Abstract
Purpose
To evaluate the safety and effectiveness of the Ahmed ClearPath® (ACP) 250 mm2 glaucoma drainage device (GDD) in managing refractory primary open-angle glaucoma (POAG).
Patients and Methods
This was a retrospective case series focused on adult patients diagnosed with severe POAG who underwent implantation of an ACP GDD. Over a 36-month follow-up period, data on intraocular pressure (IOP), the number of glaucoma medications, and complications were recorded. The primary objectives were to assess the reductions from baseline in both IOP and medication usage, through 36 months. Secondary objectives included the proportion of eyes achieving an IOP reduction of ≥ 20% from baseline at 36 months. Intraoperative and postoperative complications were also assessed.
Results
Twelve eyes from 11 patients (mean age: 71.3 ± 14.1 years) met the inclusion criteria and were included in the study. All patients had severe POAG (n=11), with the majority being Caucasian (n=8) and female (n=10). The mean (standard deviation) IOP and number of glaucoma medications at baseline were 29 (7.6) mmHg and 3 (0.9), respectively. At 36 months, mean IOP was reduced to 10.6 (5.5) mmHg (−61.8%; p= 0.0008) and mean number of medications was reduced to 0.9 (0.9) (−71.4%; p=0.0005), with 88.9% of eyes achieving an IOP reduction by ≥20%. No vision threatening complications were observed.
Conclusion
To our knowledge this is the first study to report 36-month outcomes of the novel ACP device in the treatment of refractory POAG. The safety profile and efficacy of the ACP was found to be comparable to that of other commonly utilized GDD models.
Introduction
Currently, the primary focus of glaucoma treatment revolves around reducing intraocular pressure (IOP), which is widely recognized as the sole intervention with proven efficacy in slowing disease progression.Citation1–3 IOP reduction can be achieved by pharmacotherapy, laser interventions, or incisional surgical procedures. Traditionally, glaucoma drainage devices (GDDs) have emerged as the preferred surgical option for managing refractory glaucoma, particularly in cases where previous glaucoma surgeries have not achieved failed to achieve adequate IOP reduction.Citation4–6 However, recent studies have suggested the potential for a greater role of GDD implantation in the early stages of glaucoma treatment.Citation7,Citation8 In 1969, Molteno developed the first successful GDD.Citation9 Since then, significant advancements in GDD design and technology related to size, shape and biomaterials have enhanced device effectiveness and minimized complications. The most widely used GDDs consist of the non-valved Baerveldt implant (BGI 250 and 350; Johnson & Johnson Surgical Vision), Aurolab Aqueous drainage implant (AADI; Aurolab) and valved Ahmed implants (AGV; New World Medical).
The Ahmed ClearPath® (ACP; New World Medical Inc., Rancho Cucamonga, CA, USA) is a novel valveless GDD. The device was approved by the United States Food and Drug Administration (FDA) in 2019 and has gained worldwide attention for its promising efficacy and notable success rates. The ACP comes in two available sizes (250 mm2 and 350 mm2) and both models come equipped with a preplaced 4–0 polypropylene intraluminal stent. The 350 mm2 model features a winged design and posteriorly positioned plate. The plate is designed to avoid interference with rectus muscle insertions, minimizing the risk of muscle damage or displacement during surgery. Furthermore, it aids in securely positioning the implant, reducing the likelihood of postoperative implant migration or extrusion. The 250 mm2 model is designed to fit between rectus, thus eliminating the need for extensive muscle manipulation.
The ACP offers several advantages compared to other GDDs. The ACP tube and plate are both made of barium impregnated medical-grade silicone, facilitating improved conformity to the eye’s curvature and smoother device insertion. The ACP device features a longer anteroposterior diameter and shorter horizontal diameter. By relocating the suture fixation eyelets to a more anterior position, the need for extensive posterior dissection is circumvented, thus minimizing the trauma to surrounding tissues and promoting expedited recovery times. Use of the ACP preloaded polypropylene ripcord provides controlled resistance to the outflow of aqueous humor, thereby maintaining stable IOP postoperatively and mitigating the risk of complications associated with early hypotony.Citation10 The flexibility of the material allows for easier manipulation of the device, requiring a smaller peritomy. The absence of a front plate ridge in the ACP implant gives the device a thinner profile, which can result in a lower, diffuse bleb with a larger surface area that facilitates more gradual and effective drainage of aqueous humor. The thinner device profile also lowers the risk of inflammation, fibrosis, and diplopia.
Previous studies with 6- and 24-months of follow-up have shown that ACP implantation exhibits excellent safety and high efficacy in reducing both IOP and glaucoma medication burdens. Within this study, we report on the safety and efficacy of ACP implantation in patients with refractory POAG, for up to 36 months.
Methods
This was a retrospective, nonrandomized case series performed at a tertiary hospital setting. The study was approved by the Institutional Review Board (IRB) of Mayo Clinic on August 16th, 2021. The study adhered to the Declaration of Helsinki and was in accordance with Health Insurance Portability and Accountability Act regulations. As this is a retrospective study with deidentified data, informed consent was not required. The medical records of consecutive patients who underwent ACP implantation between January 2019 and December 2021 were reviewed. Patients 18 years or older with a diagnosis of POAG uncontrolled with medical therapy with or without prior laser/surgical procedures were included in the study.
Demographic data including age, gender, race, lens status, glaucoma type, glaucoma severity, and prior glaucoma surgery were retrieved from electronic medical records. The preoperative data collection consisted of IOP, number of topical glaucoma medications taken, Snellen best-corrected visual acuity (BCVA), gonioscopy findings, and non-dilated and dilated slit lamp biomicroscopic examination of the anterior and posterior segment. Changes from baseline in IOP and number of glaucoma medications were recorded on day 1, week 1, and 1, 3, 6, 12, 18, 24 and 36 months postoperatively. Intraoperative and postoperative complications were also recorded.
A single surgeon (SD) carried out all procedures. For each eye, the ACP was implanted in the superotemporal quadrant, and the surgical procedure involved the use of ligation, fenestration, and the 4–0 polypropylene ripcord. After peritomy and posterior conjunctival dissection, the ACP device was implanted and ligated with 8–0 Vicryl near the plate and sutured 8 millimeters posterior to the surgical limbus. The anterior chamber was entered with a 23-gauge cannula through the sclera. The tube was trimmed to the appropriate length, bevel up, and inserted into the anterior chamber parallel to the iris plane. Three horizontal slits were made in the tube below the 8–0 Vicryl ligature. The scleral flap was then sutured over the silicone tube. The tube was sutured to the sclera with three 9–0 nylon sutures and carefully measured and a cut piece of Tutoplast was placed over the tube 5 to 6 millimeters posteriorly. The conjunctiva was reapproximated and secured with absorbable sutures. Patients were discharged with topical antibiotics and steroid drops.
All data were de-identified and analyzed using BlueSky Statistics v10.3.1-Pro (BlueSky Statistics LLC Chicago, IL, USA). The study employed standard descriptive statistics to report demographics and baseline characteristics. Means and standard deviations (SD) were utilized for continuous variables, while percentages were used for categorical variables. Primary study outcomes included changes from baseline in both IOP and the number of glaucoma medications at each postoperative time point. These measures were assessed using two-tailed paired t-tests, with a significance level set at P<0.05. Secondary study outcomes included the percentage of eyes achieving as ≥20% IOP reduction from baseline at 36 months. The incidence of intraoperative and postoperative complications was also assessed.
Results
Twelve eyes of 11 patients underwent 250mm2 ACP implantation and were subsequently monitored for up to 36 months. Demographic and baseline glaucoma status data are described in . The mean (SD) age was 71.3 (4.1) years. The study cohort predominantly consisted of Caucasian (72.7%) and female (90.9%) patients. Most eyes were pseudophakic (91.7%) and all had severe POAG (100%), as classified by the International Classification of Diseases 10 definition. A significant majority of the patients (83.3%) had previously undergone a glaucoma procedure. The mean follow-up time was 30 months.
Table 1 Demographic and Baseline Characteristics of Study Population
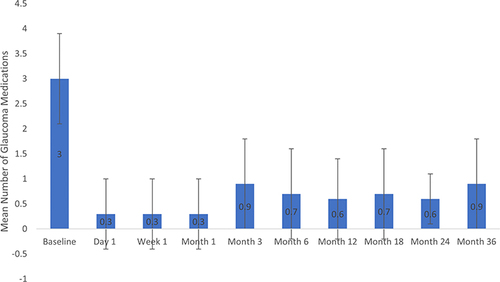
Mean IOP at baseline and each postoperative visit is given in . The mean (SD) baseline IOP was 29.0 (7.6) mmHg and through up to 36 months of follow-up ranged from 5.9 to 12.3 mmHg (). Mean percent IOP reductions across time points ranged from 54% to 79%. Statistically significant reductions in IOP occurred at each time point after surgery (p<0.05). Among the 9 eyes seen at 36 months, mean IOP was 10.6 (5.5) mmHg, a 61.8% reduction from baseline (p=0.0008), and 88.9% of eyes achieved an IOP reduction of ≥20% from baseline. Mean medication use at baseline and each postoperative visit is also given in . The mean (SD) number of medications at baseline was 3.0 (0.9) and through up to 36 months of follow-up ranged from 0.3 to 0.9 medications (). Mean percent medication reductions across each time point ranged from 70% to 90%. Statistically significant reductions in the need for glaucoma medications occurred at all time points (p<0.05). Among the 9 eyes seen at 36 months, mean medication use was 0.9 (0.9) medications per eye, a 71.4% reduction from baseline (p=0.0005). At 36 months 88.9% were using ≥1 fewer medications than at baseline and 44.4% were medication free. BCVA remained stable across all eyes throughout the follow-up period.
Table 2 Mean Intraocular Pressure and Glaucoma Medication Use at Each Study Visit
Figure 1 Mean intraocular pressure at each time point. Error bars represent standard deviation. Reductions from baseline were statistically significant at each time point (P<0.05).
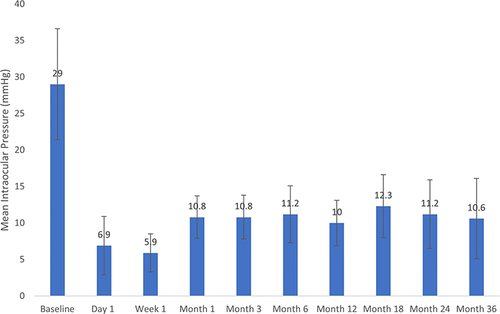
Figure 2 Mean glaucoma medication use at each time point. Error bars represent standard deviation. Reductions from baseline were statistically significant at each time point (P<0.05).
The procedure was performed without intraoperative complications in all eyes. The nature and frequency of the postoperative complications are given in . The most common complication was self-limited mild hyphema (33.3%). Four eyes (33.3%) experienced anterior chamber inflammation that resolved with conservative management. No vision-threatening complications were observed throughout the follow-up period, and there were no glaucoma surgical reinterventions required.
Table 3 The Nature and Frequency of Postoperative Complications
Discussion
Within this study, statistically significant reductions in both IOP and the requirement for glaucoma medications were observed following ACP implantation, for up to 36 months. These Results show great promise for patients with glaucoma challenging to manage under conventional pharmacotherapy or surgical interventions. Moreover, the safety profile of the ACP appears to align with that of other widely used GDD models, further supporting its clinical viability.Citation11,Citation12
The existing literature regarding ACP implantation is sparse, with few studies investigating its clinical efficacy and safety profile in adult patients. In a retrospective multicenter study, Grover et alCitation13 evaluated clinical outcomes of ACP implantation in 100 eyes of 104 patients. At 6 months, the mean IOP decreased significantly from 26.3 mmHg at baseline to 13.7 mmHg (−43%; P < 0.0001), and the mean number of glaucoma medications decreased from 3.9 at baseline to 1.9 (−47.7%; P < 0.0001).Citation13 The authors concluded there to be no significant differences in outcomes observed between the 250 mm2 and 350 mm2 ACP models or between ACP surgeries performed as standalone operations and in combination with phacoemulsification.Citation13 When compared to our 6-month results, our study yielded comparably favorable reductions in IOP (−61.5%) and glaucoma medication burden (−77.8%), but comparison is limited by intersurgeon variability and the utilization of both ACP models in Grover et al’s study. Previously, we reported 24-month safety and efficacy outcomes of the 250 mm2 ACP GDD.Citation14 Our 24-month IOP reduction outcomes (24 months: −61.4%; 36 months: −61.8%) and glaucoma medication reduction outcomes (24 months: −80.0%; 36 months: −71.4%) remain consistent with those achieved at 36 months, highlighting the long-term efficacy of the ACP GDD in managing medically and/or surgically refractory POAG.
There is a scarcity of research comparing the ACP with standard filtering surgeries or other GDDs. In a single-center, retrospective study, Shalaby et alCitation15 compared ACP implantation to BGI implantation in 128 eyes of 113 patients (ACP: 63 ACP; BGI: 65) with similar baseline characteristics over a mean follow-up period of 19.6 (10.8) months. The authors found both groups to achieve comparable final IOP, BCVA, and complication rates, with a lower number of glaucoma medications required in the ACP group (P=0.012). Similar rates of surgical failure were also observed in both groups (ACP: 9.5%, BGI: 9.2%; P=0.810). Further studies are required to more conclusively compare outcomes of the ACP to other GDDs.
Despite the small sample size in our study, it offers valuable insights into the long-term safety and efficacy outcomes of the ACP device. While larger studies are typically preferred for robust statistical analysis and generalizability, this study provides clinically useful evidence on the device’s performance over time in managing medically and/or surgically refractory POAG. Future studies with larger sample sizes could help confirm and expand upon these initial observations. Such investigations are crucial for understanding the durability of outcomes and potential complications associated with these novel implants.
Conclusion
The ACP device represents a promising leap forward in glaucoma management, offering surgeons a reliable option for complex needs of the patients with severe, refractory glaucoma. Long-term efficacy and safety of ACP implantation, as well as comparison to other GDDs and filtering surgeries, remains imperative. Prospective, randomized comparative trials are essential to determine whether these newer devices are equivalent or potentially superior to traditional surgical approaches.
Consent for Publication
This study was performed in accordance with the tenets of the Declaration of Helsinki and was in accordance with regulations of the Health Insurance Portability and Accountability Act. regulations. Patient consent was waived by the Mayo Clinic IRB and all data has been deidentified.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors report no conflicts of interest in this work.
Acknowledgments
The authors acknowledge Ms. Joyce Baker for her generous contributions to the Department of Ophthalmology, Mayo Clinic, Florida. Without her, this research would not be possible.
Data Sharing Statement
The author’s institution does not authorize data sharing. Any queries or requests should be directed to the corresponding author (SD).
Additional information
Funding
References
- Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi:10.1001/archopht.120.10.1268
- Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943–1953. doi:10.1016/s0161-6420(01)00873-9
- Van Veldhuisen PC, Ederer F, Gaasterland DE, et al. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440.
- Gedde SJ, Heuer DK, Parrish RK. Tube Versus Trabeculectomy Study G. Review of results from the Tube Versus Trabeculectomy Study. Curr Opin Ophthalmol. 2010;21(2):123–128. doi:10.1097/ICU.0b013e3283360b68
- Ramulu PY, Corcoran KJ, Corcoran SL, Robin AL. Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology. 2007;114(12):2265–2270. doi:10.1016/j.ophtha.2007.02.005
- Chen PP, Yamamoto T, Sawada A, Parrish RK, Kitazawa Y. Use of antifibrosis agents and glaucoma drainage devices in the American and Japanese Glaucoma Societies. J Glaucoma. 1997;6(3):192–196. doi:10.1097/00061198-199706000-00010
- Wilson MR, Mendis U, Paliwal A, Haynatzka V. Long-term follow-up of primary glaucoma surgery with Ahmed glaucoma valve implant versus trabeculectomy. Am J Ophthalmol. 2003;136(3):464–470. doi:10.1016/s0002-9394(03)00239-3
- Vinod K, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery: a Survey of the American Glaucoma Society. J Glaucoma. 2017;26(8):687–693. doi:10.1097/IJG.0000000000000720
- Molteno AC. New implant for drainage in glaucoma. Clinical trial. Br J Ophthalmol. 1969;53(9):606–615. doi:10.1136/bjo.53.9.606
- Langenberg K, Tran J, Koontz J, Kahook MY. Flow resistance and suture eyelet integrity of the Ahmed ClearPath glaucoma drainage device. Invest Ophth Vis Sci. 2020;61(7):3142.
- Gedde SJ, Feuer WJ, Lim KS, et al. Treatment outcomes in the primary tube versus trabeculectomy study after 3 years of follow-up. Ophthalmology. 2020;127(3):333–345. doi:10.1016/j.ophtha.2019.10.002
- Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–814 e1. doi:10.1016/j.ajo.2011.10.024
- Grover DS, Kahook MY, Seibold LK, et al. Clinical outcomes of Ahmed ClearPath implantation in glaucomatous eyes: a novel valveless glaucoma drainage device. J Glaucoma. 2022;31(5):335–339. doi:10.1097/IJG.0000000000002013
- Dorairaj S, Checo LA, Wagner IV, Ten Hulzen RD, Ahuja AS. 24-month outcomes of Ahmed ClearPath® glaucoma drainage device for refractory glaucoma. Clin Ophthalmol. 2022;16:2255–2262. doi:10.2147/OPTH.S368634
- Shalaby WS, Reddy R, Wummer B, et al. Ahmed ClearPath vs baerveldt glaucoma implant: a retrospective noninferiority comparative study. Ophthalmol Glaucoma. 2023. doi:10.1016/j.ogla.2023.12.006
