Abstract
Background
Anti-vascular endothelial growth factor (anti-VEGF) agents are widely prescribed for the treatment of neovascular age-related macular degeneration (nAMD). Although studies have investigated patient choice of anti-VEGF agent, little is known regarding factors that influence physician preference of anti-VEGF agent for their patients.
Objective
To describe physician rationale and challenges in prescribing anti-VEGF treatments for patients with nAMD.
Methods
Data were drawn from the Adelphi Real World nAMD Disease Specific Programme™, a cross-sectional survey with retrospective data capture of physicians and their patients with nAMD in the United States between October 2021 and May 2022. Physicians (n = 56) reported data for up to 13 consecutively consulting patients (n = 451), including current anti-VEGF treatments used, factors affecting physicians’ choice of anti-VEGF agent and treatment strategy, and restrictions on specific agents.
Results
Most physicians prefer employing a “treat-and-extend” treatment strategy, over “fixed interval” or “pro re nata” strategies. However, in routine clinical practice, “treat-and-extend” was reported for less than half of nAMD-diagnosed eyes. Top factors influencing physician choice of anti-VEGF agent and treatment strategy included maximizing clinical benefit (eg visual acuity gains and fluid control), patient convenience, and reducing out-of-pocket costs. However, physicians also reported facing substantial roadblocks in prescribing their choice of anti-VEGF agent, including restrictions on approved agents and gaps in insurance coverage. Persistent fluid was the most common physician-selected reason for switching a patient away from an anti-VEGF agent.
Conclusion
Physicians face barriers to prescribing their preferred anti-VEGF agents in real-world healthcare settings. Overcoming these challenges may improve treatment outcomes for patients with nAMD.
Plain Language Summary
People with wet age-related macular degeneration (wet AMD) have problems with their eyesight that can lead to blindness if left untreated. Eye doctors (ophthalmologists) use a class of medicine called anti-VEGF agents to treat people with wet AMD. However, eye doctors often face challenges in prescribing their anti-VEGF agent of choice. We surveyed eye doctors to determine the reasons why they preferred some anti-VEGF agents over others, as well as the barriers to prescribing these anti-VEGF agents. Eye doctors reported that they usually choose a specific anti-VEGF agent because it leads to better vision, has lower cost for people with wet AMD, or may reduce the number of appointments needed for people with wet AMD. Eye doctors also noted that they face challenges in treating people with wet AMD, including restrictions and limited insurance coverage for certain anti-VEGF agents. Solving these problems could help eye doctors use their medicine of choice and improve eyesight even more when they treat people with wet AMD.
Introduction
Age-related macular degeneration (AMD) is the leading cause of vision loss among individuals over 60 years of age in the United States (US),Citation1,Citation2 with a prevalence of 12.6% in adults of 40 years of age and older for early-stage AMD.Citation3 Neovascular AMD (nAMD) arises from abnormal blood vessel formation originating primarily from the choroid, which can lead to intraretinal or subretinal fluid, hemorrhage, and vision loss in patients with nAMD.Citation4 The standard-of-care for nAMD is the use of anti-vascular endothelial growth factor (anti-VEGF) agents to slow vision loss in patients.Citation5 Anti-VEGF agents approved by the US Food and Drug Administration for the treatment of nAMD include aflibercept, brolucizumab, and ranibizumab, and bevacizumab is frequently used off-label.Citation6 Most recently, faricimab, a bispecific anti-VEGF and anti-angiopoietin-2 antibody, has also been approved for nAMD.Citation7,Citation8 Although many studies have investigated patient preference for specific anti-VEGF agents,Citation9–11 little is currently understood about physician preference and the barriers faced when prescribing anti-VEGF agents in real-world clinical practice.
Four principles have been identified for the ideal anti-VEGF treatment of retinal diseases, including nAMD: “maximize and maintain visual acuity benefits for all patients”; “decide when to treat next, rather than whether to treat now”; “titrate the treatment intervals to match patients’ needs”; and “treat at each monitoring visit”.Citation13 However, in routine clinical practice, physicians may face barriers in prescribing their choice of anti-VEGF agent and must also consider the type of treatment strategy, such as the use of “treat-and-extend” (treat and monitor at every visit and extend the dosing interval if the patient and disease are stable), “fixed interval” (to treat and monitor at every visit on a fixed interval), or “pro re nata” injection regimens (to monitor at every visit and treat as needed). Choice of treatment strategy can affect clinical outcomes, as certain regimens such as “treat-and-extend” can improve visual acuity outcomes in patients with nAMD compared with a “pro re nata” strategy.Citation14,Citation15 Choice of treatment strategy also dictates the frequency and associated costs of injections that patients will receive over a 1-year and 2-year period.Citation14,Citation15 These factors may prevent physicians from prescribing agents that would otherwise have benefited patients, potentially affecting clinical outcomes for patients with nAMD.Citation16
Understanding the factors affecting physician choice of anti-VEGF agent may identify specific barriers to treatment and the potential effect on treatment outcomes for patients with nAMD. Although previous real-world studies have reported treatment strategies and drug choice in clinical practice,Citation17 little is known about the rationale of physicians prescribing these medications. The goals of this analysis are to describe: i) the preferred treatment strategies of physicians and those used in practice, ii) the factors and restrictions that affect physician prescription of anti-VEGF agents for treating nAMD, and iii) the reasons physicians have for switching between anti-VEGF agents in clinical practice.
Methods
Design
Data were drawn from the Adelphi Real World nAMD Disease Specific Programme (DSP)™, a cross-sectional survey with retrospective data collection of physicians and their patients with nAMD in the US administered between October 2021 and May 2022. The DSP offers unique insights into clinical practice and allows for the exploration of rationale for physician treatment choices for patients with nAMD, using previously published and validated methodology.Citation18–21 Eligible physicians completed both an online physician survey and online patient forms for up to 13 consecutive consulting patients with nAMD. Physicians were compensated for their time. The survey was performed in accordance with relevant guidelines and legislation;Citation22–24 ethics exemption was obtained from Pearl Institutional Review Board (#21-ADRW-121). All data were anonymized and aggregated. No medication was provided, and no tests or investigations were performed as part of this research.
Population
Physicians were eligible for inclusion if they were an ophthalmologist (with or without a retinal subspecialty), were responsible for the management and treatment decisions of patients with nAMD, were consulting with at least 20 patients with nAMD per month, and completed at least one physician-reported patient questionnaire. Data from patients were eligible for inclusion if the patients were ≥55 years of age, with a physician-confirmed nAMD diagnosis, and were not involved in an nAMD clinical trial at the time of data capture. Data from patients treated with anti-VEGF agents available in October 2021 were included in the analysis (aflibercept, brolucizumab, ranibizumab, and bevacizumab).
Outcome Measures
Within the survey, physicians reported data on their preference of treatment strategy (“treat-and-extend”, “fixed interval”, or “pro re nata”), clinical and non-clinical factors affecting general treatment decisions for patients with nAMD (multi-choice answer, subsequently grouped into clinical and non-clinical factors), and the effect of these factors on their own treatment decisions (5-point Likert scale, where 1 = “not at all influential” and 5 = “very influential”). Physicians also provided data on the formulary availability of anti-VEGF agents (a single selection from: available without restrictions, available with restrictions, or not routinely available), the types of restrictions (when applicable, multi-choice answer: only available after another option, restricted to a specific prescriber or specialty, restricted to specific hospitals or specialist units, restricted to a subset of suitable patients, prior authorization or approval required, exceptional use application required, unlicensed, but can be used per agreed restrictions, and other), and belief statements surrounding treatment decision-making (5-point Likert scale, where 1 = “strongly disagree” and 5 = “strongly agree”).
In addition, for each of the patient forms they completed, physicians provided data for the following variables: patient’s current vision status (a single selection, per eye, between mild vision impairment [20/30 to 20/60], moderate visual impairment [20/70 to 20/160], severe visual impairment [20/200 to 20/400], or profound visual impairment [20/500 to 20/1000]), time since diagnosis, the treatment strategy currently selected for the patient, dosing interval for those currently on a “fixed interval” strategy, and the ideal dosing interval for the patient, irrespective of strategy. If a patient had switched from one anti-VEGF treatment to another within the last five consultations, they were placed in a “patients who have switched anti-VEGF treatment” subgroup (these patients also had a unilateral diagnosis or were only receiving treatment in one eye). For patients who had switched anti-VEGF treatment, physicians also provided data on reasons for switching (multi-choice answer).
Statistical Analysis
The analysis of outcomes was descriptive; mean and standard deviations were reported for all variables. No hypothesis was developed or tested.
Results
Participant Characteristics
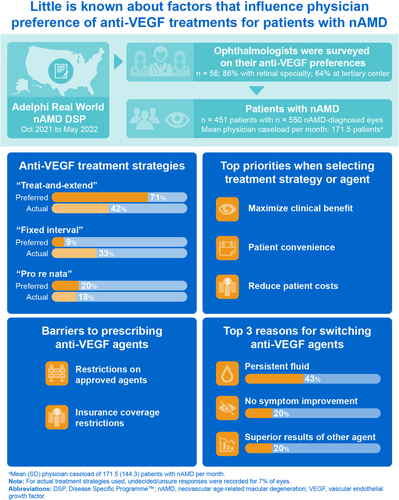
In total, 56 physicians (48 ophthalmologists with a retinal subspecialty and eight ophthalmologists without a retinal subspecialty) provided data for 451 patients with nAMD (with 550 nAMD-diagnosed eyes). Most physicians were in a group practice and practiced at a tertiary center that provides specialized medical care (). Overall, 368 patients were receiving anti-VEGF treatment in one eye only. Patient demographic and clinical characteristics are summarized in . The mean age for nAMD diagnosis was 73.4 years, and more than half of nAMD-diagnosed eyes had moderate or worse visual impairment (20/70 or worse) at diagnosis.
Table 1 Physician Profile
Table 2 Patient Demographics, Clinical Characteristics, and Treatment Profiles
Treatment Strategies for Patients with nAMD
Aflibercept was the most prescribed anti-VEGF agent (to 47% of the patients), and off-label bevacizumab was the second most prescribed (38%). Fewer than half of the patients were also prescribed supplementary eye health vitamins (42%) in addition to anti-VEGF treatment ().
Most physicians (71%) preferred a treat-and-extend treatment strategy over “fixed interval” or “pro re nata”, irrespective of the type of anti-VEGF agent prescribed, or patient factors involved (). The key reasons for this treatment strategy preference were patient convenience (selected by 61% of the physicians), efficacy (eg improvement in visual acuity, 52%), lower out-of-pocket cost for the patient (50%), and fewer patient consultations (50%).
Figure 1 Anti-VEGF treatment strategies for nAMD. Physician-stated treatment strategy preference (n = 56 physician responses) versus physician-selected treatment strategy in clinical practice for nAMD-diagnosed eyes (n = 550 eyes).
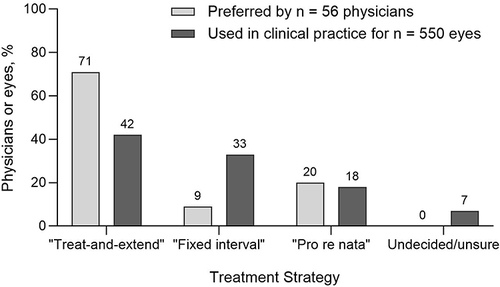
However, among treatment strategies currently implemented in patients included in the survey (n = 550 nAMD-diagnosed eyes), “treat-and-extend” was used in fewer than half of nAMD eyes (42%, ) and 7% of physicians were undecided or unsure of the treatment strategy they would employ. The key reasons for selecting the current treatment strategy for each eye (n = 515) were efficacy (selected by physicians for 80% of nAMD eyes), convenient patient dosing/consult schedule (34%), and cost (30%).
Among the eyes for which a treatment strategy had been selected (n = 512), physicians planned to change the strategy for 4% of the eyes (n = 20), with “treat-and-extend” considered as the new strategy for 65% of these eyes (13/20). Physicians did not plan to change the treatment strategy for 85% of nAMD eyes, and physicians did not know if they would change the treatment strategy for 11% of nAMD eyes.
Physicians were also asked the following question (irrespective of treatment strategy): In your opinion, for this patient, what would be the ideal treatment interval? The median ideal dosing interval preferred by physicians was 12 weeks (range of 2–52 weeks, for n = 451 patients). For those eyes on a “fixed interval” strategy (n = 183 nAMD eyes), the median interval used in clinical practice was 6 weeks.
Factors Influencing Treatment Decisions by Physicians
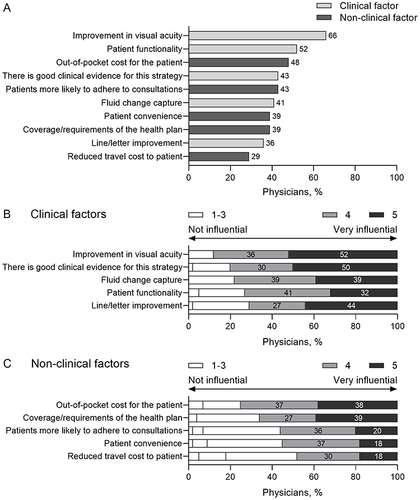
The top 10 most common physician-perceived factors influencing treatment decisions included a combination of clinical factors, such as visual acuity and patient functionality, and non-clinical factors, such as out-of-pocket cost (). The most influential clinical factors that affected physicians’ choice of anti-VEGF agent were improvements in visual acuity, clinical evidence for the prescribing strategy, and anatomical outcomes including fluid change capture, which were more influential than patient functionality and line/letter improvement (). For non-clinical factors, out-of-pocket cost and health plan coverage were more influential than perceptions of patients’ adherence, convenience, or travel costs ().
Figure 2 Factors that drive physician-reported treatment decisions. (A) Top 10 most selected physician-reported factors that affect nAMD treatment decision-making (n = 56 physician responses). (B) Physicians rated how influential clinical factors, and (C) non-clinical factors were on their own treatment decision-making, using a 5-point Likert scale (n = 56 physician responses).
Most physicians (75%) agreed that some insurers restrict and some deny patient access to products that physicians deem most appropriate for them. Physicians reported health plan coverage as a key reason in their choice of anti-VEGF agent for 17% of the patients sampled (77/451), stating they would have recommended an nAMD-approved branded agent for 65% of these patients (50/77) had it been possible.
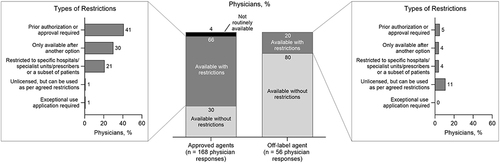
The availability and access to anti-VEGF agents also differed between approved agents and the off-label agent bevacizumab. For anti-VEGF agents approved for nAMD (aflibercept, brolucizumab, or ranibizumab), only about one-third of physician responses (51/168) indicated availability without restrictions (). Conversely, for off-label bevacizumab, most physician responses (80%, 45/56) indicated availability without restrictions (). Common restrictions on anti-VEGF agents include the physician needing a prior authorization or approval, the agent being available to the physician only after another option was tried, and the agent being restricted to a specific hospital, specialist unit, provider, or patient subset ().
Figure 3 Physician-reported availability of approved anti-VEGF agents. Availability and restrictions on approved anti-VEGF agents (aflibercept, ranibizumab, and brolucizumab; n = 168 physician responses) compared with off-label bevacizumab (n = 56 physician responses). Physicians were able to choose multiple answers for types of restrictions.
Rationale for Switching Anti-VEGF Treatments
Among patients receiving anti-VEGF treatment in one eye (n = 368 patients), 8% had been switched between anti-VEGF agents at their current, or within their last five consultations (30/368). Of these patients, 50% (15/30) were switched away from bevacizumab to another anti-VEGF agent, and over half (57%; 17/30) were switched from another anti-VEGF agent to aflibercept.
The most common physician-reported reasons for switching included persistent fluid (selected for 43% of the patients), absence of symptom improvement (20%), and superior clinical trial results of another product (20%) ().
Figure 4 Physician-reported reasons for switching/not switching anti-VEGF agent. (A) Most common reasons reported by physicians for switching away from an anti-VEGF agent (for n = 30 patients). (B) Most common reasons reported by physicians for not switching anti-VEGF agent (for n = 321 patients).
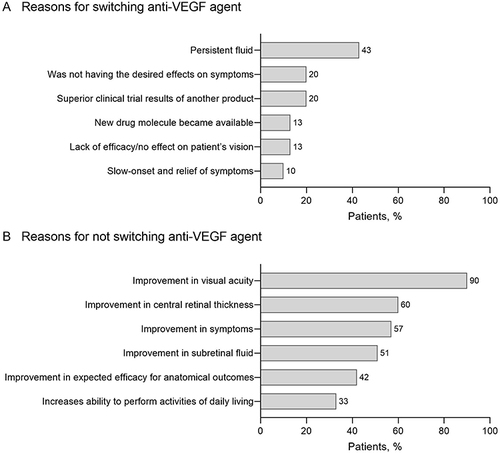
For patients who did not switch between anti-VEGF agents (321/368 patients), the most common physician-reported reasons for not switching included improvements in visual acuity (selected for 90% of the patients), central retinal thickness (60%), symptoms (57%), and subretinal fluid (51%) ().
Discussion
Using real-world data, our analysis highlights factors affecting anti-VEGF treatment decisions made by physicians for patients with nAMD. Physician preference of anti-VEGF agent and treatment strategy aims to maximize clinical benefits (including fluid reduction, which physicians may consider as important as visual gains,Citation25 and is a key reason for switching anti-VEGF agents), improve patient convenience, and reduce out-of-pocket costs for patients. However, physicians also face significant non-clinical barriers such as formulary restrictions on approved anti-VEGF agents and gaps in patient insurance coverage, which may hinder their ability to treat their patients in line with their preferences. Employing extended dosing strategies more frequently in clinical practice and revising payer policies to prioritize fluid control and minimize restrictions on approved anti-VEGF agents could lead to better treatment outcomes for patients with nAMD.
Physicians largely preferred extended dosing with a “treat-and-extend” approach for nAMD in order to reduce injection frequency, which may increase clinical benefit and patient convenience, and reduce out-of-pocket costs.Citation13,Citation26 However, we also identified a discrepancy between the median ideal dosing interval preferred by physicians, and the median interval actually used for one-third of nAMD eyes on a “fixed interval” strategy. Physicians most commonly identified efficacy as the reason for currently using a treatment strategy; however, many patients are still not on extended dosing. Although studies have shown that “treat-and-extend” may reduce the number of anti-VEGF injections required to achieve clinical benefit,Citation14 some anti-VEGF agents are still recommended to be administered monthly on a “fixed interval” basis.Citation27 Further investigation into other factors that may impact decisions around extended dosing is needed, particularly in achieving a balance between disease stability and reduced treatment burden.Citation28,Citation29
Our data also show that non-clinical barriers such as administrative controls, restricted access, and lack of coverage for approved anti-VEGF agents may all be significant barriers for physicians. Although physicians may choose an approved anti-VEGF agent for greater clinical benefit, their prescription may be limited to a second-line option only, which may explain the large difference in the unrestricted availability of off-label bevacizumab, compared with approved anti-VEGF agents. Health insurers may be enforcing step-therapy policies in which off-label bevacizumab is required to be used first before approved anti-VEGF agents can be prescribed, which may contrast with physicians’ preferred first-line agent and may be due to cost differences.Citation30 Of note, physicians indicated that fluid control was the most important reason for switching anti-VEGF agents. This reflects the American Academy of Ophthalmology’s (AAO) Preferred Practice Patterns, where optical coherence tomography (OCT) and OCT angiography are both highlighted as important screening tools to detect nAMD disease activity and guide therapy.Citation31 Further, signals of disease activity as detected through OCT may indicate disease recurrence and future vision decline.Citation32 Although improvement in visual acuity may be taken into account when assessing suboptimal clinical response to an anti-VEGF agent for step-therapy and subsequent switching,Citation33 the importance of fluid control is generally not reflected in payer policies. Thus, awareness of this important clinical endpoint among providers and payers may help reinforce provider choice and optimize patient outcomes. Future efforts by both providers and payers to align on key factors influencing anti-VEGF treatment decisions, particularly around fluid control, may be valuable for the treatment of patients with nAMD. Our analysis also supports the ideal anti-VEGF treatment principles outlined by the Vision Academy Steering Committee,Citation13 as well as statements from the American Society of Retinal Specialists (ASRS) and the AAO, who have advocated for physician choice in determining which anti-VEGF agents to prescribe for their patients.Citation34,Citation35 Importantly, in patients where coverage was reported as influencing treatment choice, physicians indicated they would have recommended a branded agent for most of these patients, had it been possible.
The analysis has several limitations. The DSP is not based on a true random sample of physicians or patients. Although minimal inclusion criteria governed the selection of the participating physicians, participation is influenced by willingness to complete the survey. In addition, only medications available during the time of the survey (October 2021) were included, and treatment proportions and strategies employed may have been affected by this selection. Data were collected between October 2021 and March 2022 during the COVID-19 pandemic. Lockdown guidelines may have affected patient consultation with physicians during this time and influenced physician prescription of treatments as well as treatment strategies. Furthermore, data capture concluded shortly after the US Food and Drug Administration approval of faricimab, which demonstrated greater durability in clinical trials than other anti-VEGF treatments.Citation7 The cross-sectional design of this survey also prevented any conclusions about causal relationships, although associations could be identified. Despite these limitations, real-world analyses play an important part in highlighting areas of concern that are not addressed in clinical trials (including a larger consulting population and restricted access to medications).Citation17,Citation36,Citation37 In addition, the use of a survey provides insight into provider rationale for choosing certain drugs and treatment strategies in clinical practice.
Conclusions
Physicians identify clinical benefit, patient convenience, and out-of-pocket costs as top factors influencing nAMD treatment decisions, including choice of treatment strategy and anti-VEGF agent. In addition, physicians reported clinical benefit as most influential in switching agents. However, physicians face non-clinical roadblocks in prescribing approved anti-VEGF agents, such as administrative controls and lack of coverage. Minimizing restrictions on approved anti-VEGF agents in payer policies could assist physicians in prescribing the appropriate medications for patients with nAMD and ultimately improve treatment outcomes in clinical practice.
Abbreviations
AAO, American Academy of Ophthalmology; AMD, age-related macular degeneration; ASRS, American Society of Retinal Specialists; CCI, Charlson Comorbidity Index; nAMD, neovascular age-related macular degeneration; OCT, optical coherence tomography; SD, standard deviation; VEGF, vascular endothelial growth factor.
Author Contributions
VH, EC, DM, EK, GS, and DT designed and/or performed the analysis. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
GY reports personal fees from 4DMT, AbbVie, Adverum, Alimera, Bausch & Lomb, Boehringer Ingelheim, Clearside Biomedical, Endogena, Genentech, Inc., Gyroscope Therapeutics, Intergalactic Therapeutics, Iridex, Janssen, jCyte, Myrobalan, NGM Biopharmaceutical, Novartis, Ocuphire, Ray Therapeutics, Regeneron, RegenXBio, Stealth Therapeutics, Thea, Topcon, West, and ZEISS. SG: personal fees from Genentech, Inc. VH, EC, DM are employees of Adelphi Real World; EK, GS, DT are employees of Genentech, Inc., a member of the Roche Group. The authors report no other conflicts of interest in this work.
Acknowledgments
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Real World nAMD DSP. Genentech, Inc., a member of the Roche Group, did not influence the original survey through either contribution to the design of questionnaires or data collection. The analysis described here used data from the Adelphi Real World nAMD DSP. The DSP is a wholly owned Adelphi Real World product. Genentech, Inc. is one of multiple subscribers to the DSP. Medical writing assistance was provided by Nilisha Fernando, PhD, of Envision Pharma Group, and funded by Genentech, Inc., a member of the Roche Group. Envision Pharma Group’s services complied with international guidelines for Good Publication Practice (GPP 2022).
Additional information
Funding
References
- National Eye Institute. Age-Related Macular Degeneration (AMD). Bethesda, MD: National Institutes of Health; 2021. Available from: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/age-related-macular-degeneration. Accessed February 15, 2023.
- BrightFocus Foundation. Age-related macular degeneration: facts & figures. Clarksburg, MD: BrightFocus Foundation; 2023. Available from: https://www.brightfocus.org/macular/article/age-related-macular-facts-figures. Accessed August 31, 2023.
- Rein DB, Wittenborn JS, Burke-Conte Z, et al. Prevalence of age-related macular degeneration in the US in 2019. JAMA Ophthalmol. 2022;140(12):1202–1208. doi:10.1001/jamaophthalmol.2022.4401
- Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond). 2016;3(1):34. doi:10.1186/s40662-016-0063-5
- Khachigian LM, Liew G, Teo KYC, Wong TY, Mitchell P. Emerging therapeutic strategies for unmet need in neovascular age-related macular degeneration. J Transl Med. 2023;21(1):133. doi:10.1186/s12967-023-03937-7
- Arepalli S, Kaiser PK. Pipeline therapies for neovascular age related macular degeneration. Int J Retina Vitreous. 2021;7(1):55. doi:10.1186/s40942-021-00325-5
- Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, Phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–740. doi:10.1016/S0140-6736(22)00010-1
- Vabysmo [Prescribing Information]. Last updated January 2023. South San Francisco, CA: Genentech Inc.
- Mueller S, Agostini H, Ehlken C, Bauer-Steinhusen U, Hasanbasic Z, Wilke T. Patient preferences in the treatment of neovascular age-related macular degeneration: a discrete choice experiment. Ophthalmology. 2016;123(4):876–883. doi:10.1016/j.ophtha.2015.12.001
- Vennedey V, Danner M, Evers SM, et al. Using qualitative research to facilitate the interpretation of quantitative results from a discrete choice experiment: insights from a survey in elderly ophthalmologic patients. Patient Prefer Adherence. 2016;10:993–1002. doi:10.2147/PPA.S101584
- Joko T, Nagai Y, Mori R, et al. Patient preferences for anti-vascular endothelial growth factor treatment for wet age-related macular degeneration in Japan: a discrete choice experiment. Patient Prefer Adherence. 2020;14:553–567.
- Ozdemir S, Finkelstein E, Lee JJ, et al. Understanding patient preferences in anti-VEGF treatment options for age-related macular degeneration. PLoS One. 2022;17(8):e0272301. doi:10.1371/journal.pone.0272301
- Lanzetta P, Loewenstein A. Vision Academy Steering Committee. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1259–1273. doi:10.1007/s00417-017-3647-4
- Rosenberg D, Deonarain DM, Gould J, et al. Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: a systematic review and meta-analysis. Eye (Lond). 2023;37(1):6–16. doi:10.1038/s41433-022-02020-7
- Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36(8):1418–1431. doi:10.1097/IAE.0000000000001142
- Wykoff CC, Clark WL, Nielsen JS, Brill JV, Greene LS, Heggen CL. Optimizing anti-VEGF treatment outcomes for patients with neovascular age-related macular degeneration. J Manag Care Spec Pharm. 2018;24(2a Suppl):S3–S15. doi:10.18553/jmcp.2018.24.2-a.s3
- Mehta H, Tufail A, Daien V, et al. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res. 2018;65:127–146. doi:10.1016/j.preteyeres.2017.12.002
- Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes - a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi:10.1185/03007990802457040
- Babineaux SM, Curtis B, Holbrook T, Milligan G, Piercy J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the disease specific programme. BMJ Open. 2016;6(8):e010352. doi:10.1136/bmjopen-2015-010352
- Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–380. doi:10.2147/DMSO.S120101
- Anderson P, Higgins V, Courcy J, et al. Real-world evidence generation from patients, their caregivers and physicians supporting clinical, regulatory and guideline decisions: an update on disease specific programmes. Curr Med Res Opin. 2023;1:1–9.
- US Department of Health and Human Services [internet]. Summary of the HIPAA Privacy Rule. Washington, DC; 2003. Available from: http://www.hhs.gov/sites/default/files/privacysummary.pdf. Accessed June 8, 2023.
- European Pharmaceutical Market Research Association (EphMRA). Code of Conduct. Basel, Switzerland; 2022. Available from: https://www.ephmra.org/code-conduct-aer. Accessed June 8, 2023.
- US Department of Health and Human Services. Health Information Technology for Economic and Clinical Health (HITECH) Act. Washington, DC; 2009. Available from: https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Accessed June 8, 2023.
- Ashraf M, Banaee T, Silva FQ, Singh RP. Switching anti-vascular endothelial growth factors in refractory neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2018;49(3):166–170. doi:10.3928/23258160-20180221-03
- Spaide R. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143(4):679–680. doi:10.1016/j.ajo.2007.02.024
- Lucentis [Prescribing Information]. South San Francisco, CA: Genentech, Inc.; 2023.
- Gemenetzi M, Patel PJ. A systematic review of the treat and extend treatment regimen with anti-VEGF agents for neovascular age-related macular degeneration. Ophthalmol Ther. 2017;6(1):79–92. doi:10.1007/s40123-017-0087-5
- Garweg JG, Gerhardt C. Disease stability and extended dosing under anti-VEGF treatment of exudative age-related macular degeneration (AMD) - a meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2021;259(8):2181–2192. doi:10.1007/s00417-020-05048-1
- Brown GC, Brown MM, Rapuano SB, Boyer D. A cost-benefit analysis of VEGF-inhibitor therapy for neovascular age-related macular degeneration in the United States. Am J Ophthalmol. 2021;223:405–429. doi:10.1016/j.ajo.2020.07.010
- Flaxel CJ, Adelman RA, Bailey ST, et al. Age-related macular degeneration preferred practice pattern®. Ophthalmology. 2020;127(1):1.
- Amissah-Arthur KN, Panneerselvam S, Narendran N, Yang YC. Optical coherence tomography changes before the development of choroidal neovascularization in second eyes of patients with bilateral wet macular degeneration. Eye (Lond). 2012;26(3):394–399. doi:10.1038/eye.2011.335
- Jhaveri CD, Glassman AR, Ferris FL, et al. Aflibercept monotherapy or bevacizumab first for diabetic macular edema. N Engl J Med. 2022;387(8):692–703. doi:10.1056/NEJMoa2204225
- American Society of Retina Specialists [internet]. Physician choice of medication. Chicago, IL; 2013. Available from: https://www.asrs.org/advocacy/physician-choice-of-medication. Accessed February 17, 2023.
- Hassan T. Step therapy undermines physician choice of AMD treatment. Conshohocken, PA: Retina Today; 2013. Available from: https://retinatoday.com/articles/2013-oct/step-therapy-undermines-physician-choice-of-amd-treatment. Accessed February 17, 2023.
- Saunders C, Byrne CD, Guthrie B, et al. External validity of randomized controlled trials of glycaemic control and vascular disease: how representative are participants? Diabet Med. 2013;30(3):300–308. doi:10.1111/dme.12047
- Katkade VB, Sanders KN, Zou KH. Real world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J Multidiscip Healthc. 2018;11:295–304. doi:10.2147/JMDH.S160029