Abstract
Herein, we report a case of abrupt suprachoroidal hemorrhage (SCH) that developed during peritoneal dialysis in a patient with proliferative diabetic retinopathy. A 53-year-old female patient visited our clinic with blurred vision due to vitreous hemorrhage and proliferative diabetic retinopathy. Her medical history included diabetes, hypertension, chronic renal failure, and she had received scheduled peritoneal dialysis. No anticoagulant agents were used. We performed combined phacoemulsification with intraocular lens implantation and vitrectomy without any complications. Two hours later, the retina was stable and the intraocular pressure (IOP) was 11 mmHg. Four hours later, while receiving peritoneal dialysis, she abruptly developed ocular pain. Examination of her eye revealed an IOP of 38 mmHg and a SCH in the entire peripheral retina and posterior pole. At 12 hours after surgery (on the same day), the SCH was found to be further aggravated, and because a “kissing retina” was imminent, silicone oil was injected. An attempted fluid–air exchange failed because there was not enough space to fill with silicone oil due to aggravation of the SCH. Sclerotomies were performed to remove the SCH, and to create space for the silicone oil injection. Two months after surgery, the silicone oil was removed and her visual acuity was found to have improved to 20/40, but the patient died of pontine hemorrhage 1 month later. SCH can occur in vitrectomized eyes due to an increase in abdominal pressure during peritoneal dialysis, because chronic renal failure patients with diabetes and hypertension have structural vulnerabilities and vascular weaknesses due to arterial sclerosis in response to the increased blood pressure.
Introduction
Suprachoroidal hemorrhage (SCH) is an accumulation of blood within the “suprachoroidal space,” which is a potential space situated between the choroid and the sclera. SCH has been reported to occur after all types of intraocular procedures.Citation1 SCH has also been reported following the Valsalva maneuver, hemodialysis, and thrombolysis with heparin and low molecular-weight derivatives for myocardial infarction.Citation2,Citation3 However, as far as we are aware, there have been no reports of SCH occurring during peritoneal dialysis. Here, we present a case of an abrupt development of SCH in a patient undergoing peritoneal dialysis after vitrectomy.
Case report
A 53-year-old female patient presented with sudden visual loss in her right eye. She had been diagnosed with diabetes and hypertension 20 years earlier. Peritoneal dialysis had been ongoing since her renal failure diagnosis the year before. No anticoagulant agents were used. She was 151 cm tall and weighed 98 kg. Best-corrected visual acuity was counting fingers in the right eye and 20/40 in the left eye. The intraocular pressure (IOP) was 19 mmHg in the right eye and 12 mmHg in the left eye. Axial length was 22.77 mm in the right eye and 22.34 mm in the left. Anterior segment examination revealed moderate nuclear sclerotic cataracts in each eye, while fundus examination revealed a dense vitreous hemorrhage in the right eye and multiple abnormal new vessels at the inferotemporal retina in the left.
Two days after presentation, we performed combined phacoemulsification with intraocular lens implantation and vitrectomy in her right eye. Vitrectomy was performed using a 23-gauge vitreous cutter driven by an Associate 2500 vitrectomy unit (DORC International, Zuidland, The Netherlands) and the DORC Two Step 23 Gauge Vitrectomy System. During vitrectomy, we identified a posterior vitreous detachment and observed new abnormally raised vessels at the peripheral retina. Panretinal endolaser photocoagulation was thus performed at the end of the surgery. Two hours after the vitrectomy, the IOP was 11 mmHg and no signs of endophthalmitis in the anterior and posterior chambers were observed. Fundus findings were the same at the end of the surgery. Four hours after vitrectomy, she began peritoneal dialysis with 1.5% Stay-Safe® (Fresenius Medical Care, Bad Hamburg, Germany). The patient usually received four 2 L exchanges of peritoneal dialysis fluid daily. She complained of sudden onset severe ocular pain and mild dyspnea approximately 10 minutes after infusion of the dialysis solution into the peritoneal cavity.
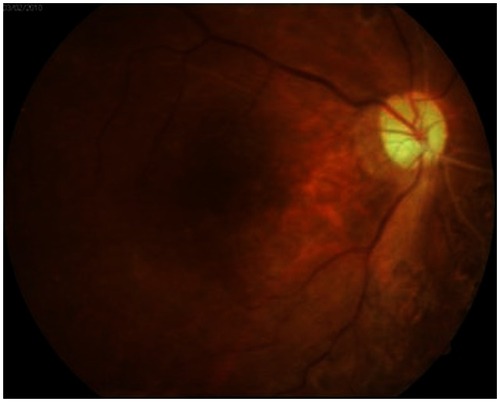
Slit-lamp examination revealed a shallow anterior chamber. The IOP was 38 mmHg in the right eye. Fundus examination of the right eye revealed an elevated, dark brown choroidal mass located in the entire retina. (). Intravenous mannitol was immediately administered, along with systemic acetazolamide, topical dorzolamide/timolol eye drops (Cosopt®, Merck, Whitehouse Station, NJ, USA), and brimonidine tartrate (Alphagan®-P, Allergan, Irvine, CA, USA). At 12 hours after the vitrectomy (the same day), the SCH was found to be further aggravated and threatened the macular architecture. A “kissing retina” was imminent. We thus performed vitreous cavity reformation and silicone oil tamponade then a secondary surgery was performed under general anesthesia.
Figure 1 (A and B) Fundus photography and fluorescein angiography images revealed vitreous hemorrhage in the right eye during preoperative examinations. (C) Intraoperative findings of the first vitrectomy. (D) Fundus photographs showed no complications, including vitreous, at the end of vitrectomy. (E and F) Four hours after surgery, fundus photography showed choroidal detachment at the posterior pole and peripheral retina.
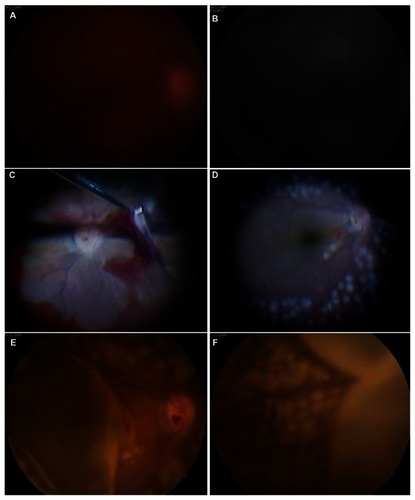
In the surgery, a 6 mm long infusion cannula was inserted 3.5 mm posterior to the limbus in an area with less choroidal detachment, an infusion catheter connection inserted, and two cannulae inserted 3.5 mm posterior to the limbus in the superiotemporal and superionasal quadrants. When we performed the fluid–air exchange, however, there was not enough space to inject the silicone oil because of the SCH. We made a full-thickness scleral incision to drain the SCH in the areas of highest choroidal detachment. Fluid–air and air–silicone oil exchange were then conducted and endolaser photocoagulation around the peripheral retina was performed (). The choroidal elevation improved, and the patient was left with only minor subretinal hemorrhages inferiorly. The retina was flat.
Figure 2 Intraoperative findings of the second vitrectomy. (A) Huge choroidal detachment and (B) “kissing retina” were observed. (C) Fundus photography showed the suprachoroidal hemorrhage removal through the sclerotomy site. (D) At the end of the vitrectomy, fundus photography showed a flat posterior pole and peripheral retina.
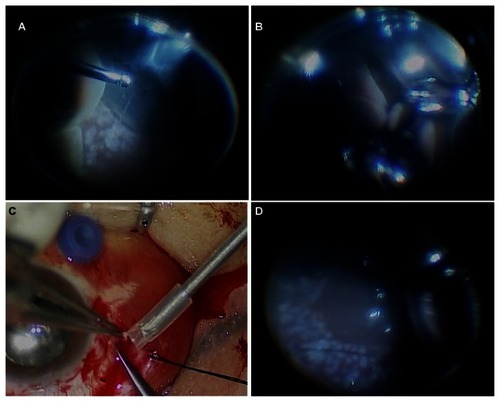
Two months after the vitrectomy, the silicone oil was removed () and the patient’s visual acuity had improved to 20/40. However, 1 month later, she died of a pontine hemorrhage.
Discussion
“SCH” is defined as hemorrhage in the suprachoroidal space that is extensive enough to forcibly extrude the intraocular contents from the eye or to move the retinal surfaces into apposition. Ophthalmic risk factors include glaucoma, aphakia, elevated IOP, axial myopia, and inflammation.Citation4 Advanced age, hypertension, and atherosclerosis are recognized systemic risk factors.Citation4 There have also been reports of SCH in patients following the Valsalva maneuverCitation5 and in those with ocular hypertension and open-angle glaucoma, and high myopia without closed-angle glaucoma.Citation6
Rossi et alCitation7 suggested the use of choroidal hemorrhage drainage through a 23-gauge vitrectomy cannula combined with 23-gauge vitrectomy and silicone oil tamponade. More recently, Rezende et alCitation8 evaluated transconjunctival drainage of serous and hemorrhagic choroidal detachment using 20- or 25-gauge transconjunctival trocar/cannula systems. They inserted the transconjunctival trocar/cannula systems into the suprachoroidal space 7.0 mm from the limbus, and reported that vitrectomy may not be necessary when the choroidal detachments are drained in this manner. Nadarajah et alCitation9 suggested that early controlled drainage of massive SCH with an expanding gas bubble (100% perfluoropropane) could help in achieving a good visual outcome. In their study, the mean drainage time of the SCH was 3.5 days after onset. The use of 100% perfluoropropane has the advantage of maintaining positive pressure while facilitating controlled drainage of the hemorrhage as the clot lyses.
This patient had chronic renal failure (CRF) associated with hypertension and diabetes mellitus. CRF is a progressive loss in renal function over a period of 3 months or longer and can be classified into five stages depending on the level of renal function disruption and kidney damage. For those at Stage 5, which is end-stage renal disease (ESRD) with less than 15% of renal function remaining, renal replacement therapies such as hemodialysis, continuous ambulatory peritoneal dialysis, or renal transplantation are required. ESRD requiring dialysis means the renal capillaries are extensively damaged and long-term damage has occurred to the systemic blood vessels. This implies that the blood vessels are vulnerable to changes in blood pressure or shearing forces because ESRD patients tend to have conditions such as blood vessel instability and sclerosis. Choroidal hemorrhage was reported in a patient with CRF in the form of a cough-induced Valsalva maneuver,Citation10 and spontaneous progressive SCH was also reported in a patient undergoing hemodialysis.Citation11 These cases clearly demonstrate the vulnerability of the vascular system in patients with ESRD. Anticoagulant and antiplatelet agents used for medical treatment and the vascular system of the patients vulnerable to unstable body fluid shifts and changes in blood pressure during hemodialysis are considered the main cause. SCH also occurred in a patient receiving ocular surgery under general anesthesia who experienced paroxysmal coughing when the depth of anesthesia was decreased.Citation12 Another case was a hemodialysis patient who developed SCH during severe coughing.Citation5 In the latter case, the Valsalva maneuver induced by severe coughing was thought to have resulted in SCH.
In patients with diabetes or hypertension accompanied by arteriosclerosis or vascular injury of the choroidal blood vessels, instability of the choroidal vascularity increases and the blood vessels are vulnerable to a shearing force, which are prime conditions for SCH. Continuous ambulatory peritoneal dialysis is a convenient and economical alternative to hemodialysis because it allows the patient to have dialysis without visiting the hospital, thereby minimizing disruption to his or her daily life. Peritoneal dialysis requires a much less restricted diet and water intake than hemodialysis and makes it easier to control blood glucose at the same time. Thus, it is used in approximately 15% of CRF patients who receive dialysis.Citation13 There are also disadvantages, however, such as a greater risk of peritonitis and protein loss compared with hemodialysis, and a risk of elevated intra-abdominal pressure when a large amount of dialysate enters the peritoneal cavity over a short period.Citation14 The reason for the increased intra-abdominal pressure during peritoneal dialysis may be the process itself. Approximately 2000 mL of glucose solution (1.50%–4.25%) is infused by gravity into the peritoneal cavity over a short period (5–10 minutes) and 4 to 6 hours later it is drained out by gravity over 20 to 30 minutes. This process is repeated three to four times a day. Dialysate used in the process can increase the intra-abdominal pressure, which can in turn induce a hernia and increase the blood pressure.Citation12–Citation14 Infusion of the peritoneal dialysate immediately elevates systolic pressure in the carotid artery, induces responsive bradycardia, and then gradually elevates the diastolic pressure of the carotid artery. The mechanism behind the conditions is reported to be venous compression in the intraperitoneal cavity that increases peripheral resistance and, in turn, increases the cardiac preload. This may persist for about 1 hour after the peritoneal dialysate is drained out, causing instability in the cardiopulmonary vascular system.Citation15–Citation19 The same mechanism was probably responsible for the SCH in the present case. In other words, the dialysis solution that infused into the peritoneal cavity increased intra-abdominal pressure, inducing an elevation in central blood pressure and responsive bradycardia, which in turn led to instability of weak choroidal vessels, which are vulnerable to shearing forces. The patient had severe abdominal obesity with a presumably high intra-abdominal pressure that was further elevated by the 2000 mL of infused dialysis solution. Neither anticoagulants nor antithrombotic agents were used in this patient, so the effect of such agents can be excluded from the possible causes.
The visual acuity of the patient improved to 20/40 after removal of the silicone oil and the retina was stabilized, but the patient died 1 month later – just 1 day after the sudden onset of a pontine hemorrhage. Systemic vascular abnormalities may already have been extensively present in the patient’s body. Although rare, peritoneal dialysis performed after 23-gauge vitrectomy could be a risk factor for the sudden onset of SCH in patients with proliferative diabetic retinopathy (PDR).
Conclusion
SCH could occur in ESRD patients due to a sudden increase in central blood pressure induced by an elevated intra-abdominal pressure during peritoneal dialysis following vitrectomy. As such, it might be safer to reduce the amount of dialysis solution or to slow down the infusion rate of the solution to prolong the time to complete peritoneal dialysis in patients who just underwent surgery, such as vitrectomy.
Disclosure
The authors report no conflicts of interest in this work.
References
- van MeurJCvan den BoschWASuprachoroidal hemorrhage following a valsalva maneuverArch Ophthalmol19931118102510268352680
- BarsamAHeatleyCJHerbertLSpontaneous suprachoroidal hemorrhage secondary to thrombolysis for the treatment of myocardial infarctionClin Experiment Ophthalmol200634217717916626438
- WongJSSpontaneous suprachoroidal haemorrhage in a patient receiving low-molecular-weight heparin (fraxiparine) therapyAust N Z J Ophthalmol199927643343410641904
- ChuTGGreenRLSuprachoroidal hemorrhageSurv Ophthalmol199943647148610416790
- HammamTMadhavanCSpontaneous suprachoroidal haemorrhage following a valsalva manoeuvreEye (Lond)200317226126212640424
- ChakMWilliamsonTHSpontaneous suprachoroidal haemorrhage associated with high myopia and aspirinEye (Lond)200317452552712802356
- RossiTBoccassiniBIossaMLesnoniGTamburrelliCChoroidal hemorrhage drainage through 23-gauge vitrectomy cannulasRetina201030117417620061907
- RezendeFAKickingerMCLiGPradoRFRegisLGTransconjunctival drainage of serous and hemorrhagic choroidal detachmentRetina201232224224922127221
- NadarajahSKonCRassamSEarly controlled drainage of massive suprachoroidal hemorrhage with the aid of an expanding gas bubble and risk factorsRetina201232354354821955989
- TajikaTYokozekiHIshimaruKNaitoTShiotaHRare case of choroidal hemorrhage complicated with hypertension due to chronic renal failureJ Med Invest20085515115518319559
- SaeedMUWongDHeimannHGibranSKSpontaneous progressive supra-choroidal haemorrhage in a patient undergoing haemodialysisGraefes Arch Clin Exp Ophthalmol2007245111741174217674016
- LimHWKoBWSongYMLeeBRSuprachoroidal hemorrhage during pars plana vitrectomy associated with valsalva maneuverJournal of the Korean Ophthalmological Society200849610221027
- BaşaranOMorayGYağmurdurMCAydoğanCKarakayaliHSix years of surgical experience with continuous ambulatory peritoneal dialysis at one centerTransplant Proc20023462039204012270306
- JungJWRyooSBChoeEKParkKJSurgical treatment of hernias in patients undergoing continuous ambulatory peritoneal dialysisJ Korean Surg Soc2009775333337
- VerbekeFVan BiesenWPletinckAVan BortelLMVanholderRAcute central hemodynamic effects of a volume exchange in peritoneal dialysisPerit Dial Int200828214214818332449
- GotloibLMinesMGarmizoLVarkaIHemodynamic effects of increasing intraabdominal pressure in peritoneal dialysisPerit Dial Int1981144143
- GotloibLGarmizoLVarkaIMinesMReduction of vital capacity due to increased intra-abdominal pressure in peritoneal dialysisPerit Dial Int1981156364
- BoonDBosWJvan MontfransGAKredietRTAcute effects of peritoneal dialysis on hemodynamicsPerit Dial Int200121216617111330561
- McIntyreCWAcute cardiovascular functional effects of peritoneal dialysis: what do we know and why might it matter?Perit Dial Int200828212312518332444