Abstract
Purpose
To investigate the relation between the quantitative iris parameters and iridotrabecular contact (ITC) in patients with primary angle-closure (PAC) and PAC glaucoma (PACG).
Materials and methods
PAC and PACG with laser peripheral iridotomy were recruited prospectively. Anterior-segment optical coherence tomography (ASOCT) was performed under light and dark conditions, and scans were taken along the vertical and horizontal axes. Iris thickness at 500 μm (IT500) and 750 μm (IT750) from the scleral spur, maximal iris thickness (MIT), and cross sections of the iris area (I-Area) were measured by using software. ITC was defined by the ASOCT as the contact between the peripheral iris and angle wall anterior to the scleral spur. The ITC+ and ITC− groups were defined as eyes that had ITC in two or more quadrants and in no or one quadrant, respectively.
Results
A total of 79 eyes of 60 patients (consisting of 48 PAC and 31 PACG) were recruited. The prevalence of superior, inferior, temporal, and nasal ITC was 44 eyes (55.7%), 48 eyes (60.8%), 18 eyes (22.8%), and 16 eyes (20.2%), respectively. These iris parameters of the inferior quadrant, which had the highest prevalence of all the quadrants, were used for the analysis. After adjusting for age, sex, pupil size, and central anterior chamber depth, mean values of IT500 and IT750 were significantly greater in the ITC+ group than the ITC− group (P<0.05). Multivariate-adjusted odds ratios of parameters for the ITC+ group compared with the ITC− group were: IT500, 1.9 (P=0.029); IT750, 2.0 (P=0.011), MIT, 1.4 (P=0.244), and I-Area, 0.97 (P=0.406), respectively, per 0.1-unit increase.
Conclusion
Peripheral iris thickness is associated with ITC in patients with angle closure.
Introduction
Primary angle-closure glaucoma (PACG) is a major cause of visual morbidity in East Asia.Citation1,Citation2 Laser peripheral iridotomy (LPI), which is first-line treatment for PACG, acts by relieving pupil block.Citation3 There have been some reports that LPI results in a significant increase in the angle width in eyes with narrow angles.Citation4–Citation6 However, despite the presence of a patent LPI, most eyes with established PACG require further treatment to control intraocular pressure (IOP), and surgical therapy is required to lower IOP in 62.9% of these eyes.Citation4,Citation7
Several biometric risk factors, including shallow anterior chamber depth (ACD), short axial length, anterior lens position, and small corneal diameter, have been related to the development of primary angle-closure (PAC).Citation8,Citation9 Among these risk factors, shallow ACD is regarded as a cardinal risk factor, but less attention has been focused on the iris. A recent study from Singapore found that quantitative iris parameters, such as iris curvature, cross-sectional area, and thickness, were independently associated with narrow angles in a community-based sample of participants.Citation10 However, the characteristics of the iris have not been extensively studied as potential risk factors for angle closure. Appositional angle closure is reversible nonpermanent angle closure that is thought potentially to increase the risk of developing peripheral anterior synechia (PAS).Citation11 Appositional angle closure is thought to play an important role in angle-closure pathogenesis.Citation12,Citation13
With anterior-segment optical coherence tomography (ASOCT), it is possible to capture a single image of the entire anterior segment and assess iris parameters more precisely. There is a report that ASOCT is highly sensitive in detecting iridotrabecular contact (ITC) between the peripheral iris and angle wall anterior to the scleral spur in patients with angle closure when compared with gonioscopy.Citation14
The aim of this study was to investigate the relation between the quantitative iris parameters and ITC in patients with PAC and PACG.
Materials and methods
This was a prospective cross-sectional study of Japanese subjects with PAC and PACG recruited from Mizoguchi Eye Clinic in Nagasaki Prefecture, Japan. Written informed consent was obtained from each subject. The study had the approval of the Institutional Review Board of the Mizoguchi Eye Clinic, and the study adhered to the Declaration of Helsinki.
This cross-sectional study included subjects aged ≥50 years who were recruited between September 2010 and March 2012. PAC was defined as eyes in which the posterior trabecular meshwork was not seen for at least 270° on indentation gonioscopy in the primary position with PAS and/or raised IOP (defined as an IOP >21 mmHg), but without glaucomatous optic neuropathy or visual field loss. PACG was defined as eyes with PAC associated with glaucomatous optic neuropathy (defined as loss of neuroretinal rim, a vertical cup:disk ratio of 0.7, or an intereye asymmetry of 0.2, and/or notching attributable to glaucoma). Patients diagnosed with secondary angle closure (such as neovascular glaucoma or uveitic glaucoma), patients who had corneal abnormalities that would affect imaging, prior history, or evidence of acute PAC in the study eye, and previous laser iridoplasty or history of intraocular surgery, and patients on miotic therapy were excluded. All subjects with angle closure had previously undergone LPI.
All subjects underwent a standardized eye examination that included visual acuity measurement, slit-lamp examination (BQ 900; Haag-Streit, Bern, Switzerland), stereoscopic optic disk examination with a 78-diopter lens (Volk Optical, Mentor, OH, USA), IOP measurement by Goldmann applanation tonometry (Haag-Streit) and gonioscopy, performed in the dark using a Goldmann two-mirror lens at magnification (×16). Indentation gonioscopy with a Sussman four-mirror lens was used to establish the presence or absence of PAS. A-scan biometry (US-800; Nidek, Gamagori, Japan) was used to measure axial length (AL), central ACD, and lens thickness (LT).
Anterior-segment optical coherence tomography
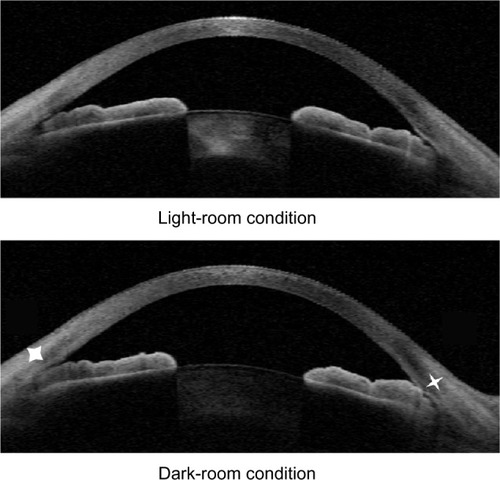
All subjects underwent imaging with ASOCT (Visante; Carl Zeiss Meditec, Jena, Germany). Imaging was performed first in the dark (0.2 lux) and then under a bright condition using a measured standardized light source. Scans were centered on the pupil and taken along the horizontal axis (nasal temporal angle at 0°–180°) and vertical axis (12 o’clock–6 o’clock at 90°–270°) using the enhanced anterior segment single protocol. To obtain the best-quality image, the examiner adjusted the saturation and noise and optimized the polarization for each scan during the examination. As several scans were acquired by the ASOCT device, the examiner chose the best images, with no motion artifacts or image artifacts due to the eyelids. The points of intersection at the anterior surface of the iris, which were defined as the point at the anterior iris surface on a line perpendicular to the trabecular meshwork of 500 microns and 750 microns, respectively, from the scleral spur, were identified. The shortest distance from these points to the posterior surface of the iris was calculated as IT500 and IT750, respectively. Maximal iris thickness (MIT) was the highest value of iris thickness along the entire iris (). Lens vault (LV) was defined as the perpendicular distance between the anterior pole of the lens and a horizontal line joining the two scleral spurs. Iris area (I-Area) was calculated as the cumulative cross-sectional area of the full length of the iris. The image-processing software WinROOF (Mitani, Fukui, Japan) was used to analyze iris images. Corneal thickness (CT) was measured by using ASOCT. All images which could not be identified as the scleral spur were excluded for this analysis. Images obtained under the dark condition were used for analysis.
Definition/diagnostic criteria
The examination was performed in a dark room below 0.2 lux and a light room. The ITC was defined as the contact on an ASOCT image if contact was visible between the peripheral iris and any part of the angle wall anterior to the scleral spur from the light-room condition to the dark-room condition (). An eye was defined as ITC+ on ASOCT if two or more of the temporal, superior, nasal, and inferior quadrants were found to have ITC. An eye was defined as ITC− if no or one quadrant was found to have ITC.
Statistical analysis
Statistical analysis was performed using the statistical package SAS version 8 (SAS Institute, Cary, NC, USA). Differences in mean values of parametric data among eyes of different subjects were examined using the independent-sample Student t-test. Multivariate-adjusted odds ratios were obtained after adjustment for age, sex, pupil size, and ACD. P-values less than 0.05 were considered statistically significant.
Results
Seventy-nine eyes of 60 patients (consisting of 48 PAC eyes and 31 PACG eyes) aged 73.2±7.6 years (mean ± standard deviation) (range 52–86 years) were included in this study. The mean spherical error was +0.6±1.6 diopters (range −4.8 to +5.5), and the mean IOP was 15.8±3.5 mmHg. The prevalence of ITC superiorly, inferiorly, temporally, and nasally was 44 eyes (55.7%), 48 eyes (60.8%), 18 eyes (22.8%), and 16 eyes (20.2%), respectively (). On quadrant-wise analysis, 20 eyes (25.3%) were found to have ITC in two quadrants, ten eyes (12.7%) in three quadrants, and three eyes (3.8%) in all quadrants. In 28 of 79 eyes (35.4%), ITC was not found in any quadrant (). ITC was found in more than two quadrants in 33 eyes (41.8%). ITC was found in no or one quadrant in 28 eyes (35.4%) and 18 eyes (22.8%), respectively. The ITC+ group consisted of 19 (39.6%) of 48 PAC eyes and 14 (45.2%) of 31 PACG eyes. There were no significant differences between the two groups in age (P=0.68), sex (P=0.61), IOP (P=0.11), ACD (P=0.51), LT (P=0.44), AL (P=0.23), LV (P=0.49), pupil size in the dark (P=0.06), refractive error (P=0.92), or CT (P=0.18) ().
Table 1 Detection of iridotrabecular contact in each quadrant by anterior-segment optical coherence tomography
Table 2 Detection of eyes with iridotrabecular contact by anterior-segment optical coherence tomography
Table 3 Comparison of iridotrabecular contact (ITC)+ group and ITC− group
The iris parameters of the inferior quadrant, which had the highest prevalence of all the quadrants, were used for the analysis. After adjusting for age, sex, pupil size, and ACD, mean values of IT500 (0.317 vs 0.275 mm, P<0.05) and IT750 (0.372 vs 0.321 mm, P<0.05) under the dark condition were significantly greater in the ITC+ group than the ITC− group, while MIT (0.521 vs 0.486 mm, P=0.19) and I-Area (1.876 vs 1.933 mm2, P=0.73) under the dark condition were not statistically significant between the two groups (). All of the points of MIT at the anterior surface of the iris under the dark condition were located farther than that of IT500 and IT750 from the peripheral iris root.
Table 4 Comparison of iris parameters in iridotrabecular contact (ITC)+ group and ITC− group
After adjusting for age, sex, pupil size, and ACD, multivariate-adjusted odds ratios of each parameter (95% confidence interval and P-value) under the dark condition for the ITC+ group compared with the ITC− group were: IT500, 1.9 (1.0–3.3, P=0.029); IT750, 2.0 (1.1–3.6, P=0.011), MIT, 1.4 (0.8–2.5, P=0.244), and I-Area, 0.97, (0.8–1.2, P=0.406) per 0.1-unit increase ().
Table 5 Relationship of iris parameters and risk of iridotrabecular contact
Discussion
Nolan et alCitation14 reported sensitivity and specificity values of ASOCT for the diagnosis of appositional angle closure of 98% and 55.3%, respectively. On the contrary, the sensitivity and specificity values of gonioscopy were 68.3% and 96.6%, respectively. Thus, the sensitivity of gonioscopy was considerably lower. The authors stated that this was caused by differences in the effect of lighting conditions during the measurement and the distortion of the anterior segment by gonioscopy. We have found that the closed angle opens on ASOCT imaging when the room lights are switched on. This suggests that it is the difference in illumination that may be the most important factor in the discrepancy between gonioscopy and ASOCT findings. In addition, there was a reportCitation14 that when performed under dark conditions, ASOCT identified 98% of subjects found to have angle closure on gonioscopy.
It has been reported that the prevalence of appositional angle closure is high at the superior and inferior quadrants and low at the nasal and temporal quadrants in PAC-suspect patients who have not undergone LPI.Citation13,Citation15 According to Bhargava et al,Citation11 appositional angle closure is temporary contact, but if this occurs repeatedly, persistent PAS develops from upward to downward. Studies have shown that LPI is effective for widening the angle and lowering the IOP.Citation4,Citation16 Furthermore, Wilensky et alCitation17 reported that appositional angle closure is the most dangerous risk factor for exacerbation of angle closure, and that LPI is effective in preventing the developing of PACG. However, in this study, superior and inferior quadrants showed ITC in as high as approximately 60% of PAC and PACG patients who underwent LPI. In addition, as high as 41.8% of the patients showed ITC in two or more quadrants, indicating that the elimination of pupillary block alone by LPI is not sufficient to reduce the prevalence of ITC. This was also revealed in the report of Ang et al,Citation18 in which 23.9% of patients showed appositional angle closure in two or more quadrants even after LPI, although the prevalence in their study was lower than that in the present study. Moreover, it was reported that such residual appositional angle closure was seen more often in patients who were found to have narrower angles and extensive appositional angle closure prior to the laser treatment. Thus, LPI cannot altogether eliminate appositional angle closure. Therefore, the presence of appositional angle closure after LPI is considered to be a risk factor for developing PACG and an important clinical finding in the management of PAC and PACG.
Alsagoff et alCitation7 reported that most eyes with established PACG require further treatment to control IOP, despite the presence of patent LPI, and 22 eyes (45.8%) eventually underwent filtering surgeries. In addition, Sihota et alCitation19 reported that 36% of PAC patients treated by LPI deteriorated during follow-up. In these studies, the relationship between the progression of glaucoma and appositional angle closure was not investigated; however, the prevalence of residual appositional angle closure roughly matched the progression rate of glaucoma after LPI in this study, and it can be speculated that appositional angle closure is one of the risk factors for exacerbation of PACG.
Shallow central ACD, short AL, and high LT are thought to be risk factors for angle closure.Citation8,Citation9 In the present study, there was no significant correlation between the presence/absence of ITC and these risk factors in patients in whom the pupillary block was relieved by LPI. In recent years, there have been many reports on iris parameters measured by ASOCT in patients with PAC and PACG. Wang et alCitation20 reported the existence of significant differences in IT750, IT2000, and MIT between PAC/PACG and normal eyes. They considered that the thicker peripheral iris is likely to contribute to angle closure, as the peripheral iris would be in closer proximity to the angle. In the present study, significant differences were observed at IT500 and IT750, ie, peripheral iris, although not at the MIT, ie, near the central iris, between the two groups. In addition, there was no significant difference in I-Area. It is considered that this is because the I-Area is the area of the whole iris in the images obtained by ASOCT, but is not a value indicating the peripheral iris configuration. This result shows that peripheral iris thickness is significantly associated with ITC, which is the most important risk factor for developing angle closure. Furthermore, it has been reported from a study of the dynamic pattern of iris configuration in narrow-angle eyes without LPI that changes in the pattern of iris bowing in light and dark rooms also affect the width of the angle.Citation21 Thus, light-induced changes in the dynamic pattern of iris configuration also affect the configuration of the angle, and it is considered that multiple factors, including the dynamic pattern of iris configuration, should be investigated in the future.
One of the limitations of this study was the cross-sectional nature of the study. Second, accommodation, which is thought to affect the configuration of the anterior segment, was not evaluated. Third, the ciliary body was not imaged by ultrasound biomicroscopy, and we did not assess iris insertion.
In summary, in this study, approximately 40% of patients had ITC in two or more quadrants even after LPI, and peripheral iris thickness was considered to be important for angle-closure pathogenesis. To arrive at a definitive conclusion, further investigation is necessary in a larger number of cases in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
- CongdonNWangFTielschJMIssues in the epidemiology and population-based screening of primary angle-closure glaucomaSurv Ophthalmol1992364114231589856
- FosterPJBaasanhuJAlsbirkPHMunkhbayarDUranchimegDJohnsonGJGlaucoma in Mongolia. A population-based survey in Hövsgöl Province, Northern MongoliaArch Ophthalmol1996114123512418859083
- SawSMGazzardGFriedmanDSInterventions for angle-closure glaucoma: an evidence-based updateOphthalmology20031101869187914522756
- LeiKWangNWangLWangBMorphological changes of the anterior segment after laser peripheral iridotomy in primary angle closureEye (Lond)20092334535018064058
- HeMFriedmanDSGeJLaser peripheral iridotomy in primary angle-closure suspects: biometric and gonioscopic outcomes: the Liwan Eye StudyOphthalmology200711449450017123610
- LimLSAungTHusainRWuYJGazzardGSeahSKAcute primary angle closure: configuration of the drainage angle in the first year after laser peripheral iridotomyOphthalmology20041111470147415288973
- AlsagoffZAungTAngLPChewPTLong-term clinical course of primary angle-closure glaucoma in an Asian populationOphthalmology20001072300230411097612
- CongdonNGYoulinQQuigleyHBiometry and primary angle-closure glaucoma among Chinese, white, and black populationsOphthalmology1997104148914959307646
- LoweRFAetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucomaBr J Ophthalmol1970541611695428641
- WangBSSakataLMFriedmanDSQuantitative iris parameters and association with narrow anglesOphthalmology2010117111719815290
- BhargavaSKLeightonDAPhillipsCIEarly angle-closure glaucoma. Distribution of iridotrabecular contact and response to pilocarpineArch Ophthalmol1973893693724697211
- KunimatsuSTomidokoroAMishimaKPrevalence of appositional angle closure determined by ultrasonic biomicroscopy in eyes with shallow anterior chambersOphthalmology200511240741215745766
- KongXFosterPJHuangQAppositional closure identified by ultrasound biomicroscopy in population-based primary angle-closure glaucoma suspects: the Liwan Eye StudyInvest Ophthalmol Vis Sci2011523970397521357394
- NolanWPSeeJLChewPTKDetection of primary angle closure using anterior segment optical coherence tomography in Asian eyesOphthalmology2007114333917070597
- KunimatsuSTomidokoroAMishimaKPrevalence of appositional angle closure determined by ultrasonic biomicroscopy in eyes with shallow anterior chambersOphthalmology200511240741215745766
- NolanWPFosterPJDevereuxJGUranchimegDJohnsonGJBaasanhuJYAG laser peripheral iridotomy treatment for primary angle closure in east Asian eyesBr J Ophthalmol2000841255125911049950
- WilenskyJTShould patients with anatomically narrow angles have prophylactic iridectomy? I. Narrow angles accompanied by slit-lamp and gonioscopic evidence of risk are indications for prophylactic laser iridectomySurv Ophthalmol19964131368827928
- AngGSWellsAPFactors influencing laser peripheral iridotomy outcomes in white eyes: an anterior segment optical coherence tomography studyJ Glaucoma20112057758321278590
- SihotaRDadaTGuptaRLakshminarayanPPandeyRMUltrasound biomicroscopy in the subtypes of primary angle closure glaucomaJ Glaucoma20051438739116148588
- WangBSNarayanaswamyAAmerasingheNIncreased iris thickness and association with primary angle closure glaucomaBr J Ophthalmol201195465020530187
- CheungCYLiuSWeinrebRNDynamic analysis of iris configuration with anterior segment optical coherence tomographyInvest Ophthalmol Vis Sci2010514040404620237248

![Figure 1 The determination of the iris thickness (IT500, IT750, and maximal iris thickness [MIT]) under the dark condition on anterior-segment optical coherence.](/cms/asset/99f1877b-f242-4720-9471-c0d4d1140a3a/doph_a_53516_f0001_c.jpg)