Abstract
Purpose
To determine the role of vascular endothelial growth factor (VEGF) in macular edema secondary to branch retinal vein occlusion (BRVO).
Patients and methods
Aqueous humor samples were collected from 52 eyes with macular edema secondary to BRVO before intravitreal drug injections and from 62 control eyes with cataract. VEGF was measured using an enzyme-linked immunosorbent assay. Fluorescein angiography showed capillary nonperfused areas (NPAs). Macular edema was evaluated by optical coherence tomography as the central retinal thickness.
Results
The mean aqueous VEGF levels in eyes with BRVO and control eyes with cataract were, respectively, 290.5 pg/mL ± 294.9 pg/mL (range 81.9 pg/mL–1567.3 pg/mL) and 118.0 pg/mL ± 50.1 pg/mL (range 24.6 pg/mL–241.1 pg/mL), which differed significantly (P < 0.0001). The mean VEGF level in eyes with BRVO without apparent NPA was 171.4 pg/mL ± 52.5 pg/mL (range 90.9 pg/mL–299.9 pg/mL), which was significantly higher than controls (P = 0.001). VEGF levels were correlated positively with the size of NPA (P = 0.0002) but not with the central retinal thickness.
Conclusion
The aqueous VEGF concentration in patients with macular edema secondary to BRVO increased significantly and was correlated significantly with the size of NPA. Aqueous VEGF increased even in eyes without apparent NPA.
Introduction
Branch retinal vein occlusion (BRVO), a common retinal vascular disease, is often associated with macular edema, which is the most frequent cause of visual impairment in these patients.Citation12 Thus, it is critical to determine the cellular and molecular factors that underlie the pathogenesis of macular edema secondary to BRVO. Intraocular levels of cytokines, such as vascular endothelial growth factor (VEGF) and interleukin (IL)-6, have been reported to increase in retinal vein occlusion.35
VEGF produced in the retina may play a key role in the pathogenesis of macular edema secondary to BRVO, similar to other retinal vascular diseases, such as proliferative diabetic retinopathy and retinopathy of prematurityCitation3 VEGF causes a marked increase in vascular permeability by provoking conformational changes in the tight junctions of the retinal vascular endothelial cell.Citation6–Citation8 Intravitreal injection of bevacizumab (Avastin; Genentech, San Francisco, CA, USA), a full-length humanized monoclonal antibody that binds and inhibits all biologically active forms of VEGF,Citation9 is effective for treating macular edema secondary to retinal vein occlusion.Citation10,Citation11 These findings suggest that VEGF may contribute to the development of macular edema secondary to BRVO.
Noma et al reported that VEGF levels increased in the vitreous fluid with macular edema associated with BRVO and were correlated positively with both the size of the nonperfused area (NPA) and the retinal thickness resulting from macular edema.Citation12–Citation14 However, these reports included eyes that had been treated previously with photocoagulation therapy, which may have affected the VEGF concentration, making an understanding of the pathogenesis difficult. Furthermore, the VEGF levels in eyes with BRVO without an NPA are poorly understood. Therefore, we measured the VEGF concentrations in the aqueous humor in a large number of treatment-naive patients with macular edema secondary to BRVO with or without NPA and investigated the relation between aqueous VEGF levels and the clinical conditions of BRVO.
Materials and methods
We prospectively measured the VEGF concentrations in the aqueous humor of 52 eyes of 52 patients (29 women, 23 men) with macular edema secondary to BRVO. The mean patient age was 68.0 years ± 10.0 years (range 39 years–90 years).
The inclusion criteria were treatment-naive BRVO in eyes that had not undergone any previous treatments, including photocoagulation therapy, sub-Tenon’s injection of triamcinolone acetonide or intravitreal injection of steroids, tissue plasminogen activator, or anti-VEGF drugs; central retinal thickness (CRT) exceeding 300 μm; best-corrected visual acuity (BCVA) of 0.5 or worse measured with the Landolt C chart; no diabetes mellitus; and no thick hemorrhages that obscured fundus visualization. For statistical analysis, the BCVA values were converted to the logarithm of the minimum angle of resolution (logMAR).
We also obtained aqueous humor from 62 eyes of 62 age-adjusted control patients (39 women, 23 men; mean age 70.9 years ± 9.3 years; range 44 years–82 years) with cataract alone. None of the 62 patients had diabetes mellitus.
Aqueous humor samples were collected from the BRVO group immediately before intravitreal injection of either triamcinolone acetonide or bevacizumab and from the control group just before cataract surgery.
All intravitreal injections were administered and samples were collected using a standard sterilization procedure that included instillation of topical povidone-iodine and antibiotic drops. All samples were stored in a deep freezer at −80°C until analysis. The VEGF concentrations were measured using an enzyme-linked immunosorbent assay (ELISA) for human VEGF (Human VEGF Quantikine ELISA Kit; R&D Systems, Minneapolis, MN, USA); the primary antibody targeted two VEGF isoforms: VEGF121 and VEGF165 The lowest detectable limit of VEGF concentration was 9 pg/mL, according to the manufacturer’s instructions.
Fluorescein angiography (FA) was performed to detect the NPAs before treatment. The sizes of the NPAs were analyzed with Scion Image software (Scion, Frederick, MD, USA) and expressed in disc areas (DAs). We divided the BRVO group into two subgroups for further analysis based on the presence (+) or absence (−) of a NPA. The NPA+ group included eyes with an NPA observed on FA, and the NPA− group included eyes without apparent NPA on FA. Optical coherence tomography (OCT) was performed using Stratus OCT (Carl Zeiss Meditec, Dublin, CA, USA), and the CRT was analyzed using the fast macular thickness-scan pattern protocol that averages the retinal thickness within 1 mm in diameter over the central fovea. Existence of subretinal fluid (SRF) was also analyzed by OCT. Sample data were analyzed using GraphPad Prism 6 software (GraphPad Software, La Jolla, CA, USA).
Unpaired t-test was used to compare the two independent groups with normal distribution. The Mann–Whitney test was used to compare the two independent groups without normal distribution. Spearman’s r correlation test was used to analyze the correlation between the VEGF concentrations and the CRT and the size of the NPA in eyes with BRVO. P < 0.05 was considered significant.
The institutional review board of Shiga University of Medical Science Hospital approved this study, which followed the principles of the Declaration of Helsinki. All participants provided written informed consent before the start of the study.
Results
Based on the presence of an NPA on FA, 52 eyes with BRVO were divided into two subgroups: NPA+ (39 eyes, 75.0%) and NPA− (13 eyes, 25.0%).
In the 52 eyes with BRVO, the mean logMAR BCVA was 0.610 ± 0.262 (range 0.301–1.398). The mean CRT was 557.3 μm ± 175.5 μm (range 205 μm–109 μm). SRF was detected in 22 (42.3%) of 52 eyes with BRVO. The mean period between the onset of BRVO and sample collection was 5.0 months ± 4.6 months (range 0 DAs–171.3 DAs). The mean size of NPA was 32.2 DAs ± 38.2 DAs (range 0 DAs–171.3 DAs).
The mean aqueous VEGF concentrations in the eyes with BRVO were 290.5 pg/mL ± 294.9 pg/mL (range 81.9 pg/mL–1567.3 pg/mL) and 118.0 pg/mL ± 50.1 pg/mL (24.6 pg/mL–241.1 pg/mL) in the control group. The mean VEGF concentration was significantly higher in eyes with BRVO than in the control cataract eyes (P < 0.0001 by Mann–Whitney test). The mean aqueous VEGF concentration in the NPA− group was 171.4 pg/mL ± 52.5 pg/mL (90.9 pg/mL–299.9 pg/mL), which was significantly higher than in the control cataract eyes (P = 0.0009 by unpaired t-test). The aqueous VEGF concentration was 330.2 pg/mL ± 330.7 pg/mL (81.9 pg/mL–1567.3 pg/mL) in the NPA+ group, which was also significantly higher than in the control cataract eyes (P < 0.0001 by Mann–Whitney test) (). The mean aqueous VEGF concentration in the NPA+ group was higher than in the NPA− group; however, the difference did not reach significance (P = 0.0852 by Mann–Whitney test) ().
Figure 1 Aqueous VEGF levels of all BRVO eyes (NPA+/−), NPA+ group, and NPA− group were significantly higher than the control cataract group.
Abbreviations: BRVO, branch retinal vein occlusion; NPA, nonperfused area; VEGF, vascular endothelial growth factor.
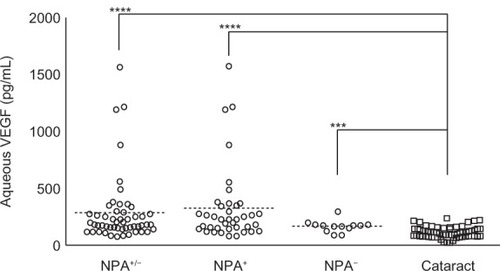
Table 1 Demographic data in BRVO subgroup
In BRVO eyes with SRF (n = 22), the mean aqueous VEGF concentration, the size of NPA, and the CRT were 220.5 pg/mL ± 90.3 pg/mL, 36.9 DAs ± 39.5 DAs, and 631.5 μm ± 199.9 μm, respectively. In BRVO eyes without SRF (n = 30), they were 341.8 pg/mL ± 374.9 pg/mL, 28.7 DAs ± 37.5 DAs, and 502.9 μm ± 134.2 μm, respectively. Statistical significance was found in CRT (P = 0.0077 by unpaired t-test), but not in the aqueous VEGF concentration (P = 0.9483 by Mann–Whitney test) and the NPA (P = 0.201 by Mann–Whitney test).
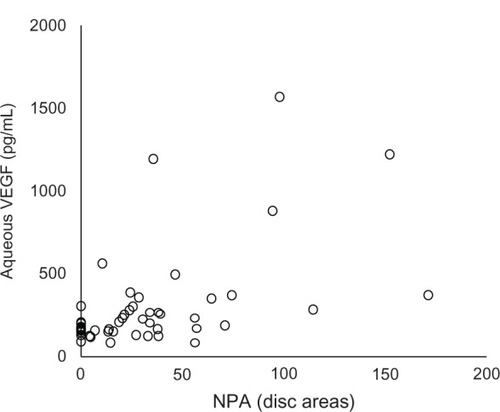
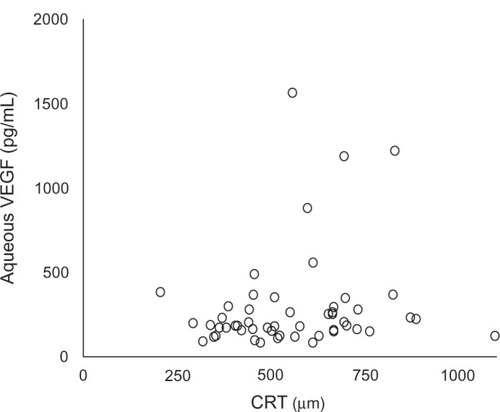
In all 52 eyes with BRVO, the size of the NPA was positively significantly correlated with the aqueous VEGF concentration (Spearman’s r = 0.488, P = 0.0002) (). However, no correlation was found between the aqueous VEGF concentration and the CRT (Spearman’s r = 0.186, P = 0.187) ().
Discussion
Macular edema secondary to BRVO is a frequent cause of visual impairment. However, no effective treatment has been established, although various treatments have been attempted.Citation11,Citation15–Citation18 To determine an effective treatment, the pathogenesis of the macular edema secondary to BRVO must be determined. VEGF is a primary cytokine that contributes to the development of macular edema. VEGF expression is promoted by hypoxia, and causes changes in the conformational structure of the tight junctions of the retinal vascular endothelial cells and induces increased vascular permeability.Citation6–Citation8 The aqueous and vitreous VEGF levels increase in eyes with proliferative diabetic retinopathy, diabetic macular edema, central retinal vein occlusion, and BRVO.Citation3,Citation12–Citation14
Noma et al reported that the aqueous VEGF level in 19 eyes with macular edema associated with BRVO was 351 pg/mL ± 273 pg/mL, which was significantly higher compared with the control group. They included nine patients who had undergone previous photocoagulation therapy before sample collection.Citation12 The previous treatment might have affected the production of VEGF in BRVO eyes.Citation3 Therefore, the current study included 52 treatment-naive eyes with BRVO with macular edema alone. The aqueous VEGF concentration in eyes with macular edema secondary to treatment-naive BRVO was 290.5 pg/mL ± 294.9 pg/mL, which was significantly higher than in the control eyes with cataract. We confirmed that the VEGF concentration was elevated in treatment-naive eyes with macular edema secondary to BRVO, which was similar to the results reported by Noma et al,Citation12 which included eyes with a history of photocoagulation therapy.
It has been reported that ischemic BRVO tends to occur in serous retinal detachment.Citation19 Therefore, we selected 13 of 52 eyes with BRVO without apparent NPA seen on FA. Even in eyes without NPA, the aqueous VEGF concentration was 171.4 pg/mL ± 52.5 pg/mL, which was significantly higher than the control group, suggesting that the presence of microischemic lesion undetectable by FA might be responsible for increased VEGF concentrations. The difference in the aqueous VEGF concentration between the NPA+ and NPA− groups did not reach significance, probably because of the small number of patients. Noma et al also reported that the aqueous VEGF level was significantly positively correlated both with the size of the NPA and the CRT in BRVO.Citation12 We also found a significant positive correlation between the aqueous VEGF concentration and the size of the NPA. However, no correlation was found between the aqueous VEGF concentration and the CRT, although we had a greater number of patients. There is no previous report about this issue.
The reason could be the varying locations of the NPA in BRVO. Retinal vein occlusion develops at various sites and in areas of different sizes. Even when the NPA is relatively large, the foveal function could be affected less if the involved location is not near the macula. A second reason could be the localized effect of VEGF in BRVO. Sawada et al, who reported that the aqueous VEGF concentration in 21 eyes with myopic choroidal neovascularization was lower than in controls, speculated that the VEGF might be localized and cause choroidal neovascularization.Citation20 Similarly, VEGF secreted from NPA distant from the macula might have little effect on the macula. Another reason could be the existence of SRF caused by other vascular permeability factors. We found that the CRT was significantly greater in BRVO eyes with SRF than without SRF, but the aqueous VEGF concentration and NPA was not different between them. This suggests that cytokines other than VEGF may be involved in BRVO. Indeed, it has been reported that inflammatory cytokines, such as IL-6, IL-8, and monocyte chemoattractant protein 1 may contribute to the pathogenesis of macular edema with BRVO, even in nonischemic BRVO.Citation21 Therefore, inflammation could also elevate the VEGF level along with inflammatory cytokines.
Conclusion
The aqueous VEGF concentration in treatment-naive eyes with macular edema secondary to BRVO increased significantly and was correlated with the size of the NPA. The aqueous VEGF concentration significantly increased even in BRVO eyes without apparent NPA, suggesting that micro-disperfusion and/or inflammatory change may increase VEGF. The location and size of the NPA and the effect of locally secreted VEGF also seem to be important, because the VEGF was not correlated with the CRT.
Acknowledgments
This study was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (24592668) and a grant from the Ministry of Health, Labour and Welfare. The authors have no proprietary interest in any aspect of this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- MichelsRGGassJDThe natural course of retinal branch vein obstructionTrans Am Acad Ophthalmol Otolaryngol197478OP166OP1774825042
- GutmanFAZegarraHThe natural course of temporal retinal branch vein occlusionTrans Am Acad Ophthalmol Otolaryngol197478OP178OP1924825043
- AielloLPAveryRLArriggPGVascular endothelial growth factor in ocular fluid of patients of diabetic retinopathy and other retinal disordersN Engl J Med1994331148014877526212
- ChenKHWuCCRoySLeeSMLiuJHIncreased interleukin-6 in aqueous humor of neovascular glaucomaInvest Ophthalmol Vis Sci1999402627263210509659
- Pe’erJFolbergRItinAGnessinHHemoIKeshetEVascular endothelial growth factor upregulation in human central retinal vein occlusionOphthalmology19981054124169499769
- DvorakHFBrownLFDetmarMDvorakAMVascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesisAm J Pathol1995146102910397538264
- VinoresSADerevjanikNLOzakiHOkamotoNCampochiaroPACellular mechanisms of blood-retinal barrier dysfunction in macular edemaDoc Ophthalmol19999721722810896335
- GardnerTWAntonettiDABarberAJLiethETarbellJAThe molecular structure and function of the inner blood-retinal barrier. Penn State Retina Research GroupDoc Ophthalmol19999722923710896336
- MulcahyMFBensonABIIIBevacizumab in the treatment of colorectal cancerExpert Opin Biol Ther20055997100516018743
- IturraldeDSpaideRFMeyerleCBIntravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term studyRetina20062627928416508427
- PaiSAShettyRVijayanPBClinical, anatomic, and electrophysiologic evaluation following intravitreal bevacizumab for macular edema in retinal vein occlusionAm J Ophthalmol200714360160617306753
- NomaHFunatsuHYamasakiMPathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6Am J Ophthalmol200514025626116086947
- NomaHMinamotoAFunatsuHIntravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusionGraefes Arch Clin Exp Ophthalmol200624430931516133018
- NomaHFunatsuHYamasakiMAqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusionEye (Lond)200822424816826241
- StefanssonEThe therapeutic effects of retinal laser treatment and vitrectomy: a theory based on oxygen and vascular physiologyActa Ophthalmol Scand20017943544011594975
- ArnarssonAStefanssonELaser treatment and the mechanism of edema reduction in branch retinal vein occlusionInvest Ophthalmol Vis Sci20004187787910711707
- SilvaRMFaria de AbreuJRCunha-VazJGBlood-retina barrier in acute retinal branch vein occlusionGraefes Arch Clin Exp Ophthalmol19952337217268566831
- SaikaSTanakaTMiyamotoTOhnishiYSurgical posterior vitreous detachment combined with gas/air tamponade for treating macular edema associated with branch retinal vein occlusion: retinal tomography and visual outcomeGraefes Arch Clin Exp Ophthalmol200123972973211760031
- YamaguchiYOtaniTKishiSSerous macular detachment in branch retinal vein occlusionRetina2006261029103317151490
- SawadaOKawamuraHKakinokiMSawadaTOhjiMVascular endothelial growth factor in the aqueous humour in eyes with myopic choroidal neovascularizationActa Ophthalmol20118945946220102348
- YoshimuraTSonodaKSugaharaMComprehensive analysis of inflammatory immune mediators in vitreoretinal diseasesPLoS One20094e815819997642