Abstract
Diquafosol is a drug used for dry eye treatment with a novel mechanism of action. It stimulates the secretion of tear fluid and mucin on the ocular surface, thus enabling us to selectively treat the tear film layer, playing an important role in the establishment of the concept of “Tear Film Oriented Therapy (TFOT)”, an effective therapeutic approach to dry eye in Japan. The 3% diquafosol ophthalmic solution has been widely used for the treatment of dry eye in clinical practice, and it is currently available in Japan and South Korea. This review provides an overview of the clinical utility of 3% diquafosol ophthalmic solution, focusing on the results of clinical studies on various types of dry eye, including aqueous-deficient dry eye, short tear film breakup time-type dry eye, and post dry eye after laser in situ keratomileusis. It also introduces the additive effect of diquafosol on sodium hyaluronate monotherapy for dry eye, and the effect of 3% diquafosol ophthalmic solution for dry eye-related conditions. Additionally, it summarizes the ocular effects of diquafosol in healthy human eyes. Lastly, the importance of improving tear film stability in dry eye treatment, as well as general advances in dry eye treatments, are described.
Introduction
Dry eye is one of the most common reasons for patient visits to eye clinics, as it affects 5%–30% of the population worldwide.Citation1,Citation2 The 2007 Report of the International Dry Eye WorkShop (DEWS) has defined dry eye as follows: dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability, with potential damage to the ocular surface.Citation3 Although the available treatments vary among the numerous counties and regions, the DEWS report suggested selecting treatments for each severity level of dry eye from a menu of therapies deemed effective in that specific area.Citation4 In general, artificial tears or lubricating drops are often used for symptom relief in mild to moderate dry eye. Tear retention agents or anti-inflammatory agents are usually used concurrently.
In Japan, cyclosporine A ophthalmic solution is not an approved treatment for dry eye. Sodium hyaluronate ophthalmic solutions have been the primary products used to treat dry eye, in conjunction with preservative-free artificial ophthalmic solutions, for many years. Two new topical pharmacologic agents have recently become commercially available for treating dry eye in Japan.Citation5 The first is 3% diquafosol ophthalmic solution (Diquas, ophthalmic solution 3%; Santen Pharmaceutical Co. Ltd, Osaka, Japan), which stimulates aqueous and mucous secretion directly on the ocular surface. The other is 2% rebamipide ophthalmic suspension (Mucosta ophthalmic suspension UD2%; Otsuka Pharmaceutical, Co., Ltd, Tokyo, Japan), which stimulates mucous secretion. These new eye drops have enabled us to selectively treat the tear film layer and increase its stability. Decreased tear film stability has been emphasized as the core mechanism of dry eye in Japan; therefore, dry eye has been treated from the perspective of improving the tear film stability. Thanks to the arrival of ophthalmic solutions that stimulate the secretion of mucin and water, we are now entering a new era of a layer-by-layer ocular surface-based diagnosis and treatment of dry eye. Recently, the Dry Eye Society of Japan advocated “Tear Film Oriented Therapy (TFOT)” as an effective therapeutic approach to dry eye ().Citation6 Based on TFOT, we expect that each layer of the ocular surface may be targeted by a selective topical therapy, thereby further stabilizing the tear film.Citation6
Figure 1 Concept diagram of TFOT.
Abbreviations: TFOT, Tear Film Oriented Therapy; OTC, over-the-counter; EGF, epidermal growth factor.
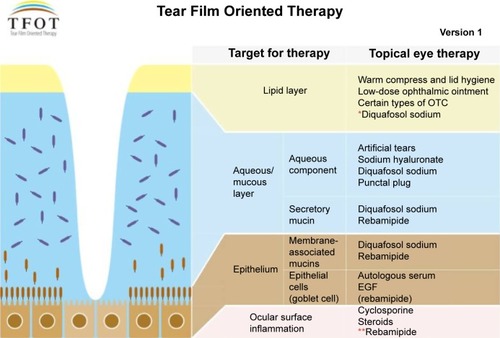
3% Diquafosol ophthalmic solution was launched at the end of 2010 as a drug for the treatment of dry eye with a novel mechanism of action involving the stimulation of tear and mucin secretion.Citation7 Diquafosol has been widely used to treat dry eye in clinical practice, and is currently approved in Japan and South Korea for dry eye treatment. It is a P2Y2 purinergic receptor agonist that activates P2Y2 receptors on the ocular surface. Diquafosol stimulates both fluid secretion from the conjunctival epithelial cells and mucin secretion from the conjunctival goblet cells directly on the ocular surface by an interaction with the P2Y2 receptors. Diquafosol may not act on the lacrimal glands directly; it did not stimulate protein secretion from isolated rabbit lacrimal glands.Citation8 On the other hand, it is known that diquafosol not only stimulates mucin secretion from the conjunctival goblet cells, but it also upregulates the expression of membrane-binding mucin genes in corneal epithelial cells.Citation9 Thus, diquafosol enhances the stabilization of the tear film and hydration of the ocular surface, independent of tear fluid secretion from the lacrimal glands.Citation10–Citation13 According to studies of animal models, diquafosol promotes tear fluid and mucin secretion leading to the improvement of corneal epithelial barrier function, and it also suppresses corneal epithelial damage induced by desiccation of the ocular surface.Citation13,Citation14
This article reviews the clinical therapeutic effects of 3% diquafosol ophthalmic solution in patients with dry eye, as well as summarizes its effects in healthy eyes.
Ocular effects in healthy eyes
Several studies have evaluated the ocular effects of using 3% diquafosol ophthalmic solution in healthy human eyes, which should be helpful in the use of diquafosol for dry eyes in clinical practice.Citation15–Citation17 Currently, an increase of the ocular surface fluid volume,Citation15 an increase in the concentration of mucin-like substance,Citation16 and the short-term effects on optical qualityCitation17 induced by diquafosol ophthalmic solution have been reported.
A significant increase of the ocular surface tear volume was observed after a single topical instillation of 3% diquafosol ophthalmic solution in healthy human eyes.Citation15 Using the reflective meniscometry technique, the radius of curvature of the lower tear meniscus was measured noninvasively. While there was no significant change with instillation of artificial tears, instillation of 3% diquafosol ophthalmic solution increased tear fluid on the ocular surface for up to 30 minutes.Citation15
The collected tear samples after the instillation of 3% diquafosol ophthalmic solution showed a transient but significant increase in sialic acid concentration in healthy human subjects.Citation16 Because sialic acid is considered a good marker for monitoring mucin in biological samples, diquafosol could possibly stimulate the secretion of mucin from the ocular tissues of human eyes.
The short-term effects of viscosity and suspensibility of dry eye drop instillation on optical quality were investigated by measurement of ocular higher-order aberrations (HOAs) and ocular forward light scattering.Citation17 Although an increase in HOAs was observed 1 minute after instillation of 3% diquafosol ophthalmic solution when compared to baseline, the increase was minimal when compared to the instillation of highly viscous 0.3% sodium hyaluronate ophthalmic solution. No significant changes in ocular forward light scattering were seen with the instillation of 3% diquafosol ophthalmic solution, although 2% rebamipide ophthalmic suspension was found to have a significant increase from baseline in this parameter. Since 3% diquafosol ophthalmic solution is functionally free from viscosity and suspensibility after instillation, and given that no remarkable short-term effect of instillation is demonstrated, this suggests that patients are more likely to comply with treatment because it will not obscure their vision.
Therapeutic efficacy in clinical trials and clinical studies
A summary of the clinical trials and clinical studies described in the remaining sections is demonstrated in .
Table 1 Summary of the clinical trials and clinical studies on the benefits of the use of 3% diquafosol ophthalmic solution
Clinical trials
There are three randomized, double-blind, multicenter, parallel-group clinical trials.Citation18–Citation20 In this section, we focus on a placebo-controlled study that was performed mainly to evaluate the efficacy and proper dosage (a Phase II study) of sodium hyaluronate ophthalmic solution,Citation19 and on an active-controlled study against 0.1% sodium hyaluronate ophthalmic solution (a Phase III study).Citation20 Improvement in corneal epithelial damage was chosen as the primary endpoint for these studies.Citation19,Citation20 The fluorescein (FL) staining score and the rose bengal (RB) staining score were used to evaluate corneal epithelial damage and conjunctival epithelial damage, respectively. In these studies, one drop of ophthalmic solution was instilled six times daily for 4 weeks or 6 weeks, for the Phase III and Phase II studies, respectively.Citation19,Citation20 In the Phase II dose-finding study,Citation19 which compared 1% and 3% diquafosol ophthalmic solutions, the efficacy of the solutions against the placebo was determined for both doses in terms of FL and RB scores. At week 6, a significant decrease in the FL and RB scores was observed for the 1% and 3% diquafosol groups when compared with the placebo group. In the FL score, dose dependency was also confirmed, suggesting that 3% was considered more effective than the 1% solution.
In the Phase III study, the FL scores were significantly improved from baseline at weeks 2 and 4, with both 3% diquafosol ophthalmic solution and 0.1% hyaluronate ophthalmic solution. Moreover, the 3% diquafosol ophthalmic solution was shown to be noninferior to the 0.1% hyaluronate ophthalmic solution at week 4 for this endpoint. A significant decrease in the RB scores was also observed for both groups at weeks 2 and 4 compared with baseline. However, the improvement from baseline to week 4 in the RB score was significantly greater with 3% diquafosol than with 0.1% hyaluronate ophthalmic solution, which showed an increased efficacy in lowering the RB score for patients with dry eye.Citation20
A recent large, multicountry, randomized clinical trial conducted in the People’s Republic of China and Singapore has reported the efficacy and safety of 3% diquafosol ophthalmic solution and compared them with those of 0.1% hyaluronate ophthalmic solution.Citation21 One drop of either ophthalmic solution was instilled six times daily for 4 weeks. In the diquafosol group, as compared with the hyaluronate group, changes in FL score were noninferior and changes in RB score were superior at week 4. Both groups improved in tear film breakup time.
Clinical studies
Aqueous-deficient dry eye
In a study evaluating the long-term efficacy of diquafosol ophthalmic solution,Citation22 patients received one drop of 3% diquafosol ophthalmic solution six times daily for 6 months. Prolonged use of diquafosol ophthalmic solution for 6 months produced significant improvement both subjectively (dry eye symptom score) and objectively (ocular staining score and tear function tests).
In a subsequent study by the same group,Citation23 the short-term (after 15 minutes of instillation) and long-term (after 4 weeks of six times daily administration) effects of diquafosol ophthalmic solution on optical quality were evaluated in aqueous-deficient dry eye. Ocular HOAs were measured with a wavefront sensor before and 15 minutes after diquafosol instillation at the baseline visit and at 4 weeks after treatment initiation. No significant change in HOAs was observed as a short-term effect of a single-drop instillation of diquafosol. However, the long-term use of diquafosol to treat dry eye reduced HOAs, as well as improved tear film stability and corneal epithelial damage.
As the majority of the patients in these studies had Sjögren’s syndrome,Citation22,Citation23 3% diquafosol may be effective for mild to moderate patients with Sjögren’s syndrome.
Short tear film breakup time-type dry eye
Clinically, the short tear film breakup time-type dry eye has been reported to be associated with a shorter tear film breakup time and dry eye symptoms without ocular surface damage and tear deficiency.Citation24,Citation25 The efficacy of 3% diquafosol ophthalmic solution in short tear film breakup time-type dry eye was examined in a few studies.Citation26–Citation28 One drop of 3% diquafosol ophthalmic solution was administered six times daily in each study.
An exploratory nonrandomized trialCitation26 showed significant improvements in subjective symptoms and tear film breakup time at both 1 month and 3 months after treatment with 3% diquafosol ophthalmic solution. In a 4-week randomized, clinical study comparing 3% diquafosol ophthalmic solution with artificial tears,Citation26 significant improvements in dry eye symptoms were noted in the diquafosol group, but not in the artificial tear group at week 2. Tear film breakup time was significantly prolonged in the diquafosol group at week 4.
In a nonrandomized study,Citation27 patients with short tear film breakup times were classified based on the presence of dry eye symptoms. Visual function, as well as tear film breakup time, were evaluated at 1 month. After the administration of 3% diquafosol ophthalmic solution, the tear film breakup time was significantly increased from baseline in the symptom-negative group, but not in the symptom-positive group. In contrast, significant improvements in functional visual acuity and ocular HOAs were observed in the symptom-positive group, but not in the symptom-negative group.
According to the recent report investigating the short-term effects on intraocular scattering,Citation28 3% diquafosol ophthalmic solution generated improvements not only in tear film breakup time, but also in intraocular scattering measurements. These measures deteriorate significantly with time in patients with short tear film breakup time-type dry eye, indicating that diquafosol is also effective for improving the optical quality of the eye.
Patients with general or “real-world” dry eye
This multicenter, prospective, noninterventional observational studyCitation29 evaluated the efficacy and safety of 3% diquafosol ophthalmic solution from over 3,000 dry eye patients. It included “real-world” dry eye patients who were excluded from clinical trials because of limitations such as age, contact lens use, and the use of eye drops other than diquafosol. The study demonstrated that the six times daily administration of 3% diquafosol ophthalmic solution was effective regardless of the severity of dry eye based on the ocular FL staining score, or on a therapeutic pattern. A total of 76.0% of the enrolled patients responded that their condition had improved.
The additive effect of diquafosol on sodium hyaluronate monotherapy
An option for adding 3% diquafosol ophthalmic solution in combination with 0.1% sodium hyaluronate ophthalmic solution has been suggested for dry eye cases where sodium hyaluronate monotherapy is insufficient.Citation30 Each eye was randomly assigned to one of the two regimens in each patient: 3% diquafosol ophthalmic solution plus 0.1% sodium hyaluronate ophthalmic solution in one eye; and 0.1% sodium hyaluronate alone in the other. The patients applied one drop of the ophthalmic solution six times daily. After 2 weeks and 4 weeks of treatment with diquafosol ophthalmic solution, significant improvements in tear film breakup time and ocular staining scores were observed. In terms of subjective symptoms, significant improvements for dry eye sensation, pain, and foreign body sensations were observed with the combination of 3% diquafosol ophthalmic solution and 0.1% sodium hyaluronate ophthalmic solutions.
A study from South Korea,Citation31 where 3% diquafosol ophthalmic solution was launched in 2013, showed the beneficial effect of the combination therapy with preservative-free 0.1% sodium hyaluronate ophthalmic solution and 3% diquafosol ophthalmic solution. Dry eye patients were randomly assigned to one of three treatment groups: preserved 0.1% sodium hyaluronate; 3% diquafosol; and a combination of 3% diquafosol and preservative-free 0.1% sodium hyaluronate for 3 months. Patients applied one drop of the ophthalmic solution four times daily. The combination therapy with 3% diquafosol and preservative-free 0.1% sodium hyaluronate significantly improved the Ocular Surface Disease Index score, ocular staining, goblet cell density, and impression cytological findings more so than either hyaluronate or diquafosol monotherapies.
Dry eye after laser in situ keratomileusis (LASIK)
The efficacy of 3% diquafosol ophthalmic solution in patients with dry eye following LASIK was examined in the following two studies.Citation32,Citation33 In one study,Citation32 post-LASIK dry eye patients were randomly assigned to one of four treatment groups: artificial tears; 0.3% sodium hyaluronate; 3% diquafosol; or a combination of 3% diquafosol and 0.3% sodium hyaluronate. Patients applied one drop of the ophthalmic solution six times daily. Distance and near uncorrected visual acuity improved after the combination therapy more so than with 0.3% hyaluronate or 3% diquafosol monotherapies. Distance functional visual acuity improved significantly, but only in the combination group 1 month after LASIK. Subjective dry eye symptoms in the combination group improved significantly when compared with those in the other groups 1 week after surgery.
Longer-term treatment with 3% diquafosol ophthalmic solution was evaluated in persistent dry eye after LASIK.Citation33 One drop of diquafosol ophthalmic solution 3% was administered six times daily. The ocular staining scores significantly improved over 12 weeks; however, the best-corrected visual acuity and tear secretion did not change. In terms of subjective symptoms, significant improvements for fatigue, dryness, grittiness, discomfort, difficulty in reading, and discomfort within the area of dryness were observed after the additional diquafosol treatment.
Both studies suggest that for LASIK-associated dry eye, the additional treatment of 3% diquafosol ophthalmic solution would be more effective than with the conventional therapy using artificial tears or sodium hyaluronate.Citation32,Citation33
Obstructive meibomian gland dysfunction (MGD)
P2Y2 receptors are also known to exist in the meibomian glands.Citation34 Obstructive MGD is a major cause of lipid layer deficiency and evaporative dry eye, and it may result in unstable tear film.
A study performing quantitative image analysis of meibomian gland morphology, as well as tear film parameters, showed the beneficial effect of diquafosol in patients with MGD.Citation35 More than 4 months of diquafosol therapy (four times daily administration) significantly improved ocular symptoms, lid margin abnormalities, ocular staining scores, and tear function tests. The mean ratio of the meibomian gland area imaged with noncontact meibography significantly increased after treatment. Diquafosol may be one of the treatment options for MGD in which conventional therapies, such as warm compresses, lid hygiene, and artificial tear eye drops or systemic medications, are often unsatisfactory or poorly tolerated.Citation36
Patients with dry eye-related conditions
The efficacy of 3% diquafosol ophthalmic solution has also been reported in dry eye-related conditions, where patients have abnormal tear film dynamics or where their symptoms are temporary.Citation37,Citation38 The effect of pretreatment with 3% diquafosol ophthalmic solution on intraoperative corneal wetting during cataract surgery has been reported.Citation37 This study also indicates that such pretreatment prevents intraoperative iatrogenic dry eye. Since pre-existing tear film abnormalities or dry eye can affect surgical outcomes, the proper management of ocular surface diseases, such as dry eye, is important prior to performing cataract surgery or refractive surgery.
According to a recent report, a transient but significant increase in lower tear meniscus height was observed after the instillation of 3% diquafosol ophthalmic solution in eyes wearing a high water content contact lens, in which the tear meniscus height was remarkably decreased.Citation38 Therefore, it would be expected that diquafosol ophthalmic solution could work as solution for contact lens-related dry eye.
Safety of diquafosol
Of the 655 subjects who were enrolled in clinical trials and received diquafosol, adverse drug reactions were reported in 155 subjects (23.7%).Citation7,Citation18–Citation20 Major adverse drug reactions included eye irritation (6.7%), eye discharge (4.7%), conjunctival injection (3.7%), eye pain (2.7%), eye pruritus (2.4%), foreign body sensation (2.1%), and eye discomfort (1.1%).Citation7 The majority of adverse reactions were of mild severity, and no serious treatment-related adverse events were reported. According to the results of the multicenter clinical study for “real-world” dry eyes,Citation29 adverse reactions were observed in 6.3% of patients, and the major adverse reactions were eye discharge, eye irritation, and eye pain. According to the results of a 6-month clinical study,Citation22 the good tolerability of diquafosol ophthalmic solution 3% was maintained in the longer term.
Conclusion
This review provides an overview of 3% diquafosol ophthalmic solution, mainly focusing on its clinical utility obtained from clinical studies. The clinical efficacy of 3% diquafosol ophthalmic solution has been confirmed for dry eye, including aqueous-deficient dry eye, short tear film breakup time-type dry eye, and post-LASIK dry eye. The additive effect of diquafosol on sodium hyaluronate monotherapy has also been demonstrated. Diquafosol has the potential to improve MGD, which is a chronic, symptomatic, ocular surface disease, and it often accompanies dry eye. The utility of diquafosol for treatment prior to cataract surgery or for contact lens wearers has also been described, through improving the quality or quantity of the tear film.
A recent studyCitation39 has evaluated the effect of lubricant eye drops containing eledoisin and carnitine in glaucoma patients with ocular discomfort symptoms, and it reported that lubricant eye drops that stimulate tear production and restore physiological osmolarity represent a promising strategy for dry eye syndrome in glaucoma patients. In this regard, 3% diquafosol ophthalmic solution might be useful in patients with chronic glaucoma suffering from iatrogenic dry eye syndrome.
Based on the pathogenesis of dry eye disease, qualitative and quantitative improvements of the tear film are crucial.Citation40 From the point of view of clinical practice, tear film instability causes dry eye symptoms, ocular surface damage, and visual disturbances. Therefore, achieving tear film stability is important for improving these symptoms or signs. As visual disturbances are noted in the definition of dry eye in the 2007 DEWS report,Citation3 more attention has been paid to the quality of vision in dry eye. In that regard, improvement in optical quality by dry eye treatment with 3% diquafosol ophthalmic solution will be helpful to manage and maintain the quality of vision in dry eye.Citation23,Citation27,Citation28
Although dry eye is a multifactorial disease of the tears and ocular surface, an increase of the availability of dry eye treatment options would enable clinicians to treat various types of dry eye more effectively in clinical practice. The successful use of diquafosol in various types of dry eye based on TFOT would be anticipated in the future.
Acknowledgments
The author wishes to thank Dr Masatsugu Nakamura (Santen Pharmaceutical Co., Ltd., Osaka, Japan) for providing assistance.
Disclosure
The author reports no conflicts of interest in this work.
References
- The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf2007529310717508117
- MizunoYYamadaMMiyakeYDry Eye Survey Group of the National Hospital Organization of JapanAssociation between clinical diagnostic tests and health-related quality of life surveys in patients with dry eye syndromeJpn J Ophthalmol201054425926520700790
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf200752759217508116
- Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf20075216317817508120
- DogruMNakamuraMShimazakiJTsubotaKChanging trends in the treatment of dry-eye diseaseExpert Opin Investig Drugs2013221215811601
- YokoiNTherapeutic guideline for dry eye: Tear Film Oriented Therapy (TFOT)Atarashii Ganka2015321916 Japanese
- NakamuraMImanakaTSakamotoADiquafosol ophthalmic solution for dry eye treatmentAdv Ther201229757958922843206
- Takaoka-ShichijoYMurakamiTNakamuraMStimulatory effect of diquafosol tetrasodium on tear fluid secretion in normal rabbitsAtarashii Ganka201128710291033 Japanese
- Takaoka-ShichijoYNakamuraMStimulatory effect of diquafosol tetrasodium on the expression of membrane-binding mucin genes in cultured human corneal epithelial cellsAtarashii Ganka2011283425429 Japanese
- LiYKuangKYerxaBWenQRosskothenHFischbargJRabbit conjunctival epithelium transports fluid, and P2Y2(2) receptor agonists stimulate CI(−) and fluid secretionAm J Physiol Cell Physiol20012812C595C60211443059
- JumblattJEJumblattMMRegulation of ocular mucin secretion by P2Y2 nucleotide receptors in rabbit and human conjunctivaExp Eye Res19986733413469778415
- MurakamiTFujiharaTHoribeYNakamuraMDiquafosol elicits increases in net Cl-transport through P2Y2 receptor stimulation in rabbit conjunctivaOphthalmic Res2004362899315017104
- FujiharaTMurakamiTFujitaHNakamuraMNakataKImprovement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye modelInvest Ophthalmol Vis Sci20014219610011133853
- FujiharaTMurakamiTNaganoTNakamuraMNakataKINS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glycoprotein in a rabbit short-term dry eye modelJ Ocul Pharmacol Ther200218436337012222766
- YokoiNKatoHKinoshitaSFacilitation of tear fluid secretion by 3% diquafosol ophthalmic solution in normal human eyesAm J Ophthalmol201415718592.e124200231
- ShigeyasuCHiranoSAkuneYYamadaMDiquafosol tetrasodium increases the concentration of mucin-like substances in tears of healthy human subjectsCurr Eye Res20141625310688
- KohSMaedaNIkedaCEffect of instillation of eyedrops for dry eye on optical qualityInvest Ophthalmol Vis Sci20135474927493323812492
- NakamuraMImanakaTNew treatment. Diquafosol tetrasodiumJpn J Ocular Pharmacol20112514246 Japanese
- MatsumotoYOhashiYWatanabeHTsubotaKDiquafosol Ophthalmic Solution Phase 2 Study GroupEfficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trialOphthalmology2012119101954196022739038
- TakamuraETsubotaKWatanabeHOhashiYDiquafosol Ophthalmic Solution Phase 3 Study GroupA randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patientsBr J Ophthalmol201296101310131522914501
- GongLSunXMaZA randomised, parallel-group comparison study of diquafosol ophthalmic solution in patients with dry eye in China and SingaporeBr J Ophthalmol Epub2015128
- KohSIkedaCTakaiYWatanabeHMaedaNNishidaKLong-term results of treatment with diquafosol ophthalmic solution for aqueous-deficient dry eyeJpn J Ophthalmol201357544044623740285
- KohSMaedaNIkedaCEffect of diquafosol ophthalmic solution on the optical quality of the eyes in patients with aqueous-deficient dry eyeActa Ophthalmol2014928e671e67524863298
- TodaIShimazakiJTsubotaKDry eye with only decreased tear break-up time is sometimes associated with allergic conjunctivitisOphthalmology199510223023097862418
- KohSMaedaNHoriYEffects of suppression of blinking on quality of vision in borderline cases of evaporative dry eyeCornea200827327527818362651
- Shimazaki-DenSIsedaHDogruMShimazakiJEffects of diquafosol sodium eye drops on tear film stability in short BUT type of dry eyeCornea20133281120112523635860
- KaidoMUchinoMKojimaTDogruMTsubotaKEffects of diquafosol tetrasodium administration on visual function in short break-up time dry eyeJ Ocul Pharmacol Ther201329659560323537148
- KobashiHKamiyaKIgarashiAMiyakeTShimizuKIntraocular scattering after instillation of diquafosol ophthalmic solutionOptom Vis Sci Epub20141230
- YamaguchiMNishijimaTShimazakiJClinical usefulness of diquafosol for real-world dry eye patients: a prospective, open-label, non-interventional, observational studyAdv Ther201431111169118125376447
- KamiyaKNakanishiMIshiiRClinical evaluation of the additive effect of diquafosol tetrasodium on sodium hyaluronate monotherapy in patients with dry eye syndrome: a prospective, randomized, multicenter studyEye (Lond)201226101363136822878452
- HwangHSSungYMLeeWSKimECAdditive effect of preservative-free sodium hyaluronate 0.1% in treatment of dry eye syndrome with diquafosol 3% eye dropsCornea201433993594125055152
- TodaIIdeTFukumotoTIchihashiYTsubotaKCombination therapy with diquafosol tetrasodium and sodium hyaluronate in patients with dry eye after laser in situ keratomileusisAm J Ophthalmol20141573616622.e124528935
- MoriYNejimaRMasudaAEffect of diquafosol tetrasodium eye drop for persistent dry eye after laser in situ keratomileusisCornea201433765966224858017
- CowlenMSZhangVZWarnockLMoyerCFPetersonWMYerxaBRLocalization of ocular P2Y2 receptor gene expression by in situ hybridizationExp Eye Res2003771778412823990
- AritaRSuehiroJHaraguchiTTopical diquafosol for patients with obstructive meibomian gland dysfunctionBr J Ophthalmol201397672572923584719
- ParanjpeDRFoulksGNTherapy for meibomian gland diseaseOphthalmol Clin North Am2003161374212683247
- MiyakeGOtaIMiyakeKZakoMIwakiMEffects of topical diquafosol pretreatment on intraoperative corneal wettingJ Cataract Refract Surg201440101682168825175269
- NagaharaYKohSMaedaNNishidaKWatanabeHProminent decrease of tear meniscus height with contact lens wear and efficacy of eye drop instillationEye Contact Lens Epub201541
- NebbiosoMEvangelistaMLibrandoAPlaterotiAMPescosolidoNIatrogenic dry eye disease: an eledoisin/carnitine and osmolyte drops studyBiomed Pharmacother201367765966323906760
- ShimazakiJRisk factors for dry eye syndromeGanka2011531115531557 Japanese
- TFOT (Tear Film Oriented Therapy) [webpage on the Internet]TokyoDry Eye Society Available from: http://www.dryeye.ne.jp/en/tfot/index.htmlAccessed May 7, 2015
