Abstract
Purpose
The objective of this study was to assess the effectiveness of intense pulsed light (IPL) therapy in individuals diagnosed with glaucoma and dry eye disease (DED).
Methods
This randomized control study recruited 22 individuals diagnosed with glaucoma, ranging in age from 33 to 82 years. These participants were undergoing treatment with hypotensive eyedrops and had clinical indications and subjective complaints associated with dry eye. Each patient underwent three sessions of IPL therapy in one eye, while the contralateral eye served as the control eye (CT). The following parameters were assessed at three time points: baseline, week-2, and week-4. These parameters include non-invasive breakup time (NITBUT), tear meniscus height (TMH), conjunctivocorneal epithelial staining score (CS), tear film lipid layer (TFLL), meibomian gland expressibility score (MGEx), Schirmer I test, ocular bulbar redness score (OBRS), and ocular surface disease index (OSDI). Intraocular pressure (IOP), best-corrected visual acuity (BCVA), and corneal endothelial cell count (ECC) were assessed for safety. The clinical trial was registered on 25/12/2023 at ClinicalTrials.gov website (NCT06158984).
Results
Comparing baseline and 4-week measurements revealed that the IPL group found significant improvements in NITBUT (IPL: 8.74±2.60 sec. vs CT: 5.76±1.75 sec. p<0.01), TMH (IPL: 0.23±0.05mm vs CT: 0.19±0.06mm, p=0.011), C.S. (IPL: 1.14±0.56 vs CT: 1.95±1.17, p=0.005), TFLL (IPL: 2.91±2.91 vs CT:3.36±0.58, p=0.047), MGEx score (IPL: 1.14±0.35 vs CT: 1.45±0.51, p=0.020) and OSDI scores (IPL: 31.77±15.59 vs 50.59±21.55, p=0.002) significantly improved. Conversely, other parameters showed no significant improvements (p>0.05).
Conclusion
The progression of ocular surface disease in individuals using topical anti-glaucoma medication may worsen if the condition is not addressed. Nevertheless, IPL therapy has the potential to result in significant improvements in both objective and subjective measures of dry eye. Best-corrected visual acuity, endothelial cell count, and intraocular pressure were determined to be within the permitted limits. No adverse events were reported during the course of the study.
Plain Language Summary
The results show that people who use topical medicines to treat glaucoma may get worse eye surface disease if they do not treat the problem. IPL treatment, on the other hand, can make a big difference in both objective and subjective dry eye tests. The vision, endothelial cell count, and the pressure inside the eye were all found to be within normal limits after the IPL treatment. Even though the people in our study had glaucoma and had been taking glaucoma medicine for it for a year and the fact that the symptoms last for a long time may also change the results. Also, DED caused by glaucoma medication is complicated, with a lot of different symptoms and signs, even in the same stage. Also, subjective complaints may not match up with clinical signs. The type, amount, and length of anti-glaucoma drugs may have affected the results.
Introduction
Dry eye disease (DED) is a complex ocular surface condition influenced by several factors. It is defined by symptoms such as pain, irritation, and vision disturbances.Citation1,Citation2 DED presents substantial challenges for individuals, resulting in limitations in social and occupational functioning, as well as diminished quality of life.Citation3 The global incidence of DED is estimated to range from 5% to 50%, with particularly high prevalence seen in China.Citation4,Citation5 Glaucoma has a global prevalence of over 70 million individuals, of whom roughly 10% have bilateral blindness, becoming it the foremost cause of permanent visual impairment on a global scale.Citation6 Glaucoma has the potential to stay asymptomatic until it reaches a severe stage, hence increasing the probability that the actual prevalence of afflicted persons surpasses the reported figures.Citation7
It is important to acknowledge that the combined impact of dry eye signs and symptoms in individuals with glaucoma contributes to the significant societal, economic, and public health impact of these conditions.Citation8 The extent of toxic or allergic responses to topical anti-glaucoma drugs is correlated with the frequency of daily instillations, length of therapy, and presence of preservatives.Citation9,Citation10 Chronic use of these ocular drops primarily leads to structural modifications characterized by impaired functionality and depletion of the goblet cell, meibomian gland, and auxiliary lacrimal glands.Citation11–13 Moreover, this phenomenon results in the disturbance of the corneal epithelium and a decrease in corneal sensitivity, ultimately causing disruption to the tear film and a reduction in the thickness of the mucus, aqueous, and lipid layers.Citation9,Citation14,Citation15
Intense pulsed light (IPL) therapy is best known for treating various dermatological conditions, including hypertrichosis, benign cavernous venous malformations, telangiectasia, port wine stains, and other pigmented lesions.Citation16,Citation17 IPL treatment involves the use of a noncoherent polychromatic light source that emits light across a wide range of wavelengths, typically between 500 and 1200 nm, targeting the sebaceous glands located on the surface of the facial skin.Citation18 It’s postulated that the photothermal impact induced by IPL therapy reduces inflammation and promotes the activation of the meibomian glands and several studies have reported its effectiveness therapy for improving signs and symptoms of DE.Citation16,Citation19 Dry eye due to glaucoma-related medication is frequently seen in eye clinics; however, it is important to highlight that the management strategy for ocular surface disease associated with glaucoma (G-OSD) is often overlooked.Citation20,Citation21 As reported by Nijm et al,Citation6 the prevalence of DED is greater in those with glaucoma compared to those without glaucoma, perhaps due to glaucoma treatment and there is a pressing need to address this problem. A self-control study found improvements in DE symptoms in glaucoma patients using IPL,Citation22 therefore, the objective of this research was to evaluate the efficacy of IPL as an adjunctive therapy for individuals receiving topical anti-glaucoma medication manifesting signs and symptoms of DED.
Materials and Methods
Study Design and Participants
This randomized control study adhered to the ethical guidelines set out by the Institutional Review Board (IRB) of He Eye Specialist Hospital in Shenyang, China, in accordance with the principles outlined in the Declaration of Helsinki (IRB (2022) K029.01). The clinical trial was registered on 25/12/2023 at ClinicalTrials.gov website (NCT06158984). Participants were recruited from the outpatient department of the Department of Ophthalmology between December and February 2024.
Experimental Design
One eye of the participant was randomly assigned to IPL (treatment) or no IPL (control) groups. IPL therapy was administered at days 0 (baseline), 14 (week-2), and 28 (week-4). A certified clinician was responsible for administrating the IPL and was not responsible for collecting data. The data collector was unaware of the participants’ eye assigned therapy when they collected data at baseline (BL), week-2 (W2), and week-4 (W4) from all participants. During this study, participants were instructed to avoid using dry eye treatments and eyedrops, including preservative-free artificial tears (). However, participants were free to opt-out of the study and use dry eye treatments and eyedrops.
Figure 1 The flowchart of the experimental framework of this study.
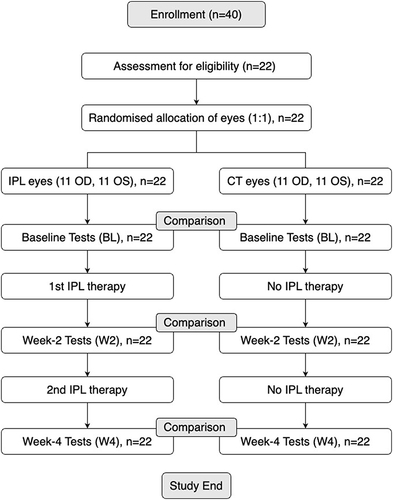
Currently, there has been one single-arm case series study assessing the impact of IPL therapy on G-OSD;Citation22 therefore, the effects of IPL therapy as a viable therapy for G-OSD has not been established. In-addition the participants enrolled for this study were not undergoing any dry eye treatment prior to enrollment. Finally, participants at any time had the right to opt-out, use other dry eye drugs and therapy, or use IPL for both eyes. However, in doing so their data would not be used for the final analysis.
All participants provided informed permission subsequent to a thorough explanation of the study’s purpose and any ramifications. The data pertaining to the participants in this research is anonymized. The research included a total of 22 participants diagnosed with glaucoma, ranging in age from 33 to 82 years. These individuals were presently undergoing treatment for glaucoma with hypotensive eyedrops, while also experiencing signs and symptoms of dry eye disease (DED). The IPL therapy was administered to one eye using a random selection process. In contrast, the other eye was designated as a control using the block randomization opaque sealed envelope technique, which was undertaken by the study’s statistician. The clinical assessors and data-collecting personnel were kept oblivious to the result of the randomization process (). The allocation sequence was produced by the statistician involved in the trial, while the primary investigator was responsible for enrolling individuals and assigning them to their respective therapies. The main objective of this research was to evaluate and contrast the alteration in tear film lipid layer (TFLL) between the two groups. The major outcome measure used for determining the sample size was the evaluation of meibomian gland function, particularly assessed by the grading of the tear film lipid layer thickness. The research performed a comprehensive analysis of multiplicity and nonparametric adjusted power calculations in order to ascertain the appropriate sample size required for detecting a clinically relevant disparity in the tear film lipid layer. The intended result was the alteration of a single grade of lipid layer. Based on the calculations, it was determined that a sample size of 15 eyeballs was required to attain a power of 80% (β = 0.2) and a two-sided statistical significance threshold of 5% (α = 0.05). The predicted standard deviation of normal values was one lipid layer grade. The study’s design, execution, reporting, and dissemination strategies did not include the involvement of patients or the general public.
The inclusion criteria for this study are as follows: (i) individuals who are under the age of 18 years; (ii) clinical diagnosis of primary open-angle glaucoma or normal tension glaucoma and have been treated with topical glaucoma eye drops for a duration of 12 months or more, in both eyes; (iii) individuals with Fitzpatrick skin types I–IV, (iv) individuals who are capable and willing to comply with treatment and follow-up obligations, and (v) individuals who have been determined to have evaporative dry eye disease (DED) based on the Asian Dry Eye Consensus.Citation23 (vi) individuals not undergoing active treatment for signs and symptoms of dry eye.
The exclusion criteria for this study included individuals who had undergone previous ocular surgery or trauma, were under the age of 18, had undergone chalazion section, were experiencing acute inflammation, had a history of blepharal and periorbital skin disease or allergies within the past month, had severe dry eyes with a corneal epithelial defect, limbic keratitis, pterygium, corneal neovascularization, or glaucoma, were breastfeeding, had rheumatic immune systemic diseases, had a history of herpes zoster infection, were pregnant, were contact lens wearers, had an allergy to fluorescein, or were using photosensitive drugs/foods. Additionally, individuals with skin Fitzpatrick scale V/VI were also excluded from the study.
Clinical Assessment
The participants were evaluated at three specific visits: before the first IPL therapy on day 0 (baseline), day 14 (week 2), and day 28 (week 4). The sequential evaluation of the patient’s eyes (both eyes) involved the following clinical assessments: best-corrected visual acuity (BCVA), non-invasive tear breakup time (NITBUT), tear film lipid layer (TFLL) quality, endothelial cell count (ECC), intraocular pressure (IOP), Schirmer I test (Liaoning Meizilin Pharmaceuticals, Panjing, China), meibomian gland expression (MGEx), and corneal staining (CS) were performed. All evaluations were conducted prior to the initiation of the IPL therapy and each subsequent visit.Citation24,Citation25 The assessment of safety was conducted by the measurement of best-corrected visual acuity (BCVA), intraocular pressure (IOP), and endothelial cell count (ECC). Additionally, thorough examinations of the cornea and conjunctiva were performed using a slit-lamp microscope at each visit. Furthermore, an assessment was conducted on several eyelash irregularities, including loss of lashes, trichiasis, and the presence of redness and irritation. All examinations were conducted before the IPL therapy at each subsequent visit.
Subjective BCVA was assessed for each eye separately using the ETDRS chart.Citation26
The measurement of IOP was conducted using a non-contact tonometer (NT-510, NIDEK, Japan).Citation27 The evaluation of ECC was conducted using a corneal endothelial counter (SP-3000P, TOPCON, Japan).Citation28
The assessment of NITBUT was conducted using the Keratograph 5M topographer (OCULUS, Germany). Three successive measurements were recorded, and the median value was recorded as the final reading.Citation29
The quality of TFLL was evaluated in a noninvasive manner utilizing TFLL interferometry with a DR-1 equipment (KOWA, Nagoya, Japan). The Yokoi dry eye (DE) severity grading system was employed to assess the results. Grade 1 indicates a somewhat grey color with a uniform distribution, grade 2 indicates a somewhat grey colour with a non-uniform distribution, grade 3 indicates a few colors with a non-uniform distribution, and grade 4 indicates many colors with a non-uniform distribution.Citation30
Meibography, also known as MG Score, is a diagnostic technique used to assess the condition of the meibomian glands. The Keratograph 5M (OCULUS, Germany), was utilized to obtain infrared photographs of the top and lower eyelids. The device was flipped over to expose the meibomian glands before capturing the images. The meibomian glands were evaluated for each eyelid and assigned a score (MG Score) based on the extent of loss. The scoring system included the following grades: grade 0 indicated no loss of meibomian glands, grade 1 indicated loss of less than one-third of the total gland area, grade 2 indicated loss of between one-third and two-thirds of the gland area, and grade 3 indicated loss of more than two-thirds of the gland area.Citation31
Meibomian gland function. Upper eyelid meibum quality and meibomian gland expression were assessed. (i) Meibum quality: Eight meibomian glands in the middle of the eyelid were graded from 0 to 3 for meibum quality. A score of 0 indicated clean meibum, 1 cloudy, 2 cloudy and grainy, and 3 thick, like toothpaste.Citation32 (ii) Evaluation of MG expression: Five middle region meibomian glands were scored from 0 to 3. A score of 0 means all glands were expressible, 1 means 3–4, 2 means 1–2, and 3 means none. We calculated the aggregate score from these eight glands’ mean scores.Citation32
Double vital staining technique, as described by Arita et al,Citation33 was employed to evaluate the extent of injury to the conjunctival and corneal epithelium. Two microliters of a preservative-free mixture comprising 1% sodium fluorescein and 1% lissamine green were dropped into the conjunctival sac. The eye was divided into three equal portions, which corresponded to the (i) temporal conjunctiva, (ii) cornea, and (iii) nasal conjunctiva. Each area was awarded a staining score ranging from 0 to 3 points. The final CS was documented to the sum of all three sections on a spectrum ranging from 0 (normal) to 9 (severe).Citation34
Treatment and Treatment Procedure
IPL system used a xenon lamp that emits IPL at 515–1200 nm on the cutaneous facial sebaceous glands. A 560- nm filter was adjusted to the appropriate setting (range of 11–14 J/cm2). The IPL treatment intensity was chosen based on the Fitzpatrick scale (scale I to III). A total of three IPL therapies were administered once at 2-week intervals, on day 0 (BL), day 14 (W2), and day 28 (W4).Citation16,Citation35,Citation36
According to Chen et al,Citation37 the ultrasonic gel was applied on the inferior border of the treated eye and the preauricular region; with all participants wearing opaque goggles during the entire IPL therapy process. The therapy included the administration of six light pulses, with each pulse slightly overlapping the previous one. These pulses were delivered starting from the preauricular region of the treated eye, extending over the cheekbones and nose, and reaching up to the inferior border of the treated eyes. Participants were instructed to refrain from wearing cosmetics on the day of treatment, and none of the individuals included in the study were habitual contact lens users. In order to mitigate the risk of face pigmentation as a result of IPL therapy, patients were duly informed and advised to refrain from direct sun exposure for a duration of one month after each IPL session.
Statistical Analysis
The mean ± standard deviation was used to describe the data, and all statistical analyses were performed using SPSS version 25 (SPSS Inc., Chicago, IL, USA). The data demonstrated a departure from normality, as shown by the Shapiro–Wilk test (p < 0.05), therefore requiring the use of nonparametric statistical techniques. The researchers used a linear mixed model to evaluate the repeated measurements of continuous variables. A generalized linear mixed model was used to conduct a statistical analysis on the repeated measurements of discrete variables. The Bonferroni post hoc analysis was used to further examine the obtained data. Statistical significance was determined to be p< 0.05, indicating a significance level.
Results
The final analysis included 44 eyes (22 participants) in the IPL group (15 O.D., 7 O.S). and 22 eyes in the control group (7 O.D., 15 O.S). The mean age was 62.09±13.40 years (range: 33 to 82 yrs). All participants had signs and symptoms indicative of evaporative DED ().
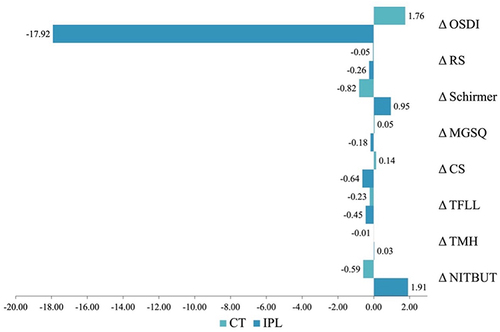
Non-invasive tear break-up time (NITBUT) values in the IPL group showed positive changes and negative changes was observed in the control group at W2 and W4; however, a statistically significant difference between the two groups was only evident at W4 (p<0.05) (). Tear meniscus height (TMH) assessment revealed no significant differences between the groups at BL and W2 test points. On W4, significant improvement (p=0.011) in TMH was observed between the IPL and the control group (). The mean corneal redness score (R.S.) in the IPL group was lower than the control group at W2; but these improvements were only found to be statistically significant at W4 (p=0.005) (). The quality of the tear-film lipid layer (TFLL) was assessed using an interferometry pattern, which assigned a numerical grade ranging from 1 to 5. There were no statistically significant differences seen between the groups at baseline (p=0.794) and W2 (p=0.757) test points; however, on W4, significant improvement (p=0.047) was observed in the IPL group (). The Meibomian gland expression (MGEx) score had no statistically significant difference at BL and W2 (p=0.764 and p=0.117, respectively) test points. However, a significant improved difference was observed in the IPL group at W4 (p=0.020) (). The Schirmer’s I test was tested at BL., W2, and W4 and no significant improvement was observed between the groups at all time points. Redness scores (RS) was assessed at BL, W2, and W4. Comparisons between the groups revealed no significant changes at all test points (p> 0.05). The mean total OSDI score was found to improve in the IPL group and deteriorate in the control group at W2 and W4 test points. The differences between the groups were statistically different at W2 (p=0.048) and W4 (p=0.002) (). and depicts the percentage change in severity of OSDI in patients diagnosed with DED who were assigned to either the IPL treatment group or the control group. illustrates the overall alteration in dry eye characteristics seen in both experimental groups, denoted by the symbol Δ, representing the difference between the data obtained at week 4 and the baseline measurements.
Table 1 Dry Eye Parameters
Figure 2 (A) Comparison of OSDI severity scale in IPL group. (B) Comparison of OSDI severity scale in Control group.
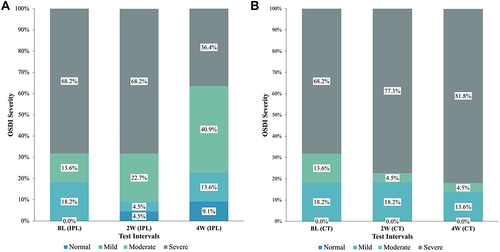
Figure 3 An overall change in dry eye parameters.
Throughout the course of the study, no instances of systemic adverse events or abnormal clinical signs and symptoms were seen in any of the groups. There was no statistically significant difference seen in either group at W2 or W4. When compared to the baseline measures, best-corrected visual acuity (BCVA), intraocular pressure (IOP), and endothelial cell count (ECC) (). There were no instances of depigmentation, blistering, swelling, redness, hair loss at the brow, eyelash loss, or ocular surface detected after the use of IPL treatment.
Table 2 Safety Parameters
Discussion
This randomized control study assessed the use of IPL therapy for managing signs and symptoms of DED due to G-OSD. Ocular surface disease, a notable ocular comorbidity, is often underestimated in glaucoma patients, with a global prevalence ranging from 40% to 59%.Citation38 In a study conducted in 2006, Tsai et al found that the occurrence rate of glaucoma among individuals with severe OSD was as high as 66%.Citation39 Our research findings indicate that after two sessions of IPL therapy conducted over four weeks, objective dry eye parameters such as NITBUT, TMH, OBR score, and TFLL score. Furthermore, subjective symptom scores obtained using the OSDI questionnaire score significantly improved earlier than objective clinical findings in the second week (p<0.05) compared to the contralateral eye (control group). While there were no significant differences observed in the meibomian glands secretion quality, Schirmer test, and redness score between the therapy and control group, nevertheless, the control eyes had their signs and symptoms deteriorate at the 4-week follow-up.
The main aim of the present investigation was to determine the efficacy of IPL therapy in individuals receiving treatment for ocular hypertension with G-OSD. The data presented in this study offer clinical evidence derived from a four-week period of follow-up, indicating that ocular surface in patients who also have a need for ongoing glaucoma therapy can be optimized with the aid of IPL therapy in our patient population. The aforementioned observations align with the single-arm case series findings of Martinez-de-la-Casa, Jose Maria et al pertaining to the use of IPL in G-OSD.Citation22 The authors concluded that the clinical signs and symptoms of dry eye can be improved using IPL therapy.
Significant NITBUT increases were expected due to improved meibomian secretions and a more stable tear film. Several studies have found considerable improvements in NITBUT following IPL.Citation40,Citation41 However, the follow-up and method employed to quantify vary among studies. The non-significant increase in NITBUT during the initial follow-up (W2) could be due to the toxicity of the preservatives in the IOP-reducing drugs or a more extended follow-up period required.Citation19 Nonetheless, at a 4-week follow-up, it revealed significant NITBUT improvements. In comparison to Martinez-de-la-Casa, Jose Maria et al (mean age: 74.6±9.0 yrs., range: 57 to 94 yrs)., the current study (mean age: 62.09±13.40 yrs., range: 33 to 82 yrs) found significant changes in NITBUT, and this could be attributed to the age of the population group as age is a significant contributing factor for DED severity.Citation42 In addition, significant improvements in dry eye signs and symptoms in the current study can be attributed to positive changes to the TFLL, as therapeutic interventions aimed at restoring or maintaining the integrity of the lipid layer play a crucial role in managing DED.Citation43 Studies have also suggested that topical OHT-reducing medication can lower tear lipid layer thickness (LLT) in glaucoma patients.Citation44
The primary focus of glaucoma treatment involves the reduction of OHT, and patients are typically prescribed IOP-lowering ophthalmic drops.Citation45 The efficacy of these treatments is contingent upon the consistent adherence of patients to prescribed regimens, which may entail the administration multiple times throughout the day.Citation45,Citation46 Regrettably, the addition of topical ocular lubricants such as hyaluronic acid and diquafosol can negatively affect patient adherence to drugs for the treatment of glaucoma.Citation47 Therefore, similar to Lipiflow, the application of IPL therapy provides an alternative to topical antibiotics (to decrease the bacterial load), steroids, or cyclosporine in mitigating inflammation, and unlike TFLL profile enhancement achieved by systemic omega-3 supplementation or oral tetracycline as the dosage does not have to be maintained daily.Citation48
The current study investigation exhibits certain limitations. It is important to note that while our study included individuals diagnosed with glaucoma who had been using anti-glaucoma drugs for an extended duration (1 year), it is possible that each patient may have experienced G-OSD at various points before their enrolment in the study. Since the duration or chronicity of the condition may also impact the outcomes. Furthermore, glaucoma-related DED is a multifactorial condition characterized by a diverse range of symptoms and indications, which can exhibit considerable variance even within the same stage. The disease does not possess a distinct and dependable indicator to signify the progression of the ailment. Moreover, it is worth noting that clinical manifestations may not necessarily align with subjective symptoms. In addition, the type, quantity, and duration of anti-glaucoma drugs employed are among several crucial aspects that impact the outcomes. The factors mentioned above were not within the scope of this study. Furthermore, antiglaucoma drugs comprise of a bioactive, inert and may contain a preservative. Active components, such as prostaglandin analogues, β-blockers, ⍺-2 adrenergic agonists, carbonic anhydrase inhibitors, pilocarpine, rho-associated kinase inhibitors, and combination drugs; this study did not collect, assess or stratify participants according to their current glaucoma treatment. Future studies will aim to account for these short comings.
Conclusion
The results suggest that dry eye disease in individuals undergoing topical treatment with anti-glaucoma medications, may worsen in the absence of appropriate intervention. Intense pulsed light, an adjuvant therapy for dry eyes, led to notable enhancements in many objective and subjective indicators of mild and moderate dry eye in individuals with glaucoma who were undergoing topical anti-glaucoma medication. The safety parameters, such as intraocular pressure, best-corrected visual acuity, and endothelial cell count, were observed to be within acceptable ranges.
Ethics Approval and Consent to Participate
The research was carried out in adherence to the principles outlined in the Declaration of Helsinki and received approval from the Institutional Review Board of He Eye Specialist Hospital, Shenyang, China (IRB (2022) K029.01). Informed consent was obtained from all participants involved in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval for the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank all participants, postgraduate students, and researchers. We also acknowledge the support from the Dry Eye and Ocular Surface Clinic and Glaucoma Clinic at HESH, Shenyang.
Data Sharing Statement
Data will be made available on reasonable request to the corresponding author.
Additional information
Funding
References
- Galor A, Feuer W, Lee DJ, et al. Prevalence and risk factors of dry eye syndrome in a United States Veterans Affairs population. Am J Ophthalmol. 2011;152(3). doi:10.1016/j.ajo.2011.02.026
- Dana R, Bradley JL, Guerin A, et al. Estimated prevalence and incidence of dry eye disease based on coding analysis of a large, all-age United States Health Care System. Am J Ophthalmol. 2019:202. doi:10.1016/j.ajo.2019.01.026
- Wang TJ, Wang IJ, Hu CC, Lin HC. Comorbidities of dry eye disease: a nationwide population-based study. Acta Ophthalmol. 2012;90(7). doi:10.1111/j.1755-3768.2010.01993.x
- Ma J, Pazo EE, Zou Z, Jin F. Prevalence of symptomatic dry eye in breast cancer patients undergoing systemic adjuvant treatment: a cross-sectional study. Breast. 2020;53. doi:10.1016/j.breast.2020.07.009
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003
- Nijm LM, Schweitzer J, Blackmore JG. Glaucoma and dry eye disease: opportunity to assess and treat. Clin Ophthalmol. 2023;17:3063–3076. doi:10.2147/OPTH.S420932
- Leite MT, Sakata LM, Medeiros FA. Managing glaucoma in developing countries. Arq Bras Oftalmol. 2011;74(2). doi:10.1590/S0004-27492011000200001
- Lee SY, Wong TT, Chua J, Boo C, Soh YF, Tong L. Effect of chronic anti-glaucoma medications and trabeculectomy on tear osmolarity. Eye. 2013;27(10):1142–1150. doi:10.1038/eye.2013.144
- Mastropasqua R, Agnifili L, Mastropasqua L. Structural and molecular tear film changes in glaucoma. Curr Med Chem. 2018;26(22). doi:10.2174/0929867325666181009153212
- Roberti G, Tanga L, Manni G, et al. Tear film, conjunctival and corneal modifications induced by glaucoma treatment. Curr Med Chem. 2019;26(22). doi:10.2174/0929867326666190517111823
- Bourne RRA, Kaarniranta K, Lorenz K, Traverso CE, Vuorinen J, Ropo A. Changes in ocular signs and symptoms in patients switching from bimatoprost–timolol to tafluprost–timolol eye drops: an open-label Phase IV study. BMJ Open. 2019;9(4):e024129. doi:10.1136/BMJOPEN-2018-024129
- Wolfram C, Stahlberg E, Pfeiffer N. Patient-reported nonadherence with glaucoma therapy. J Ocul Pharmacol Ther. 2019;35(4):223–228. doi:10.1089/jop.2018.0134
- Kim DW, Seo JH, Lim SH. Evaluation of ocular surface disease in elderly patients with glaucoma: expression of matrix metalloproteinase-9 in tears. Eye. 2020;35(3):892–900. doi:10.1038/s41433-020-0993-y
- Razeghinejad R, Lin MM, Lee D, Katz LJ, Myers JS. Pathophysiology and management of glaucoma and ocular hypertension related to trauma. Surv Ophthalmol. 2020;65(5). doi:10.1016/j.survophthal.2020.02.003
- Wong TT, Zhou L, Li J, et al. Proteomic profiling of inflammatory signaling molecules in the tears of patients on chronic glaucoma medication. Invest Ophthalmol Vis Sci. 2011;52(10). doi:10.1167/iovs.10-6532
- Fan Q, Pazo EE, You Y, et al. Subjective quality of vision in evaporative dry eye patients after intense pulsed light. Photobiomodul Photomed Laser Surg. 2020:photob.2019.4788. doi:10.1089/photob.2019.4788
- Li L, Chen J, Qin G, et al. Tear film lipid layer changes following combined effect of heated eye mask with intense pulsed light therapy for evaporative dry eye: a randomized control study. Photobiomodul Photomed Laser Surg. 2023;41(8):435–444. doi:10.1089/PHOTOB.2023.0051
- Babilas P, Schreml S, Szeimies RM, Landthaler M. Intense pulsed light (IPL): a review. Lasers Surg Med. 2010;42(2). doi:10.1002/lsm.20877
- Qin G, Chen J, Li L, et al. Efficacy of intense pulsed light therapy on signs and symptoms of dry eye disease: a meta-analysis and systematic review. Indian J Ophthalmol. 2023;71(4):1316–1325. doi:10.4103/IJO.IJO_2987_22
- Wellik SR. Glaucoma and dry eye syndrome: double trouble. Dry Eye Dis. 2023;147–152. doi:10.1016/B978-0-323-82753-9.00009-6
- Mei Kong X, Qing Zhu W, Xu Hong J, Huai Sun X. Is glaucoma comprehension associated with psychological disturbance and vision-related quality of life for patients with glaucoma? A cross-sectional study. BMJ Open. 2014;4:4632. doi:10.1136/bmjopen-2013
- Martinez-de-la-casa JM, Oribio-Quinto C, Milans-Del-Bosch A, et al. Intense pulsed light-based treatment for the improvement of symptoms in glaucoma patients treated with hypotensive eye drops. Eye Vis. 2022;9(1):12. doi:10.1186/s40662-022-00284-4
- Uchino Y, Uchino M, Dogru M, Ward S, Yokoi N, Tsubota K. Changes in dry eye diagnostic status following implementation of revised Japanese dry eye diagnostic criteria. Jpn J Ophthalmol. 2012;56(1):8–13. doi:10.1007/s10384-011-0099-y
- Zhang XM, Yang LT, Zhang Q, et al. Reliability of Chinese web-based ocular surface disease index questionnaire in dry eye patients: a randomized, crossover study. Int J Ophthalmol. 2021;14(6). doi:10.18240/ijo.2021.06.07
- Guarnieri A, Carnero E, Bleau AM, Alfonso-Bartolozzi B, Moreno-Montañés J. Relationship between OSDI questionnaire and ocular surface changes in glaucomatous patients. Int Ophthalmol. 2020;40(3):741–751. doi:10.1007/S10792-019-01236-Z/METRICS
- Mataftsi A, Koutsimpogeorgos D, Brazitikos P, Ziakas N, Haidich AB. Is conversion of decimal visual acuity measurements to logMAR values reliable? Graefes Arch Clin Exp Ophthalmol. 2019;257(7):1513–1517. doi:10.1007/s00417-019-04344-9
- Karmiris E, Tsiripidis K, Gartaganis PS, et al. Comparison of intraocular pressure obtained by Goldmann applanation tonometer, Corvis ST and an airpuff tonometer in healthy adults. Eur J Ophthalmol. 2021. doi:10.1177/11206721211069227
- Huang J, Maram J, Tepelus TC, Sadda SR, Chopra V, Lee OL. Comparison of noncontact specular and confocal microscopy for evaluation of corneal endothelium. Eye Contact Lens. 2018;44:S144–S150. doi:10.1097/ICL.0000000000000362
- Lee R, Yeo S, Aung HT, Tong L. Agreement of non-invasive tear break up time measurement between Tomey RT-7000 Auto Refractor-Keratometer and Oculus Keratograph 5M. Clini Ophthalmol. 2016;10:1785–1790. doi:10.2147/OPTH.S110180
- Hosaka E, Kawamorita T, Ogasawara Y, et al. Interferometry in the evaluation of precorneal tear film thickness in dry eye. Am J Ophthalmol. 2011;151(1):18–23.e1. doi:10.1016/j.ajo.2010.07.019
- Yeh TN, Lin MC. Repeatability of meibomian gland contrast, a potential indicator of meibomian gland function. Cornea. 2019;38(2):256. doi:10.1097/ICO.0000000000001818
- Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1(3):107–126. doi:10.1016/S1542-0124(12)70139-8
- Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmol. 2008;115(5):911–915. doi:10.1016/J.OPHTHA.2007.06.031
- Yokoi N, Takehisa Y, Kinoshita S. Correlation of tear lipid layer interference patterns with the diagnosis and severity of dry eye. Am J Ophthalmol. 1996;122(6):818–824. doi:10.1016/S0002-9394(14)70378-2
- Chen J, Qin G, Li L, et al. The combined impact of intense pulsed light combined and 3% diquafosol ophthalmic solution on evaporative dry eye: a randomized control study. Ophthalmol Ther. 2023:1–13. doi:10.1007/S40123-023-00784-Z/TABLES/3
- Dell SJ, Gaster RN, Barbarino SC, Cunningham DN. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin Ophthalmol. 2017;11:817–827. doi:10.2147/OPTH.S130706
- Chen J, Qin G, Li L, et al. Protocol for a parallel assignment prospective, randomised, comparative trial to evaluate the safety and efficacy of intense pulsed light (IPL) combined with 3% diquafosol (DQS) ophthalmic solution in dry eye syndrome. BMJ Open. 2023;13(8):e073055. doi:10.1136/BMJOPEN-2023-073055
- Nijm LM, De Benito-Llopis L, Rossi GC, Vajaranant TS, Coroneo MT. Understanding the dual dilemma of dry eye and glaucoma: an international review. Asia Pac J Ophthalmol. 2020;9(6):481–490. doi:10.1097/APO.0000000000000327
- Tsai J, Derby E, Holland E, Cornea AK. Incidence and prevalence of glaucoma in severe ocular surface disease. Cornea. 2006;25(5):530–532. doi:10.1097/01.ico.0000220776.93852.d9
- Qin G, Chen J, Li L, et al. Managing severe evaporative dry eye with intense pulsed light therapy. Ophthalmol Ther. 2023:1–13. doi:10.1007/S40123-023-00649-5/TABLES/4
- Yang L, Pazo EE, Zhang Q, et al. Treatment of contact lens related dry eye with intense pulsed light. Cont Lens Anterior Eye. 2021:101449. doi:10.1016/J.CLAE.2021.101449
- Ding J, Sullivan DA. Aging and dry eye disease. Exp Gerontol. 2012;47(7):483–490. doi:10.1016/J.EXGER.2012.03.020
- Song Y, Yu S, He X, et al. Tear film interferometry assessment after intense pulsed light in dry eye disease: a randomized, single masked, sham-controlled study. Cont Lens Anterior Eye. 2021:101499. doi:10.1016/J.CLAE.2021.101499
- Soriano D, Ferrandez B, Mateo A, Polo V, Garcia-Martin E. Meibomian gland changes in open-angle glaucoma users treated with topical medication. Optom Vis Sci. 2021;98(10):1177–1182. doi:10.1097/OPX.0000000000001782
- Quaranta L, Novella A, Tettamanti M, Pasina L, Weinreb RN, Nobili A. Adherence and persistence to medical therapy in glaucoma: an overview. Ophthalmol Ther. 2023;12(5):2227–2240. doi:10.1007/S40123-023-00730-Z
- Pérez-García P, Burgos-Blasco B, Morales-Fernández L, et al. Prescription trends for preservative free glaucoma medication in a public health system. Eur J Ophthalmol. 2024;34(1):193–203. doi:10.1177/11206721231170034
- Park MH, Kang KD, Moon J. Noncompliance with glaucoma medication in Korean patients: a multicenter qualitative study. Jpn J Ophthalmol. 2013;57(1):47–56. doi:10.1007/S10384-012-0188-6
- Hu JG, Dang VT, Chang DH, et al. Performance of a translucent activator for LipiFlow Vectored Thermal Pulse (VTP) Treatment of Meibomian Gland Dysfunction. Clin Ophthalmol. 2022;16:963–971. doi:10.2147/OPTH.S354738
