Abstract
This patient presented for surgery at the age of 32 years, 14 months after his initial complaint of metamorphopsia and visual loss in the right eye. Past tests demonstrated a whitish epiretinal membrane (ERM) with translucent stress lines over a thickened macula. Visual acuity was found on last presentation to be normal with minimal alteration on Amsler grid testing. A torn ERM was found in the center with left-over ERM temporally and rolled-over ERM nasally at the site of the epicenter with no posterior vitreous detachment. Visual recovery occurred gradually over several days 2 months prior to presentation apparently following heavy weight-lifting with a sensation of severe eye pressure. Sequential funduscopy and optical coherence tomography scans demonstrated the peeling of an ERM accompanied by normalization of foveal thickness. Valsalva maneuver had put excessive tension on ERM which tore in its center at the weakest line with gradual contraction of the ERM away from the fovea towards the peripapillary area. This is a new mechanism of self-separation of ERM induced by Valsalva. ERM in young subjects is subject to rupture and subsequent separation by tangential traction. There are three mechanisms for spontaneous separation of ERM: 1) posterior vitreous detachment with pulling of ERM by detaching vitreous (most common in adults); 2) the contracting forces of the immature ERM become stronger than its adhesions to the retina resulting in slow tangential traction on the edges of the ERM and gradual separation from the edges towards the center (remodeling common in youngsters); and 3) acute tearing of ERM at its weakest central point and retraction of part of the membrane towards the epicenter (current case report).
Introduction
Epiretinal membranes (ERMs) are characterized by wrinkling of the macular surface from cell proliferation. Most commonly ERMs are noted in adults above the age of 50 years in association with posterior vitreous detachment (PVD), laser photocoagulation, or after retinal detachment repair. A majority of ERM in adults is of the cellophane macular reflex type referring to the thin transparent variety.Citation1 ERM in children and young adults is uncommon, because a PVD is uncommon in very young subjects. Ocular trauma, pars planitis, ocular toxocariasis, ocular toxoplasmosis, combined hamartoma of the retina and retinal pigment epithelium, and Coats’ disease are leading causes of secondary ERM in children. The prevalence of ERM in subjects less than 20 years of age is around 1 in 20,000.Citation1 A majority of ERM in young subjects is of the thick white contractile variety having strong adherence to retinal vessels.Citation2,Citation3 Myofibroblasts, myoblastic differentiation of retinal pigment epithelial cells and fibrous astrocytes, as well as new collagen formation are more common in ERM of young subjects than in ERM in older subjects.Citation4 We report a young adult with ERM that underwent spontaneous release in a peculiar way (ERM tearing centrally) apparently following Valsalva maneuver.
Case report
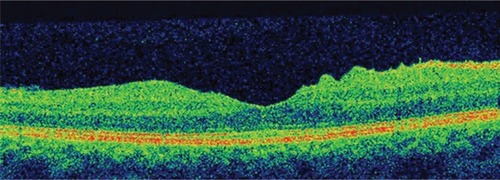
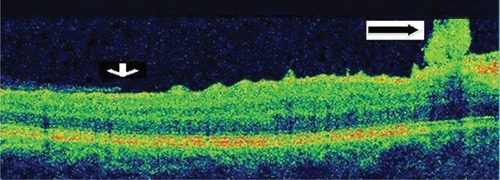
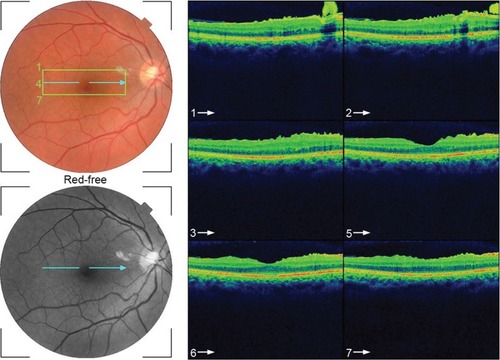
This 32 year-old healthy man complained of visual loss in the right eye 14 months prior to presentation and he was referred for surgical peeling of an ERM (–) by two retina experts. Two months before presentation, he indulged in heavy weight-lifting and noted repeatedly severe eye pressure during the exercise. Then he noted a gradual visual improvement in the right eye over several days. Two months later, ophthalmic exam revealed 6/6 uncorrected visual acuity with findings of a residual rolled-over ERM supranasal to the fovea (). The patient denied ocular rubbing or previous ocular trauma. Amsler grid revealed mild distortion temporally. Past fundus photograph 1.5 years ago revealed a dense macular gliosis with prominent radial stress line (best seen on infrared photography) throughout the posterior pole and centered around the thickest part of the membrane nasal to the disc (as noted by red-free photograph []). Intravenous fluorescein angiography done at the same time revealed obliteration of foveal avascular zone with tortuous macular capillaries and stretched out papillomacular vessels. Optical coherence tomography (OCT) done 1 year ago () revealed a diffuse ERM thin temporal to the fovea and very thick nasal to the fovea with marked thickening of the central fovea. OCT upon presentation demonstrated central peeling of ERM () accompanied by normalization of central foveal thickness (). ERM rupture could be noted with one edge ruptured temporally and the nasal edge rolling over (). The thick nasal membrane rolled over itself denoting its elastic property. There was no PVD identified by OCT or by slit lamp fundus biomicroscopy with a 90 diopter lens.
Figure 1 Fundus photograph 18 months before presentation.
Abbreviations: ERM, epiretinal membrane; ILM, internal limiting membrane.
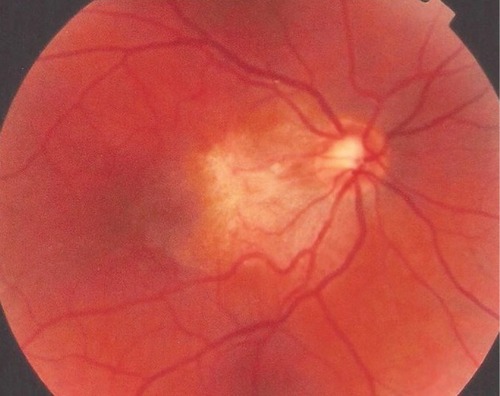
Figure 2 Red-free photograph of the right posterior pole.
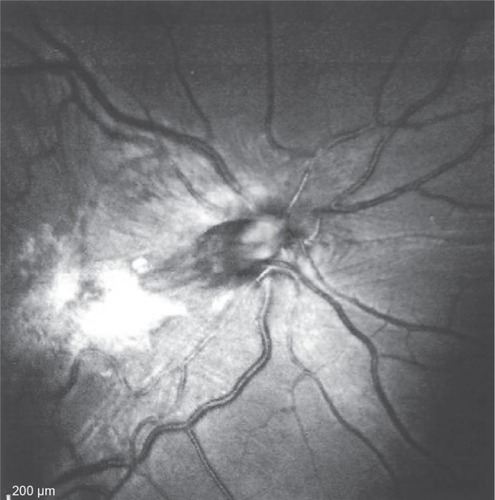
Figure 3 Infrared photograph of the right posterior pole.
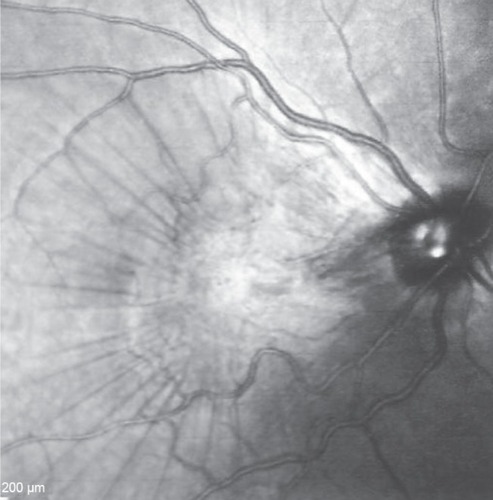
Figure 4 Arteriovenous phase of fluorescein angiography transit.
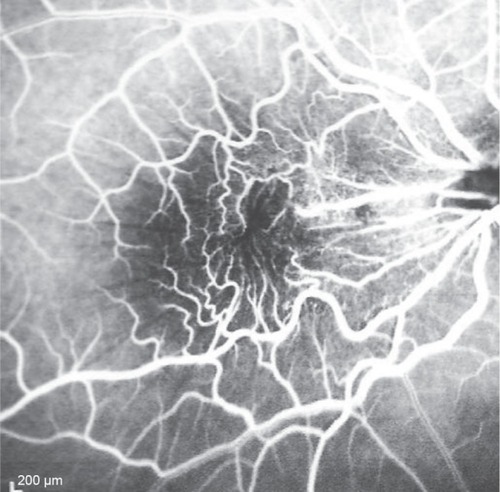
Figure 5 Optical coherence tomography done 1 year before presentation revealed a diffuse epiretinal membrane being thin temporal to the fovea and very thick nasal to the fovea (epicenter) with marked thickening of the central fovea.
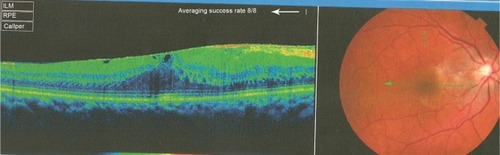
Figure 6 Fundus photograph of the right eye taken more than 1 year after the patient’s initial presentation.
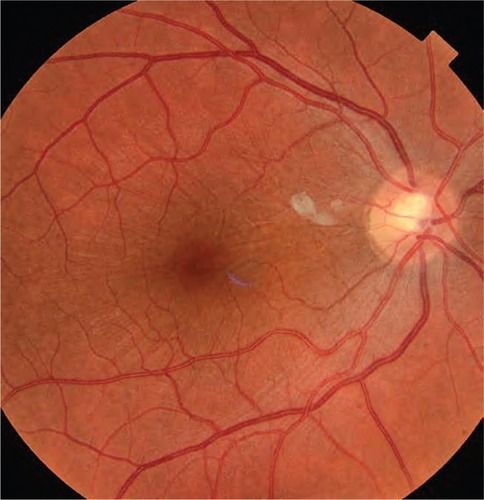
Figure 7 Serial horizontal optical coherence tomography scans demonstrate rupture of the epiretinal membrane (ERM) temporal to fovea with retraction of remaining ERM nasally and return of the normal foveal contour.
Discussion
ERM in young subjects commonly causes both metamorphopsia and reduced visual acuity from reduced axoplasmic flow, abnormal hemodynamic microcirculation, light-filtering effect of ERM, and photoreceptor distortion by tangential traction.Citation5–Citation7 Clinically, the superior and inferior vessels are narrowed while the perimacular vessels are pulled toward the epicenter with inner retinal striae. ERM in young people and children is rare and may be caused by congenital defects resulting from persistent adhesions of primary vitreous to the retina.Citation7 The clinical and ultrastructural features of juvenile macular pucker reflect a more rapidly changing, contractile tissueCitation4 compared with the usually more quiescent features in most cases involving older patients.
ERM is common in adults and rare in the young. However in adults, PVD can damage the internal limiting membrane, thereby permitting the migration of glial cells to the retinal surface.Citation6,Citation8 Alternate proposed hypothesis in adults is that an incomplete PVD provides the conditions suitable for membrane proliferation in the adhesion area between the vitreous and the retina. The cells involved in this process are retinal pigment epithelium metaplastic cells, glial cells (Muller cells and astrocytes), hyalocytes,Citation9 endothelial cells, fibroblasts, myofibroblasts, monocytes, and macrophages. It appears that several growth factorsCitation10 (platelet-derived growth factor, tissue growth factor TGF beta1 and 2, fibroblast growth factor, vascular endothelial growth factor, nerve growth factor) could stimulate glial cells to transdifferentiate into myofibroblasts and stimulate myofibroblasts to turn on their contractile actions more so in young subjects.Citation9–Citation11 Moreover the role of plasminogen and metalloproteinases in contraction of ERM has been raised.Citation12,Citation13 Spontaneous release of ERM is a rare event but is known to occur in adults and is related to the occurrence of an acute PVD that simultaneously releases the attachment between the retina and ERM (, ). Separation or peeling of ERM in young subjects is quite rare.Citation14–Citation30 It may occur spontaneously by development of a PVD,Citation23 or shortly after panretinal photocoagulation or Nd:YAG (neodymium-doped yttrium aluminum garnet) posterior capsulotomyCitation27 Meyer et alCitation15 presented six cases of spontaneous gradual “remodeling” with release of ERM in young subjects. They hypothesized that when the contracting forces of the immature ERM are stronger than its adhesions to the retina, the membrane may separate spontaneously. Only one of six cases had PVDCitation15 and one case with prior PVD also had release of ERM.Citation16 ERM release was more common in eyes with PVD:Citation23 in a study of 1,248 consecutive eyes with idiopathic ERM followed-up for around 3 years, ERM self-separation occurred in 37 eyes (3.0%), with 16 of 1,091 eyes with pre-existing PVD (1.5%) and 21 of 157 eyes without pre-existing PVD (13.4%).Citation23 A higher rate of spontaneous separation was reported in Japan: Nomoto et alCitation24 detected five patients with spontaneous separation of ERM among 92 patients with idiopathic ERM.
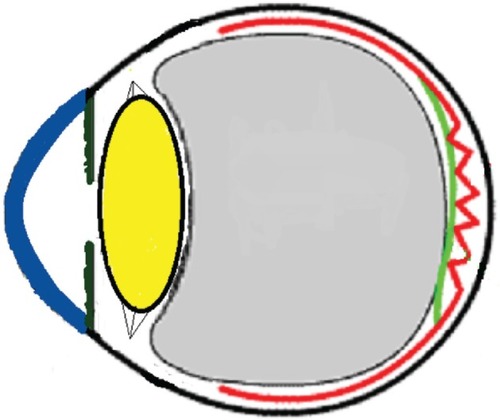
Figure 11 Schematic drawing of an adult eye with spontaneous epiretinal membrane (ERM) separation following acute posterior vitreous detachment.
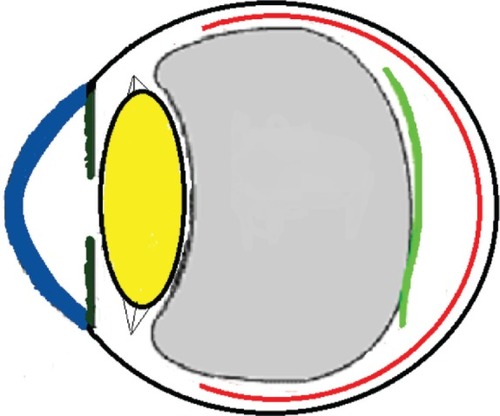
Three mechanisms (–) are proposed for spontaneous ERM separation including the two previously described ones: PVD (), remodeling (contraction of myofibroblast) (, ), and rupture of weakest line by lifting heavy objects (Valsalva) (, ). Head-down position combined with weight-lifting () and mouth closure lead to high intraocular pressure (IOP) (rise can reach up to 30 mmHg)Citation31,Citation32 and this places tension on tissues leading to compression of the vitreous against the retinal surface and hence retinal dehiscence in places of retinal lattice or in this case tearing off of the elastic fibrous tissue already under tangential traction at its weakest, thinnest point. Power athletes routinely utilize the Valsalva maneuver during weight-lifting. Valsalva maneuver comprises forcible exhalation against the closed glottis, thereby creating a sudden increase in the intrathoracic or intra-abdominal pressure. Ocular and systemic changes are secondary to the extreme pressure elevations that occur in the intra-abdominal, intrathoracic, intracranial, intraocular, and vascular compartments. The enormous pressures generated lead to elevations in intracranial pressure obstructing venous outflow leading to hemorrhage and elevations in IOP. In one study by Dickerman et alCitation32 IOPs were significantly elevated by weight-lifting by 15 mmHg from a mean of 13 to a mean of 28 with one subject’s IOP reaching 46 mmHg during maximal contraction. Macdougall et alCitation33 found severe elevations in blood pressure (BP) with mean value of 320/250 mmHg (BP exceeding 480/350 mmHg in one subject) and mouth pressures of 30–50 torr during a single maximum lift (normal mouth pressure being 15 torr). This combination of severe elevation in BP and intracranial pressure would have yielded a high incidence of ocular hemorrhage was it not for the dampening effect of high IOP. ERM sites above blood vessels appear to be under focal stress. ERM rupture during weight-lifting was not previously reported in the literature ().Citation32–Citation42
Figure 12 Schematic drawing showing gradual contraction of one edge towards the epicenter in young subjects.
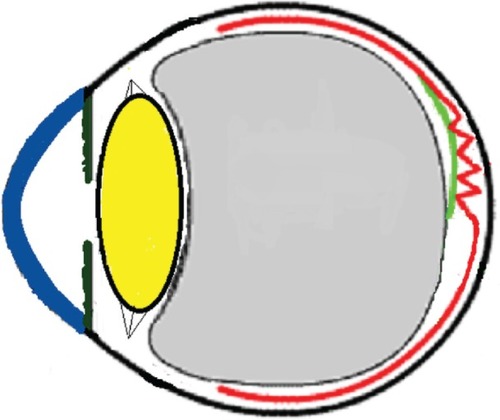
Figure 13 Schematic drawing showing further contraction of one edge resulting in epiretinal membrane release of tension on the fovea.
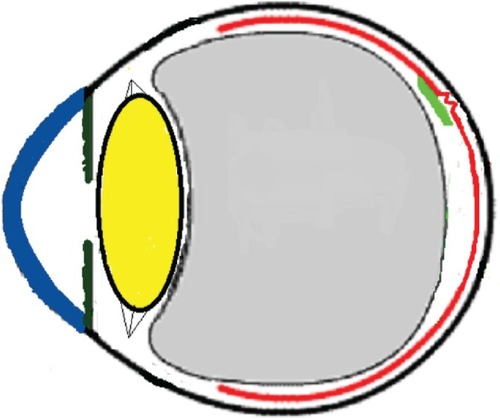
Figure 14 Schematic drawing showing the severe vitreous pressure from weightlifting putting tension on the thinnest portion of the epiretinal membrane with subsequent rupture of epiretinal membrane.
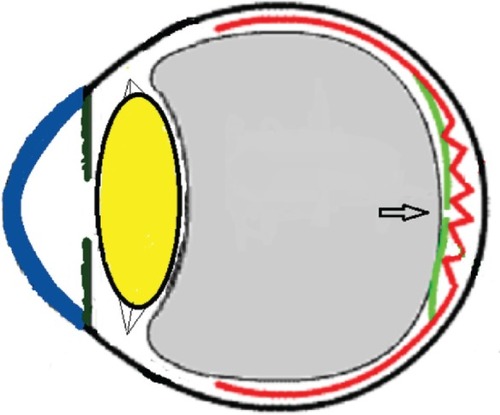
Figure 15 Schematic drawing showing the subsequent retraction and rolling over of the epiretinal membrane (ERM) towards the epicenter nasally following the rupture of ERM.
Table 1 Ocular and systemic changes in weight lifters
The relation between weight-lifting and ERM separation could be a coincidence with the natural history of ERM taking its course. The current ERM is thought to be primary and not secondary to trauma or inflammatory chorioretinal disorders. Other factors may be involved in ERM separation like rubbing of the eye or minor trauma to the eye, neither of which could be elicited in this case. A table of ocular findings in weight-lifters is enclosed ().
In conclusion, ERMs in young subjects seems strikingly different from that of those in older individuals; they appear thicker, whiter with high prevalence of myofibroblast cells, hence the contracting forces that stretch the retina. Potential maneuvers that can put stress on ERM surface include severe acute oculopression or rubbing that can raise the IOP acutely and Valsalva maneuvers, hence triggering spontaneous resolution of ERM via rupture in its thinnest meridian with subsequent contraction of the thicker side towards the epicenter.
Disclosure
The authors have no conflicts of interest related to this paper.
References
- KhajaHAMcCannelCADiehlNNMohneyBGIncidence and clinical characteristics of epiretinal membranes in childrenArch Ophthalmol2008126563263618474772
- BenhamouNMassinPSpolaoreRPaquesMGaudricASurgical management of epiretinal membrane in young patientsAm J Ophthalmol2002133335836411860973
- GastaudPJammes-VeauxHPRouhetteHDurafourgFLodsFIndications et résultats de l’éxérèse chirurgicale des membranes épimaculaires congénitales. [Indications and results of surgical removal of “congenital” epimacular membranes]J Fr Ophtalmol1999223359363 French10337594
- SmiddyWEMichelsRGGilbertHDGreenWRClinicopathologic study of idiopathic macular pucker in children and adultsRetina19921232322361410831
- BanachMJHassanTSCoxMSClinical course and surgical treatment of macular epiretinal membranes in young subjectsOphthalmology20011081232611150258
- KimmelASWeingeistTABlodiCFWellsKKIdiopathic premacular gliosis in children and adultsAm J Ophthalmol198910855785812817057
- WiseGNCongenital preretinal macular fibrosisAm J Ophthalmol19757933633651121992
- GrevenCMSlusherMMWeaverRGEpiretinal membrane release and posterior vitreous detachmentOphthalmology19889579029053174039
- KohnoRIHataYKawaharaSPossible contribution of hyalocytes to idiopathic epiretinal membrane formation and its contractionBr J Ophthalmol20099381020102619429593
- IannettiLAccorintiMMalagolaRRole of the intravitreal growth factors in the pathogenesis of idiopathic epiretinal membraneInvest Ophthalmol Vis Sci20115285786578921693611
- JoshiMAgrawalSChristoforidisJBInflammatory mechanisms of idiopathic epiretinal membrane formationMediators Inflamm2013201319258224324293
- ImmonenIVaheriATommilaPSirénVPlasminogen activation in epiretinal membranesGraefes Arch Clin Exp Ophthalmol1996234116646698950585
- WebsterLChignellAHLimbGAPredominance of MMP-1 and MMP2 in epiretinal and subretinal membranes of proliferative vitreoretinopathyExp Eye Res199968191989986746
- DesatnikHTreisterGMoisseievJSpontaneous separation of an idiopathic macular pucker in a young girlAm J Ophthalmol1999127672973110372890
- MeyerCHRodriguesEBMennelSSchmidtJCKrollPSpontaneous separation of epiretinal membrane in young subjects: personal observations and review of the literatureGraefes Arch Clin Exp Ophthalmol20042421297798515197556
- Garay-AramburuGLarrauri-AranaAResolucion espontanea de membrana epirretiniana idiopatica en un paciente joven [Spontaneous resolution of an idiopathic epiretinal membrane in a young patient]Arch Soc Esp Oftalmol20058012741743 Spanish16372220
- GaoHSalamGAChernSSpontaneous separation of idiopathic epiretinal membrane in a 7-year-old childJ AAPOS200711439339417280856
- SachdevNGuptaVGuptaASinghRSpontaneous separation of idiopathic epiretinal membrane in a young patientInt Ophthalmol200828430130217786389
- ShahPKNarendranVKalpanaNAppearance and spontaneous resolution of macular pucker after triple freeze-thaw cryotherapy for retinoblastomaJ Pediatr Ophthalmol Strabismus Epub2009625
- MulliganTGDailyMJSpontaneous peeling of an idiopathic epiretinal membrane in a young girlArch Ophthalmol199211010136713681417531
- SchadluRApteRSSpontaneous resolution of an inflammation-associated epiretinal membrane with previously documented posterior vitreous detachmentBr J Ophthalmol20079191252125317709592
- JanknechtPSpontane Ablösung eines Macular Puckers [Spontaneous resolution of a macular pucker]Klin Monatsbl Augenheilkd19972116398399 German9498192
- YangHSHongJWKimYJKimJGJoeSGCharacteristics of spontaneous idiopathic epiretinal membrane separation in spectral domain coherence tomographyRetina201434102079208724830825
- NomotoHMatsumotoCArimuraEQuantification of changes in metamorphopsia and retinal contraction in eyes with spontaneous separation of idiopathic epiretinal membraneEye (Lond)201327892493023722721
- SumersKDJampolLMGoldbergMFHuamonteFUSpontaneous separation of epiretinal membraneArch Ophthalmol19809823183207352883
- ByerNESpontaneous disappearance of early postoperative preretinal tractionArch Ophthalmol19739021331354721210
- RaySToppingTYoungLHSpontaneous peeling of epiretinal membrane associated with Nd:YAG laser injuryArch Ophthalmol2001119113713911146742
- MessnerKHSpontaneous separation of preretinal macular fibrosisAm J Ophthalmol1977831911835672
- MorelCAmelineBGuiberteauBLarocheLÉvolution spontanément favorable d’une membrane épirétinienne [Spontaneously favorable course of an epiretinal membrane]J Fr Ophtalmol2000239897900 French11084449
- EnochJMSchwartzAChangDHiroseHAniseikonia, metamorphopsia and perceived entoptic pattern: some effects of a macular epiretinal membrane, and the subsequent spontaneous separation of the membraneOphthalmic Physiol Opt19951543393437667027
- MansourAMFeghaliJGTo’meyKJaroudiNIncreased intraocular pressure with head-down positionAm J Ophthalmol19849811141156742069
- DickermanRDSmithGHLangham-RoofLMcConathyWJEastJWSmithABIntra-ocular pressure changes during maximal isometric contraction: does this reflect intra-cranial pressure or retinal venous pressure?Neurol Res199921324324610319330
- MacdougallJDTuxenDSaleDGMorozJRSuttonJRArterial blood pressure response to heavy resistance exerciseJ Appl Physiol (1985)19855837857903980383
- MattioliSDe FazioRBuiattiEPhysical exertion (lifting) and retinal detachment among people with myopiaEpidemiology200819686887118854710
- KocakNKaynakSKaynakTOnerHFCingilGUnilateral Purtscher-like retinopathy after weight-liftingEur J Ophthalmol200313439539712872799
- AhmadabadiMNKarkhanehRMirshahiAPremacular hemorrhage in Valsalva retinopathy: A study of 21 casesIranian Journal of Ophthalmology20092131116
- Chapman-DaviesALazarevicAValsalva maculopathyClin Exp Optometry20028514245
- AndroudiSAhmedMBrazitikosPFosterCSValsalva retinopathy: diagnostic challenges in a patient with pars-planitisActa Ophthalmol Scand200583225625715799745
- MattioliSCurtiSDe FazioROccupational lifting tasks and retinal detachment in non-myopics and myopics: Extended analysis of a case-control studySaf Health Work201231525722953231
- VieiraGMOliveiraHBde AndradeDTBottaroMRitchRIntraocular pressure variation during weight liftingArch Ophthalmol200612491251125416966619
- BakkeEFHisdalJSembSOIntraocular pressure increases in parallel with systemic blood pressure during isometric exerciseInvest Ophthalmol Vis Sci200950276076418836162
- DickermanRDMcConathyWJSmithGHEastJWRudderLMiddle cerebral artery blood flow velocity in elite power athletes during maximal weight-liftingNeurol Res200022433734010874679