Abstract
Laser-assisted in situ keratomileusis (LASIK) is commonly used to correct refractive defects. The procedure frequently results in dry eye symptoms, usually of short but sometimes longer duration. This study was designed to assess dry eye and ocular tolerability after LASIK in patients treated with a preservative-free lacrimal substitute (Hylabak®) or preserved lacrimal substitute (Systane®). In a single-center, investigator-masked, prospective, noninferiority, clinical study, patients undergoing LASIK surgery were randomized to receive Hylabak or Systane eye drops (one drop in each eye four times daily for 3 months). Fluorescein test scores were the primary efficacy variable and were similar on day 1 (mean 0.26 and 0.28 for Hylabak and Systane, respectively). At the final visit (day 84 ± 3) the fluorescein scores had improved to 0.11 and 0.04, respectively. The difference was not significant and thus noninferiority was established. A trend of more rapid improvement in the Hylabak group was evident. Both treatments were well tolerated and there were no serious adverse events, discontinuations for adverse events or other safety-related reasons, and no systemic adverse events. The results suggest that Hylabak is not less effective than Systane in reducing the symptoms of dry eye after LASIK surgery.
Introduction
Laser-assisted in situ keratomileusis (LASIK) is an effective procedure used to correct refractive errors such as myopia, hyperopia, and astigmatism. It provides fast and relatively painless recovery of vision, has a low probability of regression of refractive correction, is not associated with subepithelial haze, and has become a common ophthalmologic procedure.Citation1 Although complications are generally uncommon, dry eye (either temporary or permanent) has been reported in relatively high proportions of patients (up to 75% according to some reports)Citation2 and LASIK is a recognized risk factor for the development of dry eye.Citation1,Citation3–Citation5 Moreover, LASIK surgery can cause changes in the corneal environment, such as decreased corneal sensation,Citation2,Citation6 decreased tear secretion and quality, decreased corneal epithelial integrity, and reduced conjunctival goblet cell density.Citation7 Tear film stability may be adversely affected, resulting in dry eye symptoms in the 6 months following surgery.Citation1,Citation4,Citation6,Citation7 In most patients, dry eye following LASIK surgery is disturbing but temporary, although some patients continue to suffer symptoms.
First-line treatment for post-LASIK dry eye includes the same therapies as for dry eye of other etiologies, ie, artificial tears (preferably preservative-free where available), punctal occlusion, warm compresses, lid massage, and scrubs where meibomian gland dysfunction is a feature and treatment of inflammation with short courses of topical corticosteroids or cyclosporine A.
Hyaluronate is a naturally occurring glycosaminoglycan polysaccharide found in skin, connective tissue, the joints, and several components of the eye. Biological roles for hyaluronate appear to include lubricant and water-retaining functions, and it is present in human tear fluid.Citation8 It is widely employed in medicine in cataract surgery, dry eye treatment, lubrication of the joints in rheumatology, and in a wide range of skin preparations and antiaging creams.Citation9 Hyaluronate has physicochemical properties that make it a valuable component of tear replacement therapies for dry eye, including useful viscoelastic properties and mucoadhesivity. It also has a powerful ability to retain water and can heal corneal wounds.Citation10–Citation14 The efficacy of hyaluronate in the treatment of dry eye of various etiologies is well established.Citation15–Citation21
Hylabak® (Laboratoires Thea, Clermont Ferrand, France) is a preparation containing 0.15% hyaluronate presented in a preservative-free multidose dispenser system.Citation22 Until recently, preservatives (usually benzalkonium) have been required to limit microbial degradation of ophthalmic preparations. However, convincing evidence has accumulated to show that such preservatives have detrimental effects on the ocular surface.Citation23 In particular, tear film stability, goblet cell function, apoptosis of conjunctival cells, and oxidative and inflammatory processes are adversely affected.Citation23 Such changes are particularly unwelcome in patients with dry eye, and the availability of topical treatments without preservative is an important milestone in the treatment of the condition.Citation24 In addition to in vitro and in vivo evidence, clinical studies have confirmed the detrimental effects of preservatives in ocular preparations.Citation25–Citation27 Guidelines now recommend the elimination of preservative from topical dry eye products.Citation24
Systane® (Alcon Laboratories, Fort Worth, TX, USA) lubricant eye drops comprise polyethylene glycol 400 (0.4%) and propylene glycol (0.3%) demulcents, with hydroxypropyl guar polymer (0.4%) as a gelling agent and polidronium chloride (0.001%) as a preservative. An important difference between Systane and Hylabak is the presence of preservative in Systane; this may be of more importance in clinical practice than the different polymers used in the respective products.Citation24 A randomized, investigator-masked, prospective study showed this preparation to be effective in relieving symptoms of dry eye in 30 patients undergoing LASIK surgery.Citation28
The objective of the present study was to assess signs and symptoms of dry eye after LASIK in patients treated with a preservative-free lacrimal substitute (Hylabak) compared with those treated with a preserved lacrimal substitute (Systane) after 3 months. Ocular tolerability of the products was also compared between the groups.
Materials and methods
The study was conceived as a randomized, single-center, investigator-masked, prospective, noninferiority, parallel-group clinical study of 3 months’ duration. Patients undergoing bilateral LASIK surgery were randomized to receive 0.15% sodium hyaluronate preservative-free eye drops (Hylabak, in the ABAK® [Laboratoires Thea] dispenser system) or reference eye drops comprising polyethylene glycol 400, propylene glycol, and 0.001% polidronium chloride as the preservative (Systane). Each treatment was administered as one drop in each eye four times daily for 3 months. Both eyes were evaluated and treated with the same type of eye drop.
The study was conducted in compliance with Good Clinical Practice according to International Conference on Harmonisation guidelines, European directive 2001/20/CE, and the Declaration of Helsinki (2004). Ethical approval was granted by the ethics committee at the State Educational Establishment of Higher Professional Education, Saint Petersburg State Medical University and of the Federal Service for Surveillance of Healthcare and Social Development of the Russian Federation.
Study design
Treatments were prepared according to a randomization code list, and were allocated to patients in predetermined order. Qualifying patients were assigned a specific treatment number allocated in ascending order. Differences in the dispensing form of the two treatments rendered masking treatment from the patients impractical; consequently treatment allocation was masked only from the investigators. Compliance was assessed by direct questioning.
Study visits
Inclusion (day −7)
Patient inclusion was assessed 7 ± 1 days prior to LASIK surgery and the commencement of study treatment.
Surgery (day 0)
Patients who fulfilled all inclusion and exclusion criteria were randomly assigned to receive the test or reference product in their left and right eyes. Treatment was commenced on the day of surgery.
Follow-up visits
Patients were followed up the day after surgery and then at 28 and 84 days after surgery (±3 days). At each visit, the following parameters were assessed:
patients’ sensation of ocular surface dryness (burning, stinging, feeling of dryness, sandy and/or gritty sensation, light sensitivity)
global tolerance (patient assessment)
global efficacy and tolerance (investigator)
best corrected visual acuity
slit-lamp examination
fluorescein test
tear break-up time
lid-parallel conjunctival folds (LIPCOF) testCitation29
adverse events
compliance assessment.
A slit-lamp examination was also performed at the inclusion visit (day −7) and prior to surgery on day 0.
Patients
Patients aged 20–55 years and scheduled to undergo surgery to correct myopia, astigmatism, hyperopia, or a combination thereof, who had stable glasses or contact lens prescriptions for at least 2 years and who had sufficient corneal thickness (≥480 μm) were eligible for inclusion. Exclusion criteria were herpetic keratitis, glaucoma, cataract, anomalies related to eye lids or eye lid malformation, blepharitis, allergies, corneal dystrophy, history of ocular surgery, procedures such as LASIK, laser epithelial keratomileusis, or photorefractive keratectomy, moderate or serious dry eye, red eyes, keratoconus, any contraindication to LASIK surgery, known hypersensitivity to any component of the study medication, pregnancy, and premenopausal with no reliable method of birth control.
Further grounds for exclusion were any medical or surgical history, disorder, or severe acute or chronic organic disease (eg, hepatic, endocrine, neoplastic, hematologic, immunosuppressive, infectious, severe psychiatric, or relevant cardiovascular abnormality), and/or any complicating or structural factor judged by the investigator to be incompatible with the study. Noncompliant patients, those unwilling or unable to give informed consent, those involved in a clinical trial in the previous 3 months, and those who were wards of the court were also excluded. Patients were similarly excluded if they had undergone ocular surgery during the previous 12 months, as were those who had received systemic nonsteroidal anti-inflammatory drugs, immunosuppressants, corticoids, or any medication that could interfere with lacrimal function during the 3 weeks prior to the study.
No medications were permitted during the study period with the exception of drugs required in the surgical protocol (paracetamol, tobramycin, povidone-iodine, oxybuprocaine, and dexamethasone-neomycin-polymyxin B at approved doses).
Efficacy evaluation
The primary efficacy variable was reduction in mean score on the fluorescein test, evaluated using the Oxford scale,Citation30 from day 1 to day 84 ± 3 in the worst eye in the per protocol population. A noninferiority test at the alpha level of 5% was applied to test the difference in mean change in score between the treatment groups, the null hypothesis being that 0.15% hyaluronate eye drops was more than 10% inferior to Systane eye drops.
The secondary efficacy parameters were:
ophthalmologic (including slit-lamp) examination in both eyes for meibomitis or other blepharitis (evaluated on a 0–3 scale)
ocular symptoms, including sensation of dryness, burning or stinging sensations, sandy or gritty sensations, and photosensitivity were evaluated by the patient on a 0–3 scale
LIPCOF test (degree of severity of dryness syndrome was scored on a scale of 0 [no parallel fold] to 3 [several parallel folds] with height >0.2 mm)
best corrected visual acuity assessed using a Snellen chart
global efficacy assessed by the investigator on a 0–3 scale
tear film break-up time.
Assessment of safety
Safety was evaluated by global tolerance as assessed by the investigator (on a 0–3 scale) and patient questioning (“Are the eye drops comfortable?”, answered as “yes” or “no”). Ocular adverse events were recorded at all visits except on the recruitment visit (day −7). All adverse events regardless of severity that occurred between recruitment and completion were recorded, including at least a description of onset, duration, intensity, treatment required, outcome relationship to study medication, and narrative from the investigator.
Compliance and concomitant medication
Patients were questioned regarding compliance and concomitant medication at each visit after commencement of the study medication.
Statistical methods
A sample size of 42 subjects per arm was calculated to be necessary to provide an 80% probability of detecting a difference of 1 unit in mean score between the groups where the standard deviation of the scores was 2 units, the noninferiority margin was 10%, and the probability of type I error was 5%. Because each eye was to be evaluated individually and to allow for loss to follow-up, a target recruitment of 60 subjects was defined (60 eyes per arm). Noninferiority for the primary efficacy variable was considered established if the lower boundary of the 95% confidence interval (CI) for the difference in means between the two treatment groups calculated at day 84 was greater than −10%. Secondary efficacy variables were presented using descriptive statistics. Continuous efficacy variables were analyzed using the unpaired Student’s t-test and categorical efficacy variables using the chi-square test.
Results
Patient disposition and demographics
Fifty-four patients were screened and enrolled in the study, all of whom were randomized. Two patients were lost from the Systane group (one for a nonmedical reason and the other was lost to follow-up). Thus, the per protocol population comprised 27 patients in the Hylabak group and 25 patients in the Systane group (). There were no protocol deviations and no withdrawals due to adverse events or lack of efficacy. Patient demographics and baseline characteristics are shown in . There were no statistically significant differences between the groups with regard to demographic parameters or baseline characteristics. There was a preponderance of females in both treatment groups.
Figure 1 Patient disposition.
Abbreviations: ITT, intention to treat; PP, per protocol.
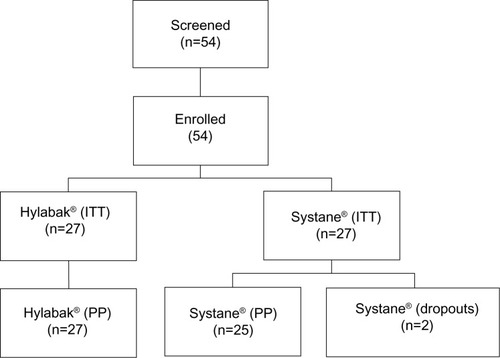
Table 1 Patient demographics and baseline characteristics
Primary efficacy variable
Fluorescein test scores were similar on day 1 (mean 0.26, 95% CI 0.11–0.41 and mean 0.28, 95% CI 0.12–0.44 for Hylabak and Systane, respectively). At the final visit (day 84 ± 3) the fluorescein scores had improved to 0.11 (95% CI 0.01–0.22) and 0.04 (95% CI −0.03 to 0.11), respectively. The improvement in fluorescein test score between baseline and day 84 ± 3 was statistically significant for Systane (P = 0.0308) but not for Hylabak; the difference between the two groups was not statistically significant, and noninferiority was achieved for Hylabak. The primary efficacy results are shown in and presented in full in .
Figure 2 Fluorescein test (Oxford scheme) score in the worst eye (per protocol sample).
Abbreviation: CI, confidence interval.
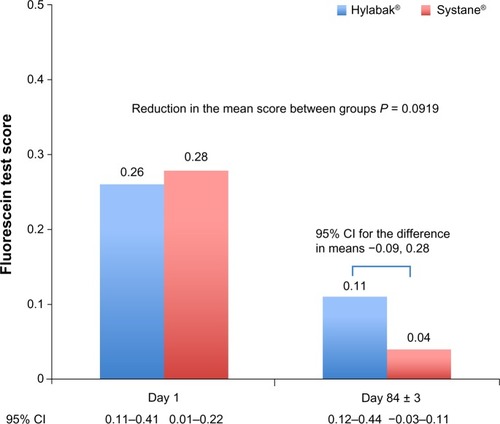
Table 2 Fluorescein test (Oxford scheme) score in the worst eye for per protocol population
The majority of the subjects in both groups were grade 0 at baseline (74.07% in the Hylabak group versus 72% in the Systane group). There was an improvement in both groups at day 84 (88.89% in the Hylabak group versus 96% in the Systane group). However, there was a trend (P = 0.0571) for a more rapid improvement in the Hylabak group than in the Systane group (reduction in the number of patients with grade 1 from 25.93% at baseline to 7.41% at day 28 in the Hylabak group versus a reduction from 25.93% to 14.81% in the Systane group).
Secondary efficacy variables
Ophthalmologic/slit-lamp examination
No subject in either group had palpebral signs (meibomitis), conjunctival discharge, chemosis, folliculopapillary conjunctivitis, filamentary keratitis, or conjunctival hyperemia at screening or during the study. Flap edema was present at baseline in two (7.41%) subjects (one with mild severity and the other with moderate severity) in the Hylabak group and in one (4%) subject (with mild severity) in the Systane group. No subjects had flap edema at the day 84 examination. One (3.7%) subject in the Hylabak group had mild but clinically nonsignificant flap folds at the day 84 examination.
Ocular symptoms
Improvement in ocular symptoms was observed in both groups during the study. In the Hylabak group, no subjects reported burning or stinging sensation. A sensation of dryness (3.7%), sandy and/or gritty feeling (11.11%), and photosensitivity (7.41%, score 1, not disturbing) were reported by very few subjects at baseline but not by any subject at days 28 and 84. In the Systane group, none of the subjects experienced stinging sensations. Similarly, burning sensation (8%), sandy and/or gritty sensation (12%), and photosensitivity (4%, score 1, not disturbing) were reported by very few subjects at baseline and none at days 28 and 84. A sensation of dryness was reported by two (8%) subjects at day 28 but by none at day 84.
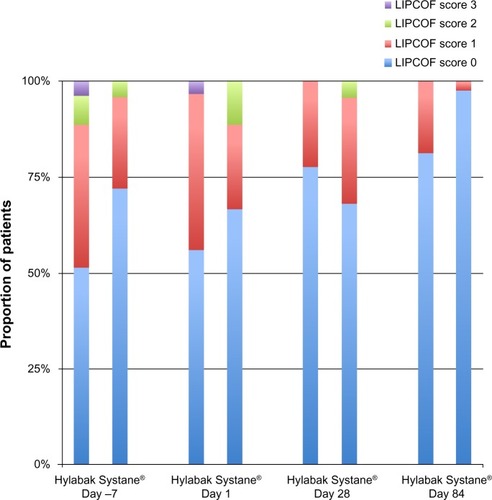
LIPCOF test
Improvement in the severity of dry eye syndrome was observed in both groups during the study (). In the Hylabak group, most of the subjects had either no parallel fold (55.56%) or one parallel fold (40.74%) at baseline. Improvement was seen at day 84, and 81.48% of subjects had no parallel fold; one subject in this group had severe parallel fold (score 3) at baseline and none had severe parallel fold at day 84. Similarly, in the Systane group at baseline, most of the subjects had either no parallel fold (64%) or one parallel fold (24%). Improvement was similarly observed at day 84, with most of the subjects (88%) having no parallel fold. None of the subjects in this group had severe parallel fold. The difference in LIPCOF test scores between the groups was not statistically significant (P > 0.05).
Visual acuity
A majority of patients in each group had a best corrected visual acuity score of 6/6 in the worst eye at baseline (59.26% in the Hylabak group versus 64% in the Systane group). The proportion of subjects with a score of 6/6 in the worst eye improved from baseline in both groups; at day 84, 81.48% in the Hylabak group and 84% in the Systane group had a score of 6/6 in the worst eye. The difference between the treatment groups was not statistically significant (P > 0.05).
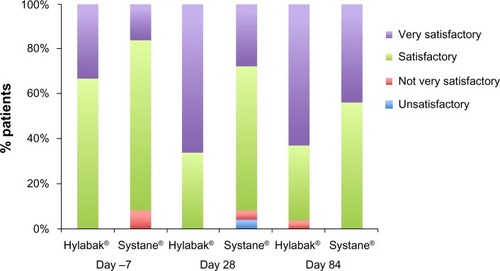
Global efficacy
The investigators rated a higher percentage of subjects in the Hylabak group to be “very satisfactory” than in the Systane group at day 1 through day 84 (). One (3.7%) subject was reported as “not very satisfactory” at day 84 in the Hylabak group; two (8%) subjects on day 1 and one (4%) subject on day 28 were reported as “not very satisfactory” in the Systane group. Efficacy was assessed as “unsatisfactory” in only one (4%) subject at day 28 in the Systane group. Based on investigator rating, Hylabak was statistically superior to Systane on day 28 (P = 0.0113) but not on day 84 (P = 0.162).
Tear film break-up time
Tear film break-up time had improved significantly from baseline (P < 0.0001) on day 84 in both groups. The difference between the groups in this regard was not statistically significant (P = 0.5619).
Safety
Fifty-two of the 54 enrolled subjects completed the study. There were no serious adverse events, no subject discontinued for adverse events or other safety-related reasons, and no adverse systemic events were reported. The safety population comprised all 54 randomized subjects. Overall, the study treatments were well tolerated, with only three adverse events (two of mild and one of moderate intensity) reported. All three adverse events were of corneal edema in both eyes. These comprised two adverse events (one mild and one moderate) in the Hylabak group and one adverse event (mild) in the Systane group. All three were assessed as unrelated to the study medication and were considered causally related to LASIK surgery. No systemic adverse events, serious adverse events, or deaths were reported during the course of the study. There were no discontinuations or withdrawals due to treatment-emergent adverse events. Both treatments were well tolerated by the study subjects. With the exception of one (3.7%) subject in the Hylabak group, all subjects reported the study treatments to be comfortable. The investigator rated tolerability as “not very satisfactory” for this subject. According to investigator assessment, a greater number of subjects scored “very satisfactory” in the Hylabak group than in the Systane group (62.96% each at day 28 and day 84, respectively, in the Hylabak group versus 37.04% each on day 28 and day 84, respectively, in the Systane group).
Discussion
The primary objective of the present study was to compare the effect of Hylabak and Systane eye drops on corneal fluorescein staining. The study indicates that Hylabak eye drops are not inferior to Systane eye drops and their overall efficacy appears to be similar, although there seem to be differences between treatments in terms of timing of the therapeutic effect.
Both groups improved during the study; 74.07% and 72% of Hylabak patients and Systane patients, respectively, had grade 0 dry eye symptoms at baseline, a proportion that increased to 88.89% and 96%, respectively, by study completion. The reduction in fluorescein score from baseline to study end was statistically significant for Systane and the difference between the Hylabak and Systane groups was not. However, improvement in fluorescein staining seemed to be more rapid in the Hylabak group than in the Systane group. This trend toward a more rapid improvement in the Hylabak group compared with the Systane group was supported by other parameters. LIPCOF score and global efficacy assessment by the investigator were both reduced more at day 28 in the Hylabak group than in the Systane group.
Clinical ocular signs were essentially absent at baseline and at study end. Although ocular symptoms of dry eye (sensation of dryness, sandy or gritty sensation, and photosensitivity) were relatively infrequent at baseline, the symptoms were reduced in both groups by the end of the study.
The number of patients with an LIPCOF test score of 0 at study end was the same in Hylabak-treated and Systane-treated patients, but the scores in Hylabak-treated patients decreased more rapidly than in Systane-treated patients (percentage of patients with LIPCOF scores of 0 were 77.78% and 68%, respectively). However, the differences were small and did not reach statistical significance. Vision correction following laser surgery was similar in the two groups, as was tear film break-up time.
Global efficacy as assessed by the investigator favored Hylabak at day 28; 66.67% of Hylabak-treated patients had a rating of “very satisfactory” compared with only 28% of Systane-treated patients, a difference that was statistically significant. Similarly, at day 84, 62.96% of Hylabak-treated patients had the highest rating for efficacy compared with 44% of Systane-treated patients, although this difference did not achieve statistical significance.
Overall, the efficacy of the two treatments appears to be similar, and so far as the primary efficacy parameter is concerned, Hylabak is shown to be noninferior to Systane. There are some suggestions that Hylabak may act more rapidly than Systane, but more highly powered studies will be required to characterize the relative efficacy of the two preparations. Both treatments were well tolerated in this post-LASIK surgery population, even on the day after surgery. A number of topical treatments are used postoperatively in LASIK surgery patients (including antibiotics, anti-inflammatory drugs, and treatments for dry eye), and many of these formulations contain preservative. Several studies have demonstrated the cytotoxicity of preservatives,Citation23 that increases with the duration of exposure, as well as the high prevalence of ocular disorders in patients treated with preserved eye drops.Citation25 Since preservative-free products are now recommended for dry eye,Citation24 the case for using them in the healing postoperative eye seems compelling.
In conclusion, preservative-free Hylabak can be considered a welcome addition to the clinical armamentarium in dry eye for patients having recently undergone LASIK surgery and has been shown to be noninferior to Systane in this population. A preservative-free formulation is preferred for the treatment of dry eye, particularly in LASIK patients.
Acknowledgments
Editorial support was provided by Dr JF Stolz who was reimbursed by Laboratoires Thea.
Disclosure
The authors report no conflicts of interest in this work.
References
- TodaISano-KatoNKomai-HoriYTsubotaKDry eye after laser in situ keratomileusisAm J Ophthalmol200113211711438046
- ShteinRMPost-LASIK dry eyeExpert Rev Ophthalmol20116557558222174730
- De PaivaCSChenZKochDDThe incidence and risk factors for developing dry eye after myopic LASIKAm J Ophthalmol2006141343844516490488
- MurakamiYMancheEEProspective, randomized comparison of self-reported postoperative dry eye and visual fluctuation in LASIK and photorefractive keratectomyOphthalmology2012119112220222422892151
- [No authors listed]The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf2007529310717508117
- Perez-SantonjaJJSaklaHFCardonaCChipontEAlioJLCorneal sensitivity after photorefractive keratectomy and laser in situ keratomileusis for low myopiaAm J Ophthalmol1999127549750410334340
- AlbietzJMLentonLMManagement of the ocular surface and tear film before, during, and after laser in situ keratomileusisJ Refract Surg2004201627114763473
- FrescuraMBerryMCorfieldACarringtonSEastyDLEvidence of hyaluronan in human tears and secretions of conjunctival culturesBiochem Soc Trans199422228S7958290
- RahMJA review of hyaluronan and its ophthalmic applicationsOptometry2011821384321146792
- SnibsonGRGreavesJLSoperNDPrecorneal residence times of sodium hyaluronate solutions studied by quantitative gamma scintigraphyEye (Lond)19904Pt 45946022226990
- SnibsonGRGreavesJLSoperNDTiffanyJMWilsonCGBronAJOcular surface residence times of artificial tear solutionsCornea19921142882931424647
- NakamuraMHikidaMNakanoTItoSHamanoTKinoshitaSCharacterization of water retentive properties of hyaluronanCornea19931254334368306665
- CamillieriGBucoloCRossiSDragoFHyaluronan-induced stimulation of corneal wound healing is a pure pharmacological effectJ Ocul Pharmacol Ther200420654855315684813
- GomesJAAmankwahRPowell-RichardsADuaHSSodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitroBr J Ophthalmol200488682182515148219
- DeLuiseVPPetersonWSThe use of topical Healon tears in the management of refractory dry-eye syndromeAnn Ophthalmol19941698238246508097
- NelsonJDFarrisRLSodium hyaluronate and polyvinyl alcohol artificial tear preparations. A comparison in patients with keratoconjunctivitis siccaArch Ophthalmol198810644844872451494
- ScrivantiMTaitiLMencucciRBardiLSalviGSyndrome sec oculaire: utilisation d’un nouveau substitut lacrymal (LO2A) [Dry Eye Syndrome; use of a new tear substitute (LO2A)]Ophtalmologie19961012427 French
- CondonPIMcEwenCGWrightMMackintoshGPrescottRJMcDonaldCDouble blind, randomised, placebo controlled, crossover, multicentre study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndromeBr J Ophthalmol199983101121112410502570
- AragonaPPapaVMicaliASantoconoMMilazzoGLong term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eyeBr J Ophthalmol200286218118411815344
- LaflammeMYSwiecaRA comparative study of two preservative-free tear substitutes in the management of severe dry eyeCan J Ophthalmol19882341741763395921
- AragonaPDi StefanoGFerreriFSpinellaRStiloASodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjogren’s syndrome patientsBr J Ophthalmol200286887988412140209
- López-GarcíaJSGarcía-LozanoIUse of containers with sterilizing filter in autologous serum eyedropsOphthalmology2012119112225223022867978
- BaudouinCLabbéALiangHPaulyABrignole-BaudouinFPreservatives in eyedrops: the good, the bad and the uglyProg Retin Eye Res201029431233420302969
- [No authors listed]Report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf20075216317817508120
- JaenenNBaudouinCPouliquenPManniGFigueiredoAZeyenTOcular symptoms and signs with preserved and preservative-free glaucoma medicationsEur J Ophthalmol200717334134917534814
- BronAChiambarettaFPouliquenPRigalDRoulandJFEfficacy and safety of substituting a twice-daily regimen of timolol with a single daily instillation of nonpreserved beta-blocker in patients with chronic glaucoma or ocular hypertensionJ Fr Ophtalmol2003267668674 French13130253
- BedenCHelleboidLMarmouzFLiardFA comparative study of the ocular tolerance after administration of anti-allergic eye drops with or without a preservativeTherapie2004592259264 French15359624
- DurrieDStahlJA randomized clinical evaluation of the safety of Systane lubricant eye drops for the relief of dry eye symptoms following LASIK refractive surgeryClin Ophthalmol20082497397919668456
- HöhHSchirraFKieneckerCRuprechtKWLid-parallel conjunctival folds are a sure diagnostic sign of dry eyeOphthalmologe1995926802808 German8563428
- BronAJEvansVESmithJAGrading of corneal and conjunctival staining in the context of other dry eye testsCornea200322764065014508260