Abstract
Background
Patients with exudative age-related macular degeneration (AMD) who did not respond to ranibizumab at the induction phase were assessed and referred to as initial non-responders.
Methods
We retrospectively reviewed the medical records of 215 patients (218 eyes) with exudative AMD. For the initial treatments, patients received three intravitreal injections of ranibizumab (IVR) every 4 weeks. Minimum follow-up period was 12 months. We defined patients with no improvement of best corrected logMAR visual acuity (BCVA), and with no decrease of central retinal thickness (CRT) at the end of the initial treatment, as initial non-responders. Patients who had previous treatment history prior to this investigation were included, but patients who had photodynamic therapy (PDT) with IVR were excluded.
Results
Twenty-two eyes (10.1%) were identified as initial non-responders. The mean BCVA of initial non-responders before IVR and after induction phase were 0.39 and 0.36, respectively. There was no significant difference between these values, however the mean BCVA decreased significantly to 0.55 at 12 months after the beginning of the induction phase (P = 0.021). The mean greatest linear dimension (GLD) of the lesion before IVR of initial non-responders was 4,121 μm. We found 16 eyes with typical AMD, and six eyes with polypoidal choroidal vasculopathy. One eye had predominantly classic choroidal neovascularization (CNV), and others had occult CNV of typical AMD. As additional treatments, twelve eyes received PDT, and in three of the eyes exudation remained after PDT.
Conclusion
Initial non-responders were more prevalent in patients with occult CNV than in patients with other CNV types. Some of the initial non-responders did not respond to PDT. This study suggested possible involvement of other factors, in addition to vascular endothelial growth factor, in the occurrence of CNV in initial non-responder patients.
Introduction
Intravitreal administration of anti-vascular endothelial growth factor (anti-VEGF) drugs has become the standard treatment for exudative age-related macular degeneration (AMD). The MARINACitation1 and ANCHORCitation2,Citation3 studies showed the efficacy of ranibizumab in patients with any lesion types of AMD at the primary 12-month assessment, and the efficacy of ranibizumab was also observed through the final 24 months assessment in the MARINA study.Citation1 Moreover, a 3-year extension of the HORIZON studyCitation4 indicated long-term safety and efficacy of intravitreal ranibizumab injections. Following the PrONTO study,Citation5,Citation6 in Japan we usually perform intravitreal injection of ranibizumab (IVR) with pro re nata regimens during the maintenance phase of ranibizumab treatment.
However, there are some patients who do not have a sufficient response to ranibizumab, and some patients fail to respond to the drug. Those patients who have inadequate responses to ranibizumab are classified into two groups: initial non-responders and tachyphylaxis patients. Initial non-responders do not respond to ranibizumab at the induction phase. In contrast, patients with tachyphylaxis respond to ranibizumab at the induction phase but the response decreases gradually after repeated administrations. In this study, initial non-responders were assessed retrospectively.
Material and methods
Two hundred and eighteen eyes in 215 patients with AMD were included in this study. Patients with exudative AMD were included and categorized on the basis of subtype of AMD. There were 162 eyes in typical AMD, 56 eyes in polypoidal choroidal vasculopathy (PCV), and no eyes in retinal angiomatous proliferation (RAP). In the patients with typical AMD, 55 eyes had predominantly classic choroidal neovascularization (CNV), 29 eyes had minimally classic CNV, and 76 eyes had occult with no classic CNV. Two patients did not undergo fluorescein angiography because they had an allergy to fluorescein. The mean age of the patients was 73.5 years (53 years - 90 years). There were 172 males and 46 females in this study.
This retrospective study was performed with informed patient’s consent and conducted in accordance with the principles of the Declaration of Helsinki. Patients received three IVR for the initial treatments every 4 weeks (from April 2009 to October 2010) at Kansai Medical University Takii and Hirakata hospitals, in Osaka, Japan. After the treatments, they received further IVR if necessary. Patients were followed up for more than 12 months. A comprehensive ophthalmic examination, including best corrected visual acuity (BCVA), and spectral domain optical coherence tomography (OCT) imaging using the RTVue-100 (Optovue Inc, Fremont, CA, USA), was performed at every visit. BCVA was measured with a Japanese standard decimal visual chart, and the logarithm of the minimum angle of resolution (logMAR) scale was used for statistical analysis.
We defined patients with no improvement of BCVA, and with no decrease of central retinal thickness (CRT) at the end of the initial treatment (1 month after the third administration) as initial non-responders to ranibizumab. A marked change in visual acuity was defined as a difference of 0.3 or more units. A marked change in CRT as measured by OCT was defined as a difference of 10% or more. An improvement of BCVA showed functional response and a decrease of CRT showed morphological response to ranibizumab. In this study, patients who had previous treatment history prior to this investigation were included but patients who had photodynamic therapy (PDT) with IVR were excluded.
Results
In this study 22 eyes of 218 eyes (10.1%) were identified as initial non-responders. There were 21 males and 1 female, and the mean age of patients was 69 years (56 years-80 years). Ten eyes (45%) had previous treatment history prior to IVR. Among them, eight eyes had a history of PDT, four eyes had injections of pegaptanib, and one eye had injection of bevacizumab. AMD subtype showed 16 eyes (9.9%) of initial non-responders out of 162 eyes with typical AMD, and six eyes (10.7%) of initial non-responders out of 56 eyes with PCV There was no significant difference between the percentages of initial non-responders in patients with typical AMD and PCV (P = 0.858; chi-square test) ().
Table 1 Characteristics of the eyes in initial non-responders
In patients with typical AMD, there were 15 eyes (19.7%) of initial non-responders out of 76 eyes with occult with no classic CNV, one eye (1.8%) of initial non-responders out of 55 eyes with predominantly classic CNV, and no patients with minimally classic CNV The number of patients with occult with no classic CNV was statistically more than that of patients with classic CNV (P = 0.002; Chi-square test) ().
The mean greatest linear dimension (GLD) of the lesion before IVR of initial non-responders was 4,121 μm, and that of all cases was 4,190 μm, with no significant difference between them (P = 0.872; Student’s t-test).
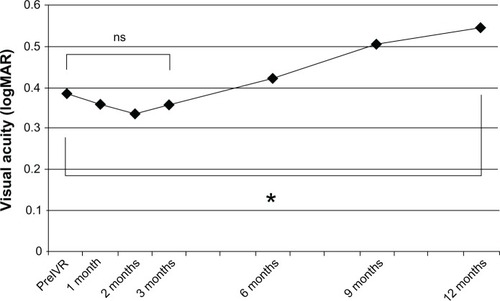
shows the change of BCVA in initial non-responders. There was no significant difference between the mean BCVA of initial non-responders before IVR (0.41; 0.39 logMAR) and the mean BCVA after induction phase (0.43; 0.36 logMAR), but this decreased significantly to 0.28 (0.55 logMAR) at 12 months after the beginning of the induction phase (P = 0.021; paired t-test).
Figure 1 Mean changes in best corrected visual acuity of initial non-responders.(/p)(p)There was no significant difference between the mean best BCVA of initial non-responders before IVR (0.39 logMAR), and the mean BCVA after initial therapy (0.36 logMAR), but it decreased significantly to 0.55 logMAR at 12 months after the beginning of initial therapy (*P = 0.021; paired t-test).
As additional treatments, twelve eyes received PDT, and in three of the eyes exudation remained after PDT. Those twelve eyes had all occult with no classic CNV. In nine eyes without any exudation after PDT, BCVA did not improve but CRT measured by OCT decreased more than 10% from the baseline.
Discussion
There have been some recent reports which show that the efficacy of IVR depended on genotype.Citation7–Citation9 Moreover, there were some unpublished reports in Japan, which indicated that 10%–30% of patients did not respond to ranibizumab throughout the overall treatment period, and these cases included more fibrovascular retinal pigment epithelial detachment and PCV patients than other cases responding to ranibizumab. In our investigation, we observed approximately 10% of initial non-responders at the induction phase. In addition, initial non-responders had more occult with no classic CNV than other lesion types.
In patients with tachyphylaxis, responses to ranibizumab deteriorated at the maintenance phase. Some reports described a possible mechanism of tachyphylaxis as the production of antibodies to ranibizumab, due to repeated injections of the drug. We hypothesize that this production of antibodies to ranibizumab could cause the deterioration of responses to the drug, and we have observed similar responses to other biological agents in patients with tachyphylaxis.Citation10,Citation11 The mechanism of refractoriness to drugs may be different from that of tachyphylaxis, because initial non-responders did not show any responses, even from the beginning of the therapy. Therefore, in initial non-responders who did not respond to ranibizumab, the cause may be less related to the production of antibody to ranibizumab than in the tachyphylaxis patients.
In initial non-responders, we often observed cases that had prolonged AMD or previous PDT therapy prior to IVR. In prolonged cases, retinal pigment epithelium (RPE) was damaged due to an extended exudative change. In cases that had received prior PDT therapy, occlusion of the choriocapillarisCitation12,Citation13 or atrophy of RPECitation14,Citation15 was reported. Therefore, in some cases of initial non-responders, the function of the RPE might have been damaged, and the ability to suppress the exudation from CNV and absorb subretinal fluid could have been compromised. In those cases, even though there was an efficacy of anti-VEGF drug, the ability to suppress the exudation may have been insufficient.
It is possible, in cases of initial non-responders that VEGF was not as involved in AMD because anti-VEGF drugs were not effective from the beginning of the therapy. Aflibercept has become available for AMD,Citation16 and this drug provides a new option of anti-VEGF therapy. In the future, the efficacy of aflibercept should be investigated in initial non-responders to ranibizumab.
In conclusion, we observed more initial non-responders in patients with occult CNV than in patients with other lesion types. In addition, some of the initial non-responders did not respond to either ranibizumab or PDT. There was no significant difference between the percentage of initial non-responders in patients with typical AMD and PCV. Our examination suggested the possible involvement of other factors in addition to VEGF in patients of initial non-responder type.
Disclosure
The authors report no conflicts of interest in this work.
References
- RosenfeldPJBrownDMHeierJSMARINA Study GroupRanibizumab for neovascular age-related macular degenerationN Engl J Med2006355141419143117021318
- BrownDMKaiserPKMichelsMANCHOR Study GroupRanibizumab versus verteporfin for neovascular age-related macular degenerationN Engl J Med2006355141432144417021319
- BrownDMMichelsMKaiserPKHeierJSSyJPIanchulevTANCHOR Study GroupRanibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR studyOphthalmology20091161576519118696
- SingerMAAwhCCSaddaSHORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age- related macular degenerationOphthalmology201211961175118322306121
- FungAELalwaniGARosenfeldPJAn optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degenerationAm J Ophthalmol20071434556583
- LalwaniGARosenfeldPJFungAEA variable dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO studyAm J Ophthalmol20091481435819376495
- TeperSJNowinskaAPilatJPaluchaAWylegalaEInvolvement of genetic factors in the response to a variable-dosing ranibizumab treatment regimen for age-related macular degenerationMol Vis2010162598260421151600
- Kloeckener-GruissemBBarthelmesDLabsSGenetic association with response to intravitreal ranibizumab in patients with neovascular AMDInvest Ophthalmol Vis Sci20115274694470221282580
- ChenHYuKDXuGZAssociation between variant Y402H in age-related macular degeneration (AMD) susceptibility gene CFH and treatment response of AMD: A meta-analysisPLoS One201278e4246422905135
- GasperiniJLFawziAAKhondkaryanABevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularizationBr J Ophthalmol2012961142021791509
- EghøjMSSørensenTLTachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumabBr J Ophthalmol2012961212321733918
- Schmidt-ErfurthUMichelsSBarbazettoIPhotodynamic effects on choroidal neovascularization and physiological choroidInvest Ophthalmol Vis Sci200243383084111867605
- MichelsSSchmidt-ErfurthUSequence of early vascular events after photodynamic therapyInvest Ophthalmol Vis Sci20034452147215412714655
- WachtlinJBehmeTHeimannHConcentric retinal pigment epithelium atrophy after a single photodynamic therapyGraefes Arch Clin Exp Ophthalmol2003241651852112734707
- Ruiz-MorenoJMMonteroJAAmatPMacular atrophy after combined intravitreal triamcinolone and photodynamic therapy to treat choroidal neovascularizationInt J Ophthalmol20103216116322553543
- HeierJSBrownDMChongVVIEW 1 and VIEW 2 Study GroupsIntravitreal aflibercept (VEGF trap-eye) in wet age-related macular degenerationOphthalmology2012119122537254823084240