Abstract
Gyrate atrophy of the choroid and retina is a rare chorioretinal dystrophy inherited in an autosomal recessive pattern. We describe the first documented case of gyrate atrophy from Australia in a 56-year-old woman with a history of previous diagnosis of retinitis pigmentosa and worsening night vision in her right eye over several years. She was myopic and bilaterally pseudophakic, and fundus examination revealed pale optic discs and extensive peripheral chorioretinal atrophy exposing bare sclera bilaterally with only small islands of normal-appearing retina at each posterior pole. Visual field testing showed grossly constricted fields, blood testing showed hyperornithinemia, and further questioning revealed consanguinity between the patient’s parents. We then used the patient’s typical retinal findings of gyrate atrophy to demonstrate the potential use of ultrawide-field fundus photography and angiography in diagnosis and monitoring response in future treatment.
Introduction
Gyrate atrophy of the choroid and retina is a rare chorioretinal dystrophy inherited in an autosomal recessive pattern. Patients typically report night blindness and/or loss of peripheral vision, usually in the second decade of life. More than 200 individuals with gyrate atrophy have been reported since it was first described in the late 19th century, with cases mainly reported from Finland, the US, Japan, and France.Citation1 Although there have been anecdotal reports of gyrate atrophy cases in Australia, we could not identify a case reported in the literature. Thus, to our knowledge, we report the first documented case of gyrate atrophy from Australia and document the clinical findings with ultrawide-field fundus photography and angiography.
Case report
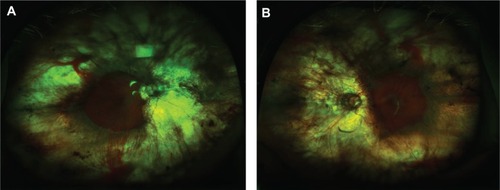
A 56-year-old woman who reported being previously diagnosed with retinitis pigmentosa and open-angle glaucoma 20 years prior was referred for ongoing worsening night vision and peripheral vision in her right eye over several years. She reported being myopic since childhood and had used latanoprost at night into both eyes over a long period. She had a past medical history of hypertension, depression, and elevated cholesterol. On examination, best-corrected visual acuity was hand movements in the right eye and 6/12 in the left eye with refractive errors of -1.75 in each eye. Intraocular pressures were 18 mmHg in each eye. Anterior segment examinations were quiet bilaterally. Ten years previously she had developed bilateral posterior subcapsular cataracts and was now bilaterally pseudophakic with patent posterior capsulotomies. Fundoscopy revealed pale optic discs and extensive peripheral chorioretinal atrophy exposing bare sclera bilaterally. Only small islands of retina at each posterior pole still appeared to be clinically normal (). These islands were sharply demarcated from the atrophic areas by a pigmented border ().
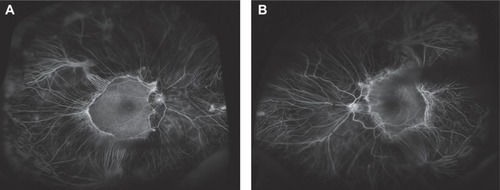
Figure 1 Ultrawide-field fundus photography of gyrate atrophy (Optos Imaging System). Extensive chorioretinal atrophy with remaining central macula islands at the posterior poles demarcated by pigmented borders.
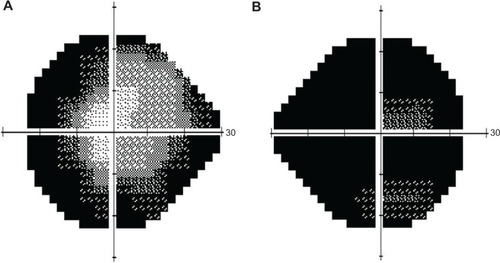
Visual field testing showed almost total constriction of the right visual field and also severe peripheral constriction of the left visual field (). Fundal fluorescein angiography confirmed significant peripheral choroidal atrophy (). Routine bloods tests were normal but serum ornithine was elevated at 206 umol/L (reference range 27–98 umol/L). Extensive visual electrodiagnostic testing, including electroretinography, pattern electroretinography, multifocal electroretinography, electro-oculography, visual evoked potential, average visual evoked potential, and Farnsworth–Munsell 100 (all performed to International Society for Clinical Electrophysiology of Vision standards), showed virtually abolished responses in scotopic and photopic conditions, confirming an advanced retinal dystrophy. On further questioning, the patient reported a history of consanguinity, with her parents being first cousins – thus potentiating an autosomal recessive inheritance and clinching the diagnosis of gyrate atrophy.
Discussion
Clinically, gyrate atrophy appears as well-circumscribed areas of atrophy of the choroidal vessels, retinal pigment epithelium, and photoreceptors in the midperipheral retina.Citation2 Typically, scalloped atrophic areas are well demarcated from the posterior pole, which although it appears clinically normal usually also has areas of photoreceptor cell loss.Citation3 Gyrate atrophy has been associated with serum hyperornithinemia due to a deficiency of the vitamin B6-dependent enzyme ornithine ketoacid aminotransferase (OAT), and the human OAT gene has been localized to chromosome 10.Citation4
Our case is typical of other gyrate atrophy cases in terms of retinal findings, myopia, early cataract formation, serum hyperornithinemia, and an autosomal recessive inheritance pattern. We have commenced treating the patient with a low-arginine diet and pyridoxine (vitamin B6), and plan to follow her up with serial visual field testing, serum ornithine levels, and retinal photography/angiography. Although the efficacy of these treatments has been variable,Citation5 we are hopeful that we will preserve the patient’s remaining visual function. In the future, diseases like gyrate atrophy with known molecular and genetic foundations hopefully may become targets for enzyme replacement treatments or gene therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- SergouniotisPIDavidsonAELenassiEDeverySRMooreATWebsterARRetinal structure, function, and molecular pathologic features in Gyrate AtrophyOphthalmology201211959660522182799
- YuanAKainesAJainAReddySSchwartzSSarrafDUltrawide-field and autofluorescence imaging of choroidal dystrophiesOphthalmic Surg Lasers Imaging201041e1e521053862
- WilsonDKWeleberRGGreenWROcular clinic-pathologic study of gyrate atrophyAm J Ophthalmol199111124331985486
- O’DonnellJCoxDShowsTThe ornithine amino-transferase gene is on human chromosome 10Invest Ophthalmol Vis Sci198526128
- Kaiser-KupferMICarusoRCValleDReedGFUse of an arginine-restricted diet to slow progression of visual loss in patients with gyrate atrophyArch Ophthalmol200412298298415249361