Abstract
Purpose
To evaluate a digital high-speed camera combined with digital morphometry software for dynamic measurements of phakic intraocular lens movements to observe kinetic influences, particularly in fast direction changes and at lateral end points.
Materials and methods
A high-speed camera taking 300 frames per second observed movements of eight iris-claw intraocular lenses and two angle-supported intraocular lenses. Standardized saccades were performed by the patients to trigger mass inertia with lens position changes. Freeze images with maximum deviation were used for digital software-based morphometry analysis with ImageJ.
Results
Two eyes from each of five patients (median age 32 years, range 28–45 years) without findings other than refractive errors were included. The high-speed images showed sufficient usability for further morphometric processing. In the primary eye position, the median decentrations downward and in a lateral direction were −0.32 mm (range −0.69 to 0.024) and 0.175 mm (range −0.37 to 0.45), respectively. Despite the small sample size of asymptomatic patients, we found a considerable amount of lens dislocation. The median distance amplitude during eye movements was 0.158 mm (range 0.02–0.84). There was a slight positive correlation (r=0.39, P<0.001) between the grade of deviation in the primary position and the distance increase triggered by movements.
Conclusion
With the use of a slit lamp-mounted high-speed camera system and morphometry software, observation and objective measurements of iris-claw intraocular lenses and angle-supported intraocular lenses movements seem to be possible. Slight decentration in the primary position might be an indicator of increased lens mobility during kinetic stress during eye movements. Long-term assessment by high-speed analysis with higher case numbers has to clarify the relationship between progressing motility and endothelial cell damage.
Introduction
Besides angle-supported intraocular lenses (ASIOLs), iris-claw intraocular lenses (ICIOLs) are also designed as anterior chamber lenses. First-generation iris-claw models were launched in 1953, and optimized designs of these are now used not only in cases of aphakia (eg, traumatic) or after intracapsular cataract extractionCitation1,Citation2 but since 1986 they have also been used in phakic eyes (phakic intraocular lenses [PIOLs]) to correct refractive errors.Citation3 Nowadays, Artisan®/Artiflex® ICIOLs (Ophthec, Groningen, the Netherlands)/Verisyse® (Abbott Medical Optics, Santa Ana, CA, USA) and Cachet® (Novartis International AG, Basel, Switzerland) ASIOLs are available in different versions depending on optic power and the need for correction of astigmatism.
Several clinical trials with variable results partially reported that the position of ICIOLs and the morphometry of the anterior chamber (eg, deep or shallow anterior chambers,Citation4 white-to-white distance, or sulcus diameter) may have consequences, eg, endothelial cell lossCitation4,Citation5 or chronic inflammations of the iris.Citation6,Citation7 To measure in more realistic conditions, investigations using Scheimpflug photography or ultrasound biomicroscopy have also been performed.Citation8,Citation9 In 1984, Miller and Doane used analog high-speed imaging to investigate movements of ASIOLs and ICIOLs (mainly Binkhorst type).Citation10 Due to technical limitations, only moderate changes of the gaze position were investigated in patients after intracapsular cataract extraction.Citation10
The aim of our pilot study was to evaluate a digital high-speed camera setting (mounted on a slit lamp) to measure the movements of PIOLs not only at a standstill or within small globe movements but also in fast direction changes in the primary position and at lateral end points.
Materials and methods
For capturing lens positions during eye movements, a high-speed charge-coupled device camera (Genie HM640; Teledyne Dalsa, Waterloo, ON, Canada) with a resolution of 640×480 pixels and speed of 300 frames per second (8 bit) was mounted (C mount) on the video adapter (f75; Haag-Streit, Köniz, Switzerland) of a conventional slit lamp (BQ900; Haag-Streit). For capturing high-speed videos, optimized software (Motion Traveller version 2.28.0.5173; Imaging Solutions, Eningen, Germany) was used. Illumination was achieved by using the total slit (8×8 mm) with the brightest illumination settings. Furthermore, a cold-light source (FlexiLux 3000; Schölly Fiberoptic, Denzlingen, Germany) was used for a more homogeneous illumination. The technical harmlessness of the setting was approved by the in-house technical assistance.
Before the high-speed video session, a standard slit-lamp examination was performed by an experienced ophthalmologist to survey for loose fixations, decentrations, and detectable abnormalities of the lens. Lentodonesis of the artificial lens was defined as 0 if absent, 1 if only slightly visible, or 2 in cases with obvious lens movements. Patients were asked whether they noticed any kind of shaking images.
Each eye of a patient was filmed separately. Sequential capturing positions were frontal without globe movement and both lateral end points of globe movements. Therefore, the slit lamp was adjusted in a parallel perspective (). Thereafter, the patients were told to move their eyes reiteratively from one end position to the other (ie, from up to down or left to right; , ). To trigger the kinetic effects of artificial lenses, horizontal and vertical eye movements were performed with and without short stops in the primary position.
Figure 1 Schematic drawing of camera positions while video capturing side glance to the right (A), primary position (B), and side glance to the left (C) for analysis of the right eye.
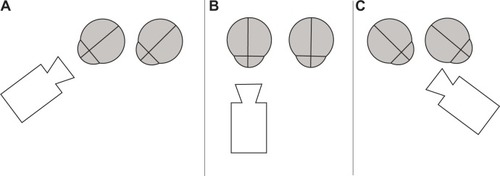
Figure 2 Images of different positions with overlays of the pupil shape and lens positions.
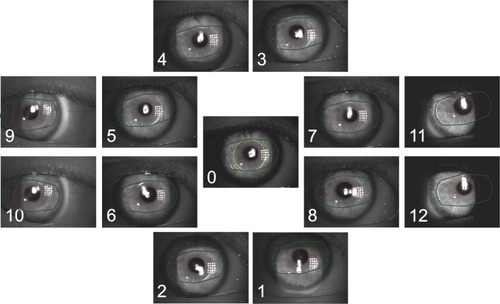
Table 1 Measurement positions and their defined coding
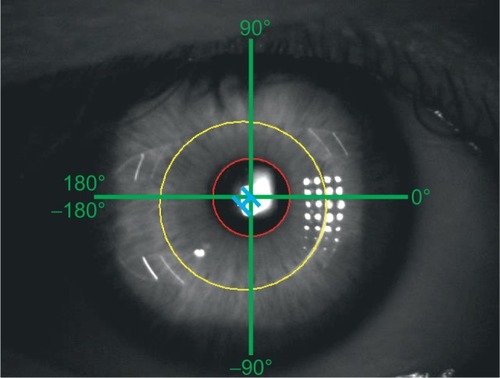
Video sequences were exported as uncompressed audio-video interleaved (AVI) files (30 frames per second). Images were exported out of the created AVI files and saved in tagged image file (TIF; resolution 640×480) format. Position measurements were performed using ImageJ (http://imagej.nih.gov/ij).Citation11 Only images with clearly delimited pupil and lens margins were included. For measurements of maximal lens deviations only video-frames with the peak of movements (backshoots and overshoots)Citation12 were used. The center of the pupil was estimated by adapting a circle within the pupil and defining its centroid. With the same method, the centroid of the lens optic was measured. Afterward, the distance and angle between the center of the lens and the center of the pupil were measured for all positions for frontal and end-point positions ().
Figure 3 Scheme of measurement conventions.
The collected data were descriptively analyzed using a statistical program (JMP 10.0; SAS Institute Inc., Cary, NC, USA). All data were anonymously analyzed in accordance with the strict German directives on information security and data protection. Written informed consent was obtained from all patients before the examination. The investigation followed the tenets of the Declaration of Helsinki. Approval of the local ethics committee (Tübingen) was obtained in advance.
Results
In this pilot study, two eyes from each of five patients (median age 32 years, range 28–45 years) were included. For further patient characteristics, see . All patients had undergone refractive surgery because of myopia or astigmatism. No other abnormalities (eg, glaucoma, lens pseudoexfoliation) existed at the time of surgery or study examination. No patients reported shaking images. The high-speed camera showed good functionality. In all cases, high-speed imaging was possible, and there were no resulting problems due to reflections or blurry images. In two patients, positions 3 and 4 were not sufficiently observable due to a small palpebral fissure.
Table 2 Patient characteristics
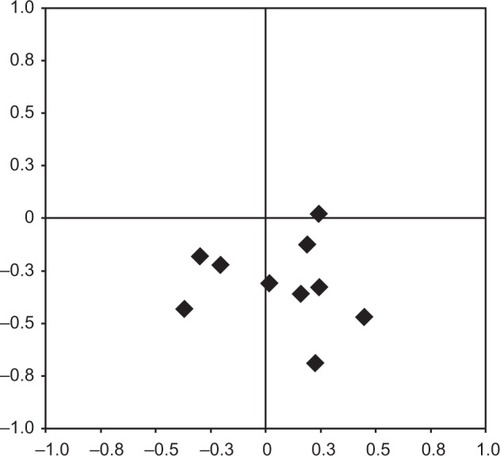
In the primary eye position, except in one eye (ASIOL), all lenses were positioned lower (negative y-values) in relation to the center of the pupils, with a median of −0.32 mm (range −0.69 to 0.024) (). Subanalysis of different lens models showed a median distance downward of −0.51 mm (range −0.69 to −0.33) for the Artiflex®, −0.34 mm (range −0.47 to −0.12) for the Artisan®, and −0.18 mm for one of two Cachet® (second one 0.024 mm upward). The median deviation in the lateral direction was 0.175 mm (range −0.37 to 0.45). The median absolute distance and angle between the center points were 0.373 mm (range 0.221–0.742) and −76.143° (range −149° to 5.711°), respectively.
Figure 4 Positions of the lens center in relation to the pupil center.
The median distance amplitude of movements that were measured in positions 1–12 was 0.158 mm (range 0.02–0.84 mm) (). Subanalysis of different lens models showed a median distance amplitude of 0.183 mm (range 0.04–0.84 mm) for the Artiflex®, 0.15 mm (range 0.02–0.47 mm) for the Artisan®, and 0.16 mm (range 0.03–0.44 mm) for the Cachet®. There was a positive correlation (r=0.39, P<0.001) between the distance of the optic center from the pupil center in the primary position and the grade of the distance increase triggered by movements.
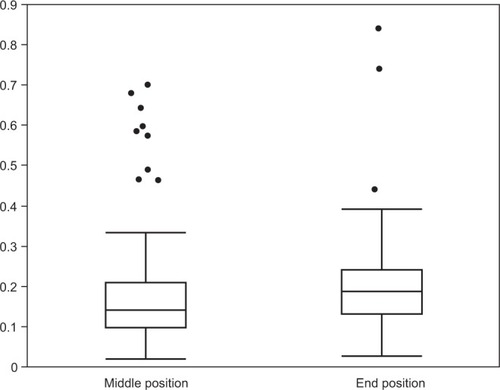
Figure 5 Median distance amplitudes in millimeters (y-axis) of the lenses in different positions (x-axis) in relation to the primary position.
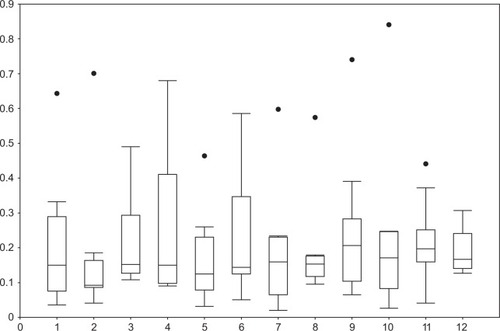
Simplifying the different positions only to middle positions (codes 1–8) and end positions (codes 9–12) for all lens types resulted in a median distance amplitude of movements of 0.14 mm (range 0.02–0.70 mm) and 0.18 mm (range 0.03–0.84 mm), respectively (). The median change of the angles was 3.96° (range −105.72° to 69.75°), which showed a negative correlation (−0.3) with median distance amplitude over all lens types.
Discussion
In this pilot study, we were able to discover the function of a slit lamp-mounted high-speed camera system and a subsequent morphometry of images to record kinetic influences on the position of ICIOLs and ASIOLs in phakic eyes. Measurement of varying ICIOL and ASIOL positions was feasible in fast direction changes and at lateral end points.
PIOLs are reversible,Citation13 maintain accommodation,Citation14 and are used in patients with high refractive errors or in cases with contraindications for corneal refractive procedures (eg, thinned cornea, scotopic pupil size).Citation1,Citation15 There are several known possible complications with PIOLs (eg, corneal endothelial cell loss, elevation of intraocular pressure)Citation16,Citation17 that can be related to their position within the eye segments.Citation18 Based on these complications, many investigations measured the location of ICIOLsCitation5,Citation9,Citation15 and ASIOLsCitation4,Citation19 and their physiological effects. Examination techniques like optical coherence tomography, biometry, or Scheimpflug imaging do not allow the recording of short-term position changes that can appear based on kinetic effects during fast eye movements. Cheng et al,Citation20 Jacobs et al,Citation21 and Miller and DoaneCitation10 demonstrated the movements of IOLs using high-speed-imaging, but not in phakic individuals and only after short-distance eye movements. As far as the authors know, position-determining investigations of PIOLs have been performed in various positionsCitation9 or during accommodation,Citation14,Citation19,Citation22 but not during fast eye movements.
Despite the small case series, we tried not to lump all lens types together, and tried a careful analysis of subgroups. The subsequent analysis of the selected “peak lens deviation” freeze images within our small series to evaluate technical feasibility showed a low-lying lens position relative to the pupil center in nine of ten eyes. Possible causes for these observations are implantation position, looseness of the iris or fixation points, shifting haptic phenomenon,Citation23 and gravity forces “pulling” the lenses to negative vertical values. Also, other investigations described decentrations of ICIOL models. Menezo et alCitation24 reported decentrations of up to 1 mm, while Pérez-Santonja et alCitation25 described a decentration greater than 0.5 mm in 14 (43%) of 32 eyes for Worst–Fechner ICIOLs.
Although the median amplitude over all measured lenses was low, our investigation showed explicit differences for single lenses. Moreover, we could see that higher movement amplitudes could be expected the higher the distance between the lens center and the pupil center at the primary position. But facing the small case numbers, we have to be careful in drawing conclusions. Causes for higher amplitudes in end positions could be loose fixations or increasing kinetic effects in cases of forceful accelerations or decelerations. Furthermore, motions of the pupil, as reported by Nyström et alCitation26 could have influenced our observations. An alternative method to define reference coordinates for deviation measurements could be seen in using Purkinje images. With this method also, movements of the natural lens in normal subjects could be observed.Citation27
We demonstrated the feasibility of our video-capturing setting using a slit lamp-mounted high-speed camera for dynamic observations of short-term lens movements in all investigated patients and in various positions of the eyes. Following our first analysis with a reduced number of patients, further measurements with our high-speed system seem to be reasonable to detect lens positions and movements in more cases and with a prolonged follow-up time, and to test parameters like interobserver agreement or correlations of lens movements and anterior chamber morphology. Hereafter, the system could be used, eg, to do further investigations of lens stability in patients with unexplained endothelial cell loss.
Limitations
The number of patients in our feasibility investigations does not allow any further statistical calculations or conclusions for diverse lens designs. Also, although the nonstandardized pupil had a limiting effect, we opted not to influence pupil movements (eg, with atropine, pilocarpine) to avoid effects on the mounting of the lens haptics of ICIOLs. Furthermore, motions of the pupils and their center could have interfered with our measurements, and with higher case numbers differences between horizontal, vertical, or oblique saccades should be evaluated.
Conclusion
With the use of a simple slit lamp-mounted high-speed camera system, the dynamic observation of movements of ICIOLs and ASIOLs is possible. The images allow detailed measurements of lens movements in various positions. Slight decentration in the primary position might be an indicator of increased lens mobility during kinetic stress during eye movements. With the small case numbers, further investigations within a bigger population are planned. Long-term assessment by high-speed analysis has to clarify the relationship between progressing motility and endothelial cell damage.
Acknowledgments
Many thanks to Dieter Schwenkel and his team from Imaging Solutions (Eningen, Germany) for the great technical assistance. We acknowledge support by Deutsche Forschungsgemeinschaft and the Open Access Publishing Fund of Tübingen University.
Disclosure
The authors report no conflicts of interest in this work.
References
- GuellJLMorralMKookDKohnenTPhakic intraocular lenses part 1: Historical overview, current models, selection criteria, and surgical techniquesJ Cataract Refract Surg201036111976199321029908
- WorstJGvan der VeenGLosLIRefractive surgery for high myopia. The Worst-Fechner biconcave iris claw lensDoc Ophthalmol1990753–43353412090409
- FechnerPUvan der HeijdeGLWorstJGIntraocular lens for the correction of myopia of the phakic eyeKlin Monbl Augenheilkd198819312934 German3184737
- SaxenaRBoekhoornSSMulderPGNoordzijBvan RijGLuytenGPLong-term follow-up of endothelial cell change after Artisan phakic intraocular lens implantationOphthalmology20081154608613 e117686520
- DoorsMCalsDWBerendschotTTInfluence of anterior chamber morphometrics on endothelial cell changes after phakic intraocular lens implantationJ Cataract Refract Surg200834122110211819027569
- Pérez-SantonjaJJIradierMTBenítez del CastilloJMSerranoJMZatoMAChronic subclinical inflammation in phakic eyes with intraocular lenses to correct myopiaJ Cataract Refract Surg19962221831878656382
- SenthilSReddyKPA retrospective analysis of the first Indian experience on Artisan phakic intraocular lensIndian J Ophthalmol200654425125517090877
- YuAYLinZDChenXQCaiXYLiuYZLuoSKPosition of myopic iris-claw phakic intraocular lens by Scheimpflug photography and ultrasound biomicroscopyEye (Lond)200822223323917435684
- SchöpferKBergerAKorbCStoffelnsBMPfeifferNSekundoWPosition-dependent accommodative shift of retropupillary fixated iris-claw lensesGraefes Arch Clin Exp Ophthalmol2012250121827183422527324
- MillerDDoaneMGHigh-speed photographic evaluation of intraocular lens movementsAm J Ophthalmol19849767527596731539
- RasbandWSImageJBethesda (MD)US National Institutes of Health1997
- DeubelHBridgemanBFourth Purkinje image signals reveal eye-lens deviations and retinal image distortions during saccadesVision Res19953545295387900293
- GüellJLMorralMGrisOGaytanJSisquellaMManeroFFive-year follow-up of 399 phakic Artisan-Verisyse implantation for myopia, hyperopia, and/or astigmatismOphthalmology200811561002101217980432
- GüellJLMorralMGrisOGaytanJSisquellaMManeroFEvaluation of Verisyse and Artiflex phakic intraocular lenses during accommodation using Visante optical coherence tomographyJ Cataract Refract Surg20073381398140417662431
- KohnenTCichockiMKossMJPosition of rigid and foldable iris-fixated myopic phakic intraocular lenses evaluated by Scheimpflug photographyJ Cataract Refract Surg200834111412018165090
- KohnenTKookDMorralMGüellJLPhakic intraocular lenses: part 2: results and complicationsJ Cataract Refract Surg201036122168219421111322
- LovisoloCFReinsteinDZPhakic intraocular lensesSurv Ophthalmol200550654958716263370
- TaneriSOehlerSKochJMHeiligenhausAHypermotility of an iris-fixated anterior chamber phakic intraocular lens due to nontraumatic iris laxityEur J Ophthalmol201222348148421959679
- KlaprothOKRehrmannJKohnenTDynamic positional change and defocus curve of a phakic foldable anterior-chamber angle-supported intraocular lens during accommodationOphthalmology201312071373137923523163
- ChengHJacobsPPriceNMoarIAnterior chamber profile and lens implant movement – a high-speed cinematographic studyTrans Ophthalmol Soc U K1982102Pt 121236963059
- JacobsPMChengHPriceNCPseudophakodonesis and corneal endothelial contact: direct observations by high-speed cinematographyBr J Ophthalm19836710650654
- SekundoWBissmannWTietjenABehaviour of the phakic iris-claw intraocular lens (Artisan/Verisyse) during accommodation: an optical coherence biometry studyEur J Ophthalmol200717690490818050115
- DoorsMEgginkFAWebersCANuijtsRMLate-onset decentration of iris-fixated phakic intraocular lenses: a case seriesAm J Ophthalmol200914769971003, 1003.e1–e219327741
- MenezoJLAviñoJACisnerosARodriguez-SalvadorVMartinez-CostaRIris claw phakic intraocular lens for high myopiaJ Refract Surg19971365455559352483
- Pérez-SantonjaJJBuenoJLZatoMASurgical correction of high myopia in phakic eyes with Worst-Fechner myopia intraocular lensesJ Refract Surg1997133268281 discussion 281–2649183759
- NyströmMHoogeIHolmqvistKPost-saccadic oscillations in eye movement data recorded with pupil-based eye trackers reflect motion of the pupil inside the irisVision Res201392596624096093
- SchaeffelFBinocular lens tilt and decentration measurements in healthy subjects with phakic eyesInvest Ophthalmol Vis Sci20084952216222218436854