Abstract
Purpose
To evaluate the anatomic and functional effect of microincision vitrectomy surgery (MIVS) with internal limiting membrane (ILM) peeling for macular edema secondary to branch retinal vein occlusion (BRVO).
Methods
The medical records of 101 eyes of 101 patients who had undergone MIVS with ILM peeling for macular edema secondary to BRVO were studied. Patients were classified into ischemic and non-ischemic BRVO based on angiograph. The best-corrected visual acuity (BCVA) and central foveal thickness (CFT), determined by spectral domain optical coherence tomography, were evaluated at baseline and at 1, 3, 6, and 12 months postoperatively.
Results
Preoperative mean logarithm of the minimum angle of resolution (logMAR) BCVA ± standard deviation (SD) was 0.52±0.43 and mean CFT ± SD was 489.4±224.9 μm. Postoperative mean BCVA ± SD values were 0.41±0.35, 0.35±0.41, 0.29±0.36, and 0.25±0.41, and mean CFT values were 370.1±148.9, 327.5±157.5, 310.9±154.9, and 274.4±135.3 μm at 1, 3, 6, 12 months, respectively. The mean BCVA was significantly improved at 3, 6, and 12 months postoperatively (all P<0.05), and the mean CFT was significantly decreased at all postoperative follow-up time points (all P<0.05). At the 12-month postoperative evaluation, BCVA had improved by 0.2 logMAR units in 50 eyes (60.0%) with ischemic BRVO and in nine eyes (50.0%) with non-ischemic BRVO. Six eyes (6.0%) experienced recurrence or persistence of macular edema at 12 months postoperatively.
Conclusion
MIVS with ILM peeling for macular edema secondary to BRVO is effective in improving visual acuity and foveal morphology with low recurrence of macular edema.
Keywords:
Introduction
Branch retinal vein occlusion (BRVO) is the second most common vascular disorder of the eye, and macular edema is a major cause of severe visual loss in BRVO. Macular edema has been reported to be present in 60% of BRVO cases.Citation1,Citation2 A previous study on the natural course of BRVO reported that only 14% of the eyes with chronic macular edema secondary to BRVO retained a visual acuity of 20/40 or better by the end of the follow-up period.Citation3
The Branch Vein Occlusion Study Group recommended grid laser photocoagulation be used to treat macular edema secondary to BRVO.Citation4 However, grid laser photocoagulation cannot be used in eyes with intraretinal hemorrhage in the fovea or the foveal capillary area. In addition, grid laser photocoagulation can result in scotoma. Therefore, better ways to treat macular edema secondary to BRVO are being sought via numerous therapeutic trial studies performed retrospectively or prospectively.
Vitrectomy with internal limiting membrane (ILM) peeling has been suggested as a potential treatment because it is generally believed that vitreous traction on the macula leads to fluid accumulation in the retina and removal of posterior hyaloid may improve oxygenation of the retina.Citation5–Citation8 At present, 23-, 25-, or 27-gauge pars plana vitrectomy, called microincision vitrectomy surgery (MIVS), is becoming safe and convenient due to improved surgical instrumentation combined with sophisticated surgical procedures. Surgeons can now perform vitrectomy less invasively, which in turn results in less patient discomfort and faster postoperative recovery than conventional 20-gauge vitrectomy.Citation9 Thus, MIVS appears to be an effective way to treat macular edema secondary to BRVO. Several studies have reported the efficacy of vitrectomy.Citation10–Citation13 However, previous studies mainly evaluated conventional 20-gauge vitrectomy, and studies of MIVS for BRVO had limitations such as small sample sizes or short follow-up periods. Furthermore, these reports rarely made mention of ischemic severity despite the ability to classify BRVO as ischemic and non-ischemic via angiograph.
Thus, the purpose of this study was to evaluate the best-corrected visual acuity (BCVA) and central foveal thickness (CFT) in more than 100 eyes observed postoperatively over 12 months after MIVS with ILM peeling for macular edema secondary to ischemic or non-ischemic BRVO.
Materials and methods
We retrospectively reviewed the medical records of patients who were initially treated for macular edema secondary to BRVO at Yokohama City University Medical Center between June 2007 and March 2012. All patients received complete information regarding the advantages and disadvantages of the available treatment. Informed consent was obtained from all patients who decided to undergo vitrectomy, and all participants provided their written informed consent to participate in this study. The institutional review committee at Yokohama City University Medical Center approved the study protocol, and the procedures used conformed to the tenets of the Declaration of Helsinki.
Inclusion criteria were: 1) macular edema, including the fovea, secondary to BRVO detected as macular thickening by spectral domain optical coherence tomography (OCT); 2) Snellen BCVA less than 20/20; 3) age >40 years; 4) CFT >250 μm; and 5) follow-up period >12 months. Exclusion criteria were: 1) prior laser photocoagulation, intravitreal injection of triamcinolone acetonide or intravitreal injection of antibodies against vascular endothelial growth factor (VEGF); 2) prior ocular surgery (excepting cataract surgery); 3) moderate or severe cataract that could cause vision decrease; and 4) glaucoma, diabetic retinopathy, or any other disease that could cause vision reduction.
All patients underwent a standard ophthalmologic examination, including measurement of BCVA with a Landolt chart at 5 meters, slit-lamp microscope examination, measurement of intraocular pressure, and dilated indirect slit-lamp biomicroscopy at all visits. CFT was measured by spectral-domain OCT (Cirrus® high-definition OCT; Carl Zeiss Meditec AG, Jena, Germany). CFT was measured manually as the distance between the surface of the ILM and the retinal pigment epithelium in the center of the fovea. The mean BCVA and the mean CFT were evaluated at baseline and at 1, 3, 6, and 12 months postoperatively. Fluorescein angiography was performed at baseline, and ischemic BRVO was defined angiographically as eyes with at least ten disc areas of retinal capillary non-perfusion.Citation14 Intraoperative and postoperative complications were recorded.
The surgery consisted of 25-gauge microincision vitrectomy (ACCURUS® or CONSTELLATION® Vision system; Alcon Laboratories Inc., Fort Worth, TX, USA) under local anesthesia. After removal of the posterior hyaloid membrane, the ILM was removed at the macular area in all cases.
Statistical analysis was performed with SPSS statistical software (v14.0; IBM Corporation, Armonk, NY, USA). Snellen visual acuity was converted to the logarithm of the minimum angle of resolution (logMAR). Preoperative and 12-month postoperative BCVA and CFT were compared by the nonparametric Wilcoxon signed-rank test. A P-value <0.05 was considered to be statistically significant. An improvement or decline of BCVA was defined as a change greater than or less than 0.2 logMAR units. A recurrence of macular edema was determined if CFT was >250 μm and if there was evidence of persistent or recurrent macular edema deemed to be affecting the patient’s visual acuity according to the investigator’s evaluation.Citation15
Results
The medical records of 101 eyes from 101 BRVO patients were studied. Patient demographics and clinical status are summarized in . Preoperative mean logMAR BCVA ± standard deviation (SD) of all eyes was 0.52±0.43. Postoperative BCVA values were 0.41±0.35, 0.35±0.41, 0.29±0.36, and 0.25±0.41 at 1, 3, 6, and 12 months after the vitrectomy, respectively (). BCVA values at 3, 6, and 12 months after the vitrectomy were significantly (P<0.01) better than preoperative BCVA values. BCVA values were 0.54±0.44, 0.42±0.37, 0.37±0.43, 0.31±0.37, and 0.27±0.42 in ischemic BRVO eyes, and 0.39±0.36, 0.38±0.31, 0.26±0.33, 0.22±0.35, and 0.19±0.38 in non-ischemic BRVO eyes at baseline and 1, 3, 6, and 12 months after the vitrectomy, respectively (). The percentage of patients who had improvement in BCVA values at the 12-month postoperative examination (ischemic and non-ischemic) were 60.0% and 50.0%, respectively; those unchanged were 36.1% and 44.4%, respectively; and those with diminished values were 3.6% and 5.6%, respectively. A Snellen BCVA of ≥20/40 is generally sufficient to support reading and driving and is considered an excellent outcome. The percentage of patients with Snellen equivalent BCVA ≤20/40 at baseline and ≥20/40 at 12 months after the vitrectomy was 63.4% in all cases.
Figure 1 Changes of BCVA after surgery.
Abbreviations: BCVA, best-corrected visual acuity; logMAR, logarithm of the minimum angle of resolution; SEM, standard error of the mean.
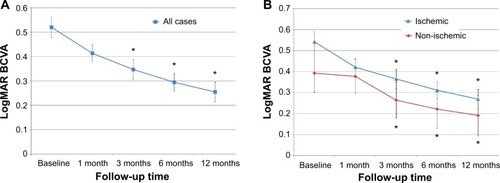
Table 1 Patient demographic data and preoperative clinical status
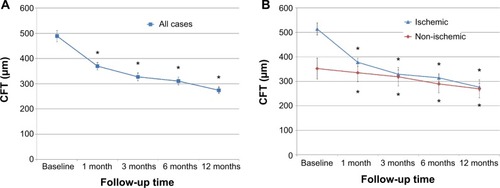
Preoperative average CFT (mean ± SD) of all eyes was 489.4±224.9 μm. Postoperative CFT were 370.1±148.9 μm, 327.5±157.5 μm, 310.9±154.9 μm, and 274.4±135.3 μm at 1, 3, 6, and 12 months after the vitrectomy, respectively () Mean CFT decreased significantly 1 month after surgery, and postoperative CFT was significantly decreased from the baseline thickness at all times evaluated. In ischemic BRVO, preoperative CFT were 514.3±228.9 μm, with postoperative CFT of 378.0±151.8 μm, 329.3±160.8 μm, 314.7±158.8 μm, and 279.2±152.7 μm at 1, 3, 6, and 12 months after vitrectomy, respectively. Also, in non-ischemic BRVO, preoperative CFT was 352.4±166.6 μm, with postoperative CFT of 335.3±141.5 μm, 318.7±147.8 μm, 289.5±142.9 μm, and 269.5±142.8 μm at 1, 3, 6, and 12 months after vitrectomy, respectively (). Five eyes in ischemic BRVO patients had persistent or recurrent macular edema at 12 months postoperatively. One eye in a non-ischemic BRVO patient had persistent macular edema during the follow-up period.
Figure 2 Changes in CFT after surgery.
Abbreviations: CFT, central foveal thickness; SEM, standard error of the mean.
No serious intraoperative complications, such as retinal detachment or choroidal hemorrhage, occurred. No retinal detachment or endophthalmitis was seen, although vitreous hemorrhage (1.0%) and iris neovascularization (1.0%) were seen in one eye of an ischemic BRVO patient during the follow-up period.
Discussion
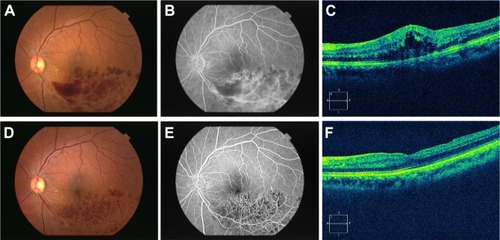
Our results demonstrate that MIVS with ILM peeling for BRVO effectively reduced macular edema and significantly improved visual acuity. Overall, macular edema was significantly decreased with the passage of time, and visual acuity continued to improve over the follow-up period compared with visual acuity prior to surgery. MIVS with ILM peeling was beneficial in terms of anatomic and functional effects in both ischemic and non-ischemic BRVO patients ().
Figure 3 Clinical findings before and after surgery.
In this study, 25-gauge instruments were used to minimize surgical-induced trauma.Citation16 Many vitreoretinal procedures that do not involve extensive intraocular tissue dissection, such as epiretinal membrane peeling and macular hole or macular edema surgery, are likely to benefit from a less invasive approach because much of the surgical trauma in those cases may be related to the conjunctival and scleral incisional procedures.Citation17 To increase the feasibility and safety of operations to treat macular edema, surgical invasiveness should be decreased. We used a MIVS operating system that allowed for self-sealing transconjunctival sclerotomies, which have been reported to be associated with decreased surgical time, less postoperative inflammation, and faster postoperative recovery.Citation9,Citation18,Citation19 The absence of intraoperative and postoperative complications suggests that this technique is safe and effective for treating macular edema. Therefore, this study indicates that MIVS is helpful in reducing macular edema associated with BRVO and for improvement of visual acuity. MIVS has the potential to increase treatment efficiency in these disorders by both hastening postoperative recovery and improving outcomes due to the simplified surgical procedure, which minimizes surgically induced trauma, and provides associated decreases in operating time, postoperative inflammatory response, and convalescence period.
Recently, VEGF has been shown to be a key molecule in the pathogenesis of macular edema secondary to BRVO.Citation20 Several studies revealed that the intraocular level of VEGF is significantly increased in eyes with BRVO and that its level is correlated with the severity of macular edema.Citation21,Citation22 Additionally, vitreous fluid levels of VEGF and other inflammatory factors are excessively increased in the eyes of ischemic BRVO patients.Citation23 Accordingly, anti-VEGF agents have been shown to be useful for patients with macular edema, and the intravitreal injection of anti-VEGF agents is fast becoming the standard treatment for macular edema secondary to BRVO.Citation15,Citation24,Citation25 However, for sustained effective treatment, multiple injections of the anti-VEGF agents are needed over time. Ischemic BRVO with larger hypoperfusion leads to higher levels of VEGF expression and thus results in many repeated injections of anti-VEGF agents. Such repeated interventions might lead to increased risk of severe complications such as endophthalmitis, retinal detachment, or cerebral infarction.Citation26,Citation27 On the other hand, vitrectomy can complete the treatment in just one procedure. A recent study has shown that clearance of VEGF is increased after vitrectomy, suggesting that vitrectomy may lead to resolution of macular edema by reducing the level of the intravitreal VEGF, especially in ischemic BRVO.Citation28 However, vitreous hemorrhage and iris neovascularization were seen in one eye of an ischemic BRVO patient during the follow-up period. A single vitrectomy might be inadequate in the case of severe ischemic BRVO, and peripheral scatter laser photocoagulation may be required. In addition, vitrectomy has some limitations. First, vitrectomy is an unsuitable procedure to be done on an outpatient basis. Second, vitrectomy requires the surgeon to be skilled in advanced techniques compared to anti-VEGF therapy or laser photocoagulation.
ILM peeling was performed in all cases and this procedure has been reported to release tangential traction, which helps to resolve macular edema.Citation10,Citation29 ILM removal has also been reported to facilitate the diffusion of macromolecules, including mediators such as VEGF, from the retina to the vitreous cavity.Citation30 Furthermore, removing the ILM at the time of vitrectomy may reduce the risk of postoperative epiretinal membrane proliferation. Through these mechanisms, vitrectomy with ILM peeling could achieve long-term resolution of macular edema associated with BRVO. Meanwhile, our findings showed that reduction of macular edema and the subsequent BCVA improvement was sustained for at least 12 months after the vitrectomy.
To our knowledge, although several studies have reported outcomes of vitrectomy for BRVO, only one study mentioned the severity of the ischemia; however, the sample size was very small. Raszewska-Steglinska et al reported outcomes of vitrectomy with ILM peeling in eleven ischemic BRVO eyes and three non-ischemic BRVO eyes.Citation11 As far as studies on MIVS that have included 23-, 25-, or 27-gauge vitrectomy for BRVO, none report whether the BRVO was ischemic or non-ischemic. The current study found that MIVS effectively reduced macular edema and improved visual acuity in both ischemic and non-ischemic BRVO.
In this study, the percentage of patients with Snellen equivalent BCVA ≤20/40 at baseline and ≥20/40 at 12 months after the vitrectomy was 63.4% in all cases. A previous study has reported that the percentage of patients with Snellen equivalent BCVA >20/40 in natural course was 41.7% at 6 months postoperatively.Citation24 The higher percentage of patients with improving BCVA in our study may provide a backing for the efficacy of MIVS with ILM peeling for BRVO.
There are some limitations to our study. First, this study was a retrospective, non-randomized, single-center study. Thus, we cannot eliminate the possibility that there may have been a bias in the choice of patients. Secondly, combined cataract surgery might affect visual outcomes, although patients with moderate or severe cataracts that could have caused vision decrease were excluded. Thirdly, a control group managed with laser or observation was absent.
Conclusion
In conclusion, the present study suggests that MIVS with ILM peeling for ischemic or non-ischemic BRVO is effective in improving visual acuity and foveal morphology and, moreover, results in few recurrences of macular edema. A large, randomized study with a control group and long observation period is necessary in order to prove the effectiveness of MIVS for BRVO.
Acknowledgments
We are deeply grateful to Professor Kamei, whose enormous support and insightful comments were invaluable during the course of our study. We would also like to express gratitude to the staff of our hospitals for their support and warm encouragement.
Disclosure
The authors report no conflicts of interest in this work.
References
- GreerDVConstableIJCooperRLMacular oedema and retinal branch vein occlusionAust J Ophthalmol1980832072097425970
- FinkelsteinDIschemic macular edema. Recognition and favorable natural history in branch vein occlusionArch Ophthalmol199211010142714341417544
- GutmanFAZegarraHThe natural course of temporal retinal branch vein occlusionTrans Am Acad Ophthalmol Otolaryngol1974782OP178OP1924825043
- Argon laser photocoagulation for macular edema in branch vein occlusionThe Branch Vein Occlusion Study GroupAm J Ophthalmol19849832712826383055
- TrempeCLTakahashiMTopilowHWVitreous changes in retinal branch vein occlusionOphthalmology19818876816877267038
- KadoMTrempeCLRole of the vitreous in branch retinal vein occlusionAm J Ophthalmol1988105120242447780
- AvundukAMCetinkayaKKapicioğluZKayaCThe effect of posterior vitreous detachment on the prognosis of branch retinal vein occlusionActa Ophthalmol Scand19977544414429374257
- StefánssonEThe therapeutic effects of retinal laser treatment and vitrectomy. A theory based on oxygen and vascular physiologyActa Ophthalmol Scand200179543544011594975
- ParkJCRamasamyBShawSLingRHPrasadSA prospective and nationwide study investigating endophthalmitis following pars plana vitrectomy: clinical presentation, microbiology, management and outcomeBr J Ophthalmol20149881080108624686917
- KumagaiKFurukawaMOginoNLarsonEUemuraALong-term visual outcomes after vitrectomy for macular edema with foveal hemorrhage in branch retinal vein occlusionRetina200727558458817558320
- Raszewska-SteglinskaMGozdekPCisieckiSMichalewskaZMichalewskiJNawrockiJPars plana vitrectomy with ILM peeling for macular edema secondary to retinal vein occlusionEur J Ophthalmol20091961055106219882567
- SatoTSawadaKIwahashi-ShimaCBandoHIkedaTEmiK25-gauge vitrectomy versus intravitreal bevacizumab for macular edema secondary to branch retinal vein occlusion: 1 year follow-upAnn Acad Med Singapore201241729429922892606
- NomaHShimadaKMimuraTVisual function after pars plana vitrectomy in macular edema with branch retinal vein occlusionInt Ophthalmol201333322723623160823
- [No authors listed] Baseline and early natural history reportThe Central Vein Occlusion StudyArch Ophthalmol19931118108710957688950
- HeierJSCampochiaroPAYauLRanibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trialOphthalmology2012119480280922301066
- FujiiGYDe JuanEJrHumayunMSA new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgeryOphthalmology20021091018071812 discussion 181312359598
- ZhangZHLiuHYWimpissingerBAvitabileTXuXLiuKTransconjunctival sutureless vitrectomy versus 20-gauge vitrectomy for vitreoretinal surgery: a meta-analysis of randomized controlled trialsGraefes Arch Clin Exp Ophthalmol2013251368168822695935
- IbarraMSHermelMPrennerJLHassanTSLonger-term outcomes of transconjunctival sutureless 25-gauge vitrectomyAm J Ophthalmol2005139583183615860288
- ShimadaHNakashizukaHMoriRMizutaniYHattoriT25-gauge scleral tunnel transconjunctival vitrectomyAm J Ophthalmol2006142587187317056376
- NomaHMimuraTEguchiSAssociation of inflammatory factors with macular edema in branch retinal vein occlusionJAMA Ophthalmol2013131216016523411880
- NomaHFunatsuHYamasakiMPathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6Am J Ophthalmol2005140225626116086947
- NomaHMinamotoAFunatsuHIntravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusionGraefes Arch Clin Exp Ophthalmol2006244330931516133018
- NomaHFunatsuHYamasakiMAqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusionEye (Lond)2008221424816826241
- CampochiaroPAHeierJSFeinerLBRAVO InvestigatorsRanibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III studyOphthalmology2010117611021112.e120398941
- MitryDBunceCCharterisDAnti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusionCochrane Database Syst Rev20131CD00951023440840
- TanoYOhjiMEXTEND-I Study GroupLong-term efficacy and safety of ranibizumab administered pro re nata in Japanese patients with neovascular age-related macular degeneration in the EXTEND-I studyActa Ophthalmol201189320821721232078
- BresslerNMBoyerDSWilliamsDFCerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trialsRetina20123291821182823011184
- NomaHFunatsuHMimuraTEguchiSShimadaKVisual prognosis and vitreous molecules after vitrectomy for macular edema with branch retinal vein occlusionClin Ophthalmol2011522322921386915
- MaJYaoKZhangZTangX25-gauge vitrectomy and triamcinolone acetonide-assisted internal limiting membrane peeling for chronic cystoid macular edema associated with branch retinal vein occlusionRetina200828794795618698296
- JosifovaTSchneiderUHenrichPBSchraderWEye disorders in diabetes: potential drug targetsInfect Disord Drug Targets200882707518537702
