y, et al. Retinal neuron is more sensitive to blue light-induced damage than glia cell due to DNA double-strand breaks. Cells. 2019;8(1):68. doi:10.3390/cells8010068
- Ratnayake K, Payton JL, Meger ME, et al. Blue light-triggered photochemistry and cytotoxicity of retinal. Cell Signal. 2020;69:109547. doi:10.1016/j.cellsig.2020.109547
- Contín MA, Benedetto MM, Quinteros-Quintana ML, Guido ME. Light pollution: the possible consequences of excessive illumination on retina. Eye. 2016;30(2):255–263. doi:10.1038/eye.2015.221
- Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Surv Ophthalmol. 2006;51(5):461–481. doi:10.1016/j.survophthal.2006.06.009
- Forest DL, Johnson LV, Clegg DO. Cellular models and therapies for age-related macular degeneration. Dis Model Mech. 2015;8(5):421–427. doi:10.1242/dmm.017236
- Vila N, Siblini A, Esposito E, et al. Blue-light filtering alters angiogenic signaling in human retinal pigmented epithelial cells culture model. BMC Ophthalmol. 2017;17(1):198. doi:10.1186/s12886-017-0592-2
- Abdouh M, Lu M, Chen Y, et al. Filtering blue light mitigates the deleterious effects induced by the oxidative stress in human retinal pigment epithelial cells. Exp Eye Res. 2022;217:108978. doi:10.1016/j.exer.2022.108978
- Yu WY, Shan SSW, Lakshmanan Y, et al. Selective blue-filtering spectacle lens protected primary porcine RPE cells against light emitting diode-induced cell damage. PLoS One. 2022;17(5):e0268796. doi:10.1371/journal.pone.0268796
- Fernandez-Vega Cueto A, Del Olmo-Aguado S, Garcia-Perez E, et al. Protector role of intraocular lenses under artificial light conditions. Ophthalmic Res. 2022;65(3):276–286. doi:10.1159/000521306
- Di Cesare S, Maloney S, Fernandes BF, et al. The effect of blue light exposure in an ocular melanoma animal model. J Exp Clin Cancer Res. 2009;28(1):48. doi:10.1186/1756-9966-28-48
- Marshall JC, Gordon KD, McCauley CS, de Souza Filho JP, Burnier MN. The effect of blue light exposure and use of intraocular lenses on human uveal melanoma cell lines. Melanoma Res. 2006;16(6):537–541. doi:10.1097/CMR.0b013e3280112b86
- Logan P, Bernabeu M, Ferreira A, Burnier MN. Evidence for the role of blue light in the development of uveal melanoma. J Ophthalmol. 2015;2015:386986. doi:10.1155/2015/386986
- Achiron A, Elbaz U, Hecht I, et al. The effect of blue-light filtering intraocular lenses on the development and progression of neovascular age-related macular degeneration. Ophthalmology. 2021;128(3):410–416. doi:10.1016/j.ophtha.2020.07.039
- Hamel T, Rheault J, Simonyan D, Bourgault S, Rochette PJ. The influence of blue-filtering intraocular lenses implant on exudative age-related macular degeneration: a case-control study. Clin Ophthalmol. 2021;15:2287–2292. doi:10.2147/OPTH.S300461
- Lee JS, Li PR, Hou CH, et al. Effect of blue light-filtering intraocular lenses on age-related macular degeneration: a nationwide cohort study with 10-year follow-up. Am J Ophthalmol. 2022;234:138–146. doi:10.1016/j.ajo.2021.08.002
- Pipis A, Touliou E, Pillunat LE, Augustin AJ. Effect of the blue filter intraocular lens on the progression of geographic atrophy. Eur J Ophthalmol. 2015;25(2):128–133. doi:10.5301/ejo.5000520
- Nagai H, Hirano Y, Yasukawa T, et al. Prevention of increased abnormal fundus autofluorescence with blue light-filtering intraocular lenses. J Cataract Refract Surg. 2015;41(9):1855–1859. doi:10.1016/j.jcrs.2015.01.017
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi:10.1001/jama.2014.3192
- Hecht I, Kanclerz P, Achiron A, Elbaz U, Tuuminen R. The effect of blue-light filtering intraocular lenses on the development and progression of glaucoma. J Glaucoma. 2023;32(6):451–457. doi:10.1097/IJG.0000000000002220
- Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–6412. doi:10.1523/JNEUROSCI.21-16-06405.2001
- Turner PL, Mainster MA. Circadian photoreception: ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92(11):1439–1444. doi:10.1136/bjo.2008.141747
- Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006;90(6):784–792. doi:10.1136/bjo.2005.086553
- Landers JA, Tamblyn D, Perriam D. Effect of a blue-light-blocking intraocular lens on the quality of sleep. J Cataract Refract Surg. 2009;35(1):83–88. doi:10.1016/j.jcrs.2008.10.015
- Zambrowski O, Tavernier E, Souied EH, et al. Sleep and mood changes in advanced age after blue-blocking (yellow) intra ocular lens (IOLs) implantation during cataract surgical treatment: a randomized controlled trial. Aging Mental Health. 2018;22(10):1351–1356. doi:10.1080/13607863.2017.1348482
- Brondsted AE, Sander B, Haargaard B, et al. The effect of cataract surgery on circadian photoentrainment: a randomized trial of blue-blocking versus neutral intraocular lenses. Ophthalmology. 2015;122(10):2115–2124. doi:10.1016/j.ophtha.2015.06.033
- Brondsted AE, Haargaard B, Sander B, et al. The effect of blue-blocking and neutral intraocular lenses on circadian photoentrainment and sleep one year after cataract surgery. Acta Ophthalmol. 2017;95(4):344–351. doi:10.1111/aos.13323
- See LC, Li PR, Lin KK, Hou CH, Lee JS. Effect of blue light-filtering intraocular lenses on insomnia after cataract surgery: a nationwide cohort study with 10-year follow-up. Am J Ophthalmol. 2022;239:26–36. doi:10.1016/j.ajo.2022.01.012
- Feng X, Xu K, Hao Y, Qi H. Impact of blue-light filtering intraocular lens implantation on the quality of sleep in patients after cataract surgery. Medicine. 2016;95(51):e5648. doi:10.1097/MD.0000000000005648
- Lee TM, Loh EW, Kuo TC, et al. Effects of ultraviolet and blue-light filtering on sleep: a meta-analysis of controlled trials and studies on cataract patients. Eye. 2021;35(6):1629–1636. doi:10.1038/s41433-020-01132-2
- Wahl S, Engelhardt M, Schaupp P, Lappe C, Ivanov IV. The inner clock-blue light sets the human rhythm. J Biophotonics. 2019;12(12):e201900102. doi:10.1002/jbio.201900102
- Hammond BR, vanDellen M. The effects of intraocular lens implant type on mood: a response to Zambrowski et al. Aging Mental Health. 2019;23(2):171–172. doi:10.1080/13607863.2017.1399351
- Cho Y, Ryu SH, Lee BR, et al. Effects of artificial light at night on human health: a literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int. 2015;32(9):1294–1310. doi:10.3109/07420528.2015.1073158
- Bennett S, Alpert M, Kubulins V, Hansler RL. Use of modified spectacles and light bulbs to block blue light at night may prevent postpartum depression. Med Hypotheses. 2009;73(2):251–253. doi:10.1016/j.mehy.2009.01.049
- Choi K, Shin C, Kim T, Chung HJ, Suk HJ. Awakening effects of blue-enriched morning light exposure on university students’ physiological and subjective responses. Sci Rep. 2019;9(1):345. doi:10.1038/s41598-018-36791-5
- Jniene A, Errguig L, El Hangouche AJ, et al. Perception of sleep disturbances due to bedtime use of blue light-emitting devices and its impact on habits and sleep quality among young medical students. BioMed Res Int. 2019;2019:7012350. doi:10.1155/2019/7012350
- Lee CS, Gibbons LE, Lee AY, et al. Association between cataract extraction and development of dementia. JAMA Intern Med. 2022;182(2):134–141. doi:10.1001/jamainternmed.2021.6990
- Lad M, Sedley W, Griffiths TD. Sensory loss and risk of dementia. Neuroscientist. 2022;30(2):247–259. doi:10.1177/10738584221126090
- Steinemann A, Bromundt V, Chellappa SL, et al. Evaluation of visual comfort and mental effort under different light conditions for ultraviolet-absorbing and additional blue-filtering intraocular lenses for cataract surgery. Klin Monbl Augenheilkd. 2019;236(4):398–404. doi:10.1055/a-0810-0302
- Lu Y, Qi H. Evaluate the protective effect of antioxidants on retinal pigment cell hazard induced by blue light: a mini-review. Food Rev Int. 2022. doi:10.1080/87559129.87552022.82098317
- Marie M, Bigot K, Angebault C, et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018;9(3):287. doi:10.1038/s41419-018-0331-5
- Arunkumar R, Calvo CM, Conrady CD, Bernstein PS. What do we know about the macular pigment in AMD: the past, the present, and the future. Eye. 2018;32(5):992–1004. doi:10.1038/s41433-018-0044-0
- Stringham JM, Hammond BR, Wooten BR, Snodderly DM. Compensation for light loss resulting from filtering by macular pigment: relation to the S-cone pathway. Optom Vis Sci. 2006;83(12):887–894. doi:10.1097/01.opx.0000249976.00534.2d
- Johnson EJ, Avendano EE, Mohn ES, Raman G. The association between macular pigment optical density and visual function outcomes: a systematic review and meta-analysis. Eye. 2021;35(6):1620–1628. doi:10.1038/s41433-020-01124-2
- Hammond B. The dietary carotenoids lutein and zeaxanthin in pre-and-postnatal development. Funct Food Rev. 2012;4(3):130–137.
- Beluska-Turkan K, Korczak R, Hartell B, et al. Nutritional gaps and supplementation in the first 1000 days. Nutrients. 2019;11(12):2891. doi:10.3390/nu11122891
- Zimmer JP, Hammond BR. Possible influences of lutein and zeaxanthin on the developing retina. Clin Ophthalmol. 2007;1(1):25–35.
