Abstract
Purpose
This study aimed to determine whether switching from balanced salt solution (BSS) to vancomycin 20 g/mL BSS for incision hydration and eye pressurization reduces the rate of postcataract endophthalmitis.
Methods
This was a patient safety/quality improvement project, including all patients undergoing cataract surgery at the Kaiser Permanente Colorado Ophthalmology Department from January 2002 to December 2014. Throughout the study, patients received vancomycin 20 μg/mL in the irrigating solution. During the baseline period from 2002 to 2005, surgeons pressurized eyes and hydrated incisions with plain BSS. During the intervention period from 2006 through 2014, surgeons switched from BSS to the vancomycin/BSS irrigating solution for eye pressurization and incision hydration.
Results
A total of 57,263 cataract operations were performed by 24 surgeons at seven surgical centers: 12,400 in the baseline period and 44,863 in the intervention period. The rate of post-cataract endophthalmitis declined significantly from 5/12,400 (rate: 0.4/1,000) in the baseline period to 1/44,863 (rate: 0.022/1,000) during the intervention period (odds ratio [OR]: 18.1, 95% confidence interval [CI]: 2.11–154.9; χ2=13.5, P=0.00024). Accounting for an estimated 2.05-fold risk reduction due to confounding variables, the risk reduction attributed to the intervention remained significant: (adjusted OR: 8.78, 95% CI: 1.73–44.5; χ2=10.06, P=0.0015). Since 2009, we have not experienced any cases of postcataract endophthalmitis after 32,753 operations.
Conclusion
We experienced a significant reduction in postcataract endophthalmitis when we switched from BSS to the vancomycin/BSS irrigating solution for incision hydration and eye pressurization. The pharmacokinetics profile indicates that this switch was important for effective prophylaxis.
Introduction
Postcataract endophthalmitis is an uncommon, potentially devastating complication of cataract surgery. In the Endophthalmitis Vitrectomy Study, 31% of patients had visual acuity 20/200 or worse at 3 months after diagnosis.Citation1 The most recently reported rate of postcataract endophthalmitis among US Medicare members was 1.11/1,000 in 2004.Citation2 Because US surgeons operate approximately 3.5 million cataract cases annually,Citation3,Citation4 there are approximately 3,885 endophthalmitis cases, causing severe, permanent iatrogenic vision loss in approximately 1,200 eyes each year. Internationally, the rate is as much as ten times higher.Citation5
Risk factors for endophthalmitis include age >85 years,Citation2 posterior capsule rupture,Citation6 clear corneal incisions (particularly when they leak on postoperative day 1),Citation6,Citation7 the use of silicone (rather than acrylic or polymethyl methacrylate) intraocular lenses (IOLs),Citation6 low surgeon caseload,Citation2 the failure to use povidone–iodine on the ocular surface, in addition to the povidone–iodine skin preparation,Citation8 and the failure to use intracameral antibiotics.Citation6,Citation9–Citation12 This study addresses the questions of safety and efficacy of vancomycin irrigation for endophthalmitis prophylaxis, while accounting for the confounders of declining intraoperative complication rates, declining silicone IOL use, and increasing surgeon caseloads in Kaiser Permanente Colorado Ophthalmology Department.
Vancomycin is the most widely used intracameral antibiotic in the US.Citation13 According to the 2012 Annual Survey of United States members of the American Society of Cataract and Refractive Surgery, approximately 50% of cataract cases were operated using intracameral antibiotics, evenly split between irrigation and injection.Citation13 In these cases, 56% of surgeons used vancomycin (approximately 980,000 cases, assuming the survey results can be extrapolated nationwide), while 31% used moxifloxacin and 13% used cefuroxime.Citation13 Although cefuroxime has been packaged and approved for intracameral use in Europe (Aprokam, Thea, Clermont-Ferrand, France), no intracameral antibiotic has been packaged or approved in the US.
Since 2002, every cataract patient in the Kaiser Permanente Colorado Ophthalmology Department has received vancomycin 20 μg/mL in the irrigation fluid.Citation14 When we diagnosed four cases of postcataract endophthalmitis in 2005, we reevaluated our protocols, with particular attention to the pharmacokinetics of vancomycin prophylaxis.
Pharmacokinetics
Contamination of the anterior chamber is common after cataract surgery, although the colony counts are low (20–60 colony forming units [CFU]/mL).Citation15 The most common postcataract endophthalmitis pathogen is coagulase-negative Staphylococcus.Citation16,Citation17 Vancomycin’s mean inhibitory concentration for 90% of Staphylococcus endophthalmitis pathogens (MIC90) was 3–4 μg/mL in two recent series.Citation18,Citation19 Vancomycin is a slow killer, requiring 4.6 hours to reduce Staphylococcus epidermidis colony counts by a factor of 100 under ideal growth conditions.Citation20 Mutant, highly resistant, slime-forming Staphylococcus epidermidis has a 1-hour delay in killing, while vancomycin dislodges the bacteria from the lens implant, particularly from a silicone lens implant.Citation21 This may partially explain the increased risk of endophthalmitis with silicone lens implants.Citation6 It is not surprising that vancomycin failed to sterilize aqueous in experiments lasting up to 120 minutes:Citation15,Citation22–Citation24 the contact time was shorter than vancomycin’s known kill time.Citation20,Citation21,Citation25
If surgeons hydrate the incisions and pressurize eyes with the irrigating solution, the vancomycin concentration at the end of surgery is 20 μg/mL. After surgery, the vancomycin concentration drops through two-step kinetics: the half-life is 1.80 hours to 1.87 hours in the first 2 hours,Citation15,Citation22 then dilution slows, with a half-life of 3.27 hours for the next 16 hours.Citation26 On the basis of these measurements, it takes approximately 6.45 hours for the vancomycin concentration to drop from 20 μg/mL to the MIC90 of 4 μg/mL.
However, if surgeons hydrate incisions and pressurize eyes with plain balanced salt solution (BSS), some of the vancomycin is washed out of the eye.Citation27 In a recent series of ten of our cataract cases, these steps required an added volume Va of 0.6–1.5 mL (mean: 1.05 mL). The final concentration Cf of a diluted solution is Cf = Ci(Vi/[Vi + Va]), where Vi is the initial volume (0.54 mL for the anterior chamber of the pseudophakic eye) and Ci is the initial concentration.Citation28 The concentration of vancomycin drops from 20 μg/mL to 5.2–9.7 μg/mL immediately after hydration and pressurization with plain BSS. After surgery, the concentration drops further, falling below the MIC90 of 4 μg/mL in 52 minutes to 181 minutes, which is probably insufficient for complete sterilization of the aqueous.Citation15,Citation20–Citation25
Our analysis indicates that the choice of fluid used for incision hydration and eye pressurization is an important factor in the efficacy of endophthalmitis prophylaxis with vancomycin irrigation. If these steps are performed with the vancomycin/BSS irrigating solution, the vancomycin concentration is predicted to exceed the MIC90 for a sufficient time for effective endophthalmitis prophylaxis. However, if these steps are performed with plain BSS, the vancomycin concentration could decrease below the MIC90 before the effective kill time, and prophylaxis failures could occur. In early 2006, we switched from using BSS to using the vancomycin/BSS irrigating solution for incision hydration and eye pressurization, as recommended by others.Citation15,Citation29
Methods
This was a patient safety/quality improvement project. It was a prospective, interventional case series of all cataract operations in the Kaiser Permanente Colorado Ophthalmology Department from January 2002 to December 2014. Kaiser Permanente is a prepaid, community-based, health maintenance organization. Our Institutional Review Board reviewed the methods and waived the approval requirements, determining that this quality improvement project was not human subject research.
Since 2002, all cataract surgeries in our department have been performed using vancomycin in the irrigating solution (BSS, Alcon, Fort Worth, TX, USA). Compounding is done at our in-house, licensed compounding pharmacies, which also compound our bevacizumab for intravitreal injection and systemic chemotherapy agents. In brief, vancomycin powder (Hospira, Inc, Lake Forest, IL, USA), is reconstituted per manufacturer instructions to 50 mg/mL with sterile water for injection in a laminar flow hood. It is then diluted 5:1 in normal saline and divided in a sterile manner into single-use syringes containing 10 mg vancomycin/1.0 mL normal saline. Each lot is tested for sterility by incubation for 14 days, using positive controls. Compounding costs for drug acquisition and technician salary and benefits are <$2.00 per dose. The vancomycin mixture is injected by the circulating nurse into the 500 mL BSS bottle immediately before each surgical case, giving a final concentration of 20 μg/mL.Citation14 Most patients receive a preoperative cellulose pledget (Weck-Cel, Becton Dick-inson, East Rutherford, NJ, USA) soaked with equal parts current-generation fluoroquinolone antibiotics, cyclopentolate 1%, phenylephrine 10%, and diclofenac 1% in the conjunctival cul-de-sac 45 minutes before surgery.Citation29 In the operating room, all patients receive povidone/iodine 5% (Betadine, Alcon) preparation on the eyelid skin, eyelashes, and ocular surface before surgery.Citation8
Throughout the study, at the end of surgery, all eyes were pressurized to a physiologic intraocular pressure, and incisions were hydrated. Each incision was carefully checked for aqueous leakage, either with a cellulose surgical spear or Seidel tested with fluorescein or Betadine solution. Leaky incisions were rehydrated or sutured. During the baseline period from 2002 to 2005 (baseline period), surgeons pressurized eyes and hydrated incisions with plain BSS. Starting in early 2006 (the intervention period), surgeons switched from using plain BSS to using the vancomycin/BSS irrigating solution for eye pressurization and incision hydration. We instructed the scrub technicians to draw up all syringes for these steps from the irrigating fluid. In our operating rooms, there are no syringes with plain BSS on the instrument table, eliminating the possibility of an incorrect syringe being passed to the surgeon.
Outpatient topical antibiotics were prescribed by every surgeon throughout the study. From 2002 to 2012, the majority of surgeons prescribed current-generation fluoroquinolones, while others prescribed tobramycin or neomycin/polymyxin/dexamethasone. In late 2012, we noted reports of increasing fluoroquinolone resistance by endophthalmitis isolates,Citation18,Citation30 but good coverage of Gram-positive eye infection isolates with trimethoprim.Citation31 In early 2013, we uniformly switched outpatient antibiotics to polymyxin B/trimethoprim drops four times daily, starting the day before surgery and continuing for a week after surgery.
Our retinal surgeons reported endophthalmitis cases (pain, erythema, corneal edema, decreased vision, and panuveitis) to our Infection Control Department. Suspected endophthal-mitis patients received vitreous and aqueous taps for cultures, as well as intravitreal antibiotic injections (vancomycin 1 mg/0.1 mL and ceftazidime 2.25 mg/0.1 mL or amikacin 400 μg/0.1 mL). Patients with light perception or worse visual acuity typically underwent pars plana vitrectomy, per the Endophthalmitis Vitrectomy Study protocol.Citation1
Starting in 2005, we queried our electronic health record (HealthConnect, Epic, Madison, WI, USA) to ascertain every case of endophthalmitis (International Classification of Diseases, Ninth Edition, ICD-9, 360.0×), regardless of cause. Both authors reviewed each case of endophthalmitis, and those occurring within 6 weeks of surgery, in the operated eye, without other intraocular procedures, trauma, or pathology, were classified as acute postcataract endophthalmitis. The presence or absence of intraoperative complications, including posterior capsule tear and vitreous loss, were recorded by surgeons in the electronic record for every case, as was the lens implant manufacturer, model, and power. Each chart was electronically audited for completeness, and surgeons were contacted to complete any missing data.
Odds ratios (ORs), confidence intervals (CIs), and χ2 tests for statistical significance (P<0.05) were computed from standard formulas,Citation32 using Microsoft Excel 2013 (Redmond, WA, USA). We estimated the confounding effects of our changing incidence of three risk factors for endophthalmitis: communication with the vitreous (posterior capsule tear and/or vitreous loss),Citation6 surgeon caseload,Citation2 and the use of silicone IOLs.Citation6 We multiplied the published ORs for endophthalmitis (approximately equal to relative risk [RR] for rare events in large samples),Citation32 for each risk factor by the change in incidence of the risk factor, to develop weighted ORs. We multiplied the three weighted ORs to estimate the reduction in endophthalmitis due to risk factor modification. Then we estimated the effect of the intervention, without the confounding risk factor modifications, by dividing the raw ORs by the OR estimated for risk factor modification.
Results
From 2002 to 2014, 24 surgeons operated on 57,263 cataracts at seven surgical venues. The rate of posterior capsule rupture declined from 1.9% to 0.9%, surgeons’ cataract caseload increased by a factor of 2.67, and the use of silicone IOLs decreased from approximately 60% to 2%. Six cases of postcataract endophthalmitis occurred (). gives the details of the cases. One case of postcataract endophthalmitis was reported in 2003, with limited details. One case of chronic uveitis, operated in 2003, which resolved after pars plana vitrectomy, lensectomy, and removal of capsule plaques, and which grew Propionibacterium acnes in 2005, was classified as chronic postcataract endophthalmitis. Three of six cases occurred after operations complicated by posterior capsule tear and/or vitreous loss.
Figure 1 Endophthalmitis cases during the study period.
Abbreviation: BSS, balanced salt solution.
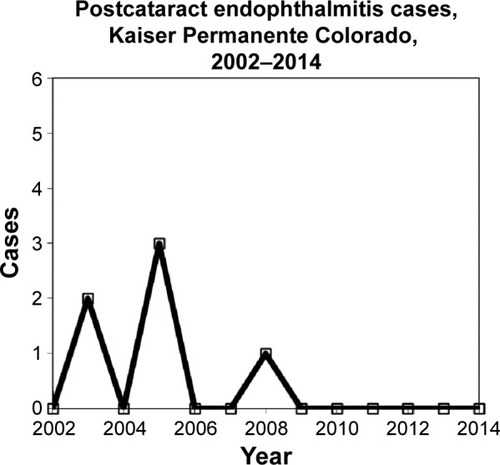
Table 1 Details of endophthalmitis cases
summarizes the rates and statistics. From 2002 to 2005 (baseline), there were five cases of postcataract endophthalmitis after 12,400 cataract operations. From 2006 through 2014 (intervention), we experienced one case of endophthalmitis after 44,863 cataract operations. The rate of postcataract endophthalmitis was significantly higher in the baseline period (0.40/1,000) than in the intervention period (0.022/1,000) (raw OR: 18.1, 95% CI: 2.11–154.9; χ2=13.5, P=0.00024). Since 2009, we have experienced no cases of endophthalmitis after 32,753 cataract operations. After switching from majority use of fourth-generation fluoroquinolone outpatient antibiotics to uniform use of polymyxin B/trimethoprim in early 2013, we continued to have no postcataract endophthalmitis for the following 13,205 cases.
Table 2 Cataract cases and endophthalmitis rates for the periods 2002–2005 and 2006–2014
summarizes the estimated effects of the following known confounders: decreasing use of silicone IOLs, decreasing communication with the vitreous, and increasing surgeon caseload. The confounders are estimated to reduce the endophthalmitis rate by a factor of 2.05, in total. Accounting for the confounders, the estimated effect of the intervention remained significant (adjusted OR: 8.78, 95% CI: 1.73–44.5; χ2=10.06, P=0.0015).
Table 3 Estimated endophthalmitis risk reductions due to confounding variables
Discussion
We experienced a significant reduction in postcataract endophthalmitis after we switched from plain BSS to the vancomycin/BSS irrigation fluid for eye pressurization and incision hydration, while maintaining our standard infection control practices. This may be the first study to show a statistically significant decrease in postcataract endophthalmitis with vancomycin irrigation.Citation14,Citation27,Citation33 Our experience of absence of endophthalmitis after 32,753 cataract operations from 2009 through 2014 is, to our knowledge, the longest, largest, multisurgeon, multivenue series of its kind.
The pharmacokinetic analysis indicates that switching from plain BSS to vancomycin/BSS for irrigation, pressurization, and incision hydration maintains the vancomycin concentration greater than the MIC90 for >6 hours. This switch may be necessary for effective endophthalmitis prophylaxis using vancomycin irrigation.Citation20,Citation21,Citation25 Other authors draw opposite conclusions about the sufficiency of the vancomycin dwell time, based on the rate of aqueous turnover.Citation34 But our calculations are based on published measurements of vancomycin concentrations versus time in postoperative cataract patients,Citation15,Citation22,Citation26 as well as published kill times for Staphylococci,Citation20,Citation21,Citation26 enhancing their validity. Experimental studies failing to show sterilization of aqueous after exposure to vancomycin for 30 minutes to 120 minutesCitation15,Citation22–Citation24 probably suffered from inadequate time for vancomycin to kill pathogens.Citation20,Citation21,Citation25 Other studies failing to show a significant decrease in postcataract endophthalmitis with vancomycin irrigationCitation27,Citation33 probably suffered from flaws in technique: hydrating incisions and pressurizing eyes with plain BSS instead of the vancomycin/BSS irrigating solution.Citation27
Our study has additional strengths, which enhance the accuracy of discernment of endophthalmitis cases. Using an electronic health record probably reduced recall bias, because all cases of endophthalmitis were reviewed, regardless of cause. Mandatory surgeon reporting of the presence or absence of complications on every case also probably decreased recall bias. Our health system is a prepaid, closed network, so it is unlikely that patients would obtain care for endophthalmitis outside our network, without outside providers filing claims with diagnoses for reimbursement. This is expected to reduce ascertainment bias.
Injecting compounded vancomycin, typically 1 mg/0.1 mL, directly into the anterior chamber at the end of surgery would give a sufficient concentration to kill nearly all Gram-positive endophthalmitis pathogens.Citation12,Citation27,Citation29,Citation33 The high initial concentration provides an extended time above the MIC90,Citation26 potentially sterilizing late contamination from wound leaks, blunting an important risk factor for endophthalmitis.Citation7 But in agreement with US and European surgeons,Citation35,Citation36 the majority of surgeons in our Department have been reluctant to inject compounded intracameral antibiotics, citing the possibilities of concentration errors,Citation37–Citation40 pathogenic contamination,Citation41 and toxic anterior segment syndrome.Citation42–Citation44 Mixing compounded vancomycin into the BSS bottle dilutes its concentration (and any contaminants, toxins, and formulation errors) 500:1, potentially minimizing risk compared to direct injections. For example, contamination with 10,000 CFU/mL of pathogens in the compounded vancomycin would be diluted 500:1 in the irrigating bottle, to 20 CFU/mL (the lower end of normal intraoperative contamination).Citation15 An osmolarity error of 100 mOsm/mL would be diluted 500:1 in the irrigating bottle to nearly physiologic 300.6 mOsm/mL. A five-fold concentration in mixing error, eg, by omitting the second dilution step,Citation37,Citation40 giving a concentration of 50 mg/mL, would be diluted 500:1 in the irrigating bottle, to 100 μg/mL. However, both this and higher concentrations have proven safe in thousands of operations.Citation12,Citation27,Citation29 Errors of pH in compounding, or contamination with detergents or bacterial toxins, which are risk factors for toxic anterior segment syndrome,Citation42–Citation44 would be diluted 500:1 in the irrigating bottle, reducing the risk. We strongly believe that intracameral antibiotics should be prepared in a licensed, inspected compounding pharmacy, rather than by the staff of the operating room. However, surgeons at centers without access to such compounding pharmacies may wish to consider vancomycin irrigation over direct injection of compounded drugs for these safety reasons.
Vancomycin offers additional significant efficacy and safety advantages relative to other intracameral antibiotics. Gram-positive endophthalmitis pathogens have not developed resistance to vancomycin,Citation16–Citation19 although they have developed resistance to moxifloxacinCitation18,Citation30 and cefuroxime.Citation10,Citation19 Recent reports of life-threatening anaphylaxis with intracameral cefuroxime are concerning.Citation45,Citation46 Some centers using intracameral cefuroxime are forced to perform skin prick testing, change antibiotics, or pretreat patients having beta-lactam allergies with antihistamines.Citation47 We are unaware of such severe side effects with intracameral vancomycin. We use vancomycin irrigation, pressurization, and hydration in every cataract patient and do not pretreat any patients with antihistamines. In agreement with others’ results,Citation48 we have not noted an increase in cystoid macular edema with vancomycin irrigation, although it was noted in a teaching hospital with prolonged cases.Citation49
Three percent to 8% of endophthalmitis cases are caused by Gram-negative bacteria,Citation16,Citation17 but we have never experienced a case of Gram-negative postcataract endophthalmitis. We hypothesize that povidone–iodine antisepsis, the fluoroquinolone-soaked topical pledgets, and topical antibiotic drops have been sufficient to eliminate Gram-negative bacteria in our center. Gram-negative endophthalmitis isolates have not developed resistance to fluoroquinolones or trimethoprim–sulfamethoxazole.Citation17
The American Academy of Ophthalmology and the Centers for Disease Control and Prevention have urged caution in using vancomycin irrigation in the hospital setting, citing the possibility of inducing vancomycin resistance.Citation50 However, fears of inducing vancomycin resistance in the population from use during outpatient eye surgery may be unfounded.Citation25,Citation51 With irrigation, if the 10 μg dose of vancomycin in the anterior chamber were absorbed systemically, the serum concentration would be 10 μg/3,500 mL or 2.9 ng/mL. With an intracameral injection of 1 mg, the serum vancomycin concentration would be 290 ng/mL. These concentrations are almost certainly insufficient to induce systemic resistance.Citation52 Measurements of antimicrobial susceptibilities of viridans group Streptococci isolated from the conjunctiva, nasopharynx, and oropharynx of patients did not show resistance to vancomycin after cataract surgery with vancomycin irrigation.Citation53 Finally, some argue that prophylaxis should use a drug with a different mechanism of action than the drug used for treatment, to avoid treatment failures if endophthalmitis cases occur.Citation34 However, if vancomycin prophylaxis essentially eradicates postcataract endophthalmitis (one case after 44,863 operations in our intervention period), the effect of a hypothetical treatment failure with vancomycin in a case of endophthalmitis becomes statistically small.
Some question the financial value of intracameral antibiotics.Citation34,Citation43 However, a detailed cost–benefit study showed an 18-fold greater value of intracameral cefuroxime prophylaxis versus that with topical fourth-generation fluoroquinolone antibiotics.Citation54 Our internal compounding pharmacy’s cost of <$2.00 per dose for vancomycin drug acquisition and pharmacy technician salary and benefits, as well as the retail price of compounded vancomycin (Leiter’s Pharmacy, San Jose, CA, USA), compare favorably with the average acquisition price of $102.81–$124.84 for topical fourth-generation fluoroquinolones.Citation55
A randomized clinical trial with thousands of patients, such as the European Society of Cataract and Refractive Surgeons endophthalmitis trial,Citation9 would be the most definitive tool to determine whether irrigation, pressurization, and hydration with the vancomycin/BSS irrigating fluid significantly reduces postcataract endophthalmitis. However, such a study was far beyond the scope of this quality improvement project. Compared to a randomized clinical trial, any patient safety/quality improvement project such as this interventional case series is inherently limited by the lack of a control group and the effects of confounding variables. Our decreasing posterior capsule rupture and vitreous loss rates,Citation6,Citation10 our increasing surgical volume,Citation2,Citation5 and our switch from silicone to acrylic lens implantsCitation6,Citation9,Citation21 probably contributed to our drop in the endophthalmitis rate.
We performed a sensitivity analysis to quantify the effects of these confounders. summarizes these effects. A recent meta-analysis of over six million cataract cases quantified the RR and ORs of several risk factors.Citation6 Posterior capsule tear/vitreous loss is associated with a 6.5-fold increase in endophthalmitis.Citation6 Our 1.0% reduction in capsule tears and/or vitreous loss, from 1.9% to 0.9% of cases, would be expected to reduce our endophthalmitis rate by a factor of 1+ (6.5×0.01) =1.065. Silicone IOLs are associated with a 3.0-fold increased risk for endophthalmitis versus polymethyl methacrylate or acrylic lenses.Citation6 Our 58% reduction in the use of silicone lenses, from 60% of cases to 2% of cases, would be expected to reduce our endophthalmitis rate by a factor of 3.0×0.58=1.74. Low annual surgical caseload has been associated with an increasing endophthalmitis rate, with an RR of 1.27 per 225 cases.Citation2 Our mean surgical volume increased by 88 cases, from 221 in the baseline period to 309 in the intervention period. This would be expected to reduce our endophthalmitis rate by a factor of 1+ (0.27×[88/225]) =1.106. Some confounders are correlated: compared to surgeons with low annual caseloads, surgeons with high caseloads have lower capsule tear rates,Citation56 and they use intracameral antibiotics more frequently.Citation13 Nonetheless, we multiplied the effects of the three expected confounders, to give their maximum, total estimated effect on our endophthalmitis rate: 1.74×1.065×1.106=2.05. We might expect as much as a 2.05-fold reduction in endophthalmitis due to these confounders. Clearly, these confounders cannot explain the 18-fold decrease in the endophthalmitis rate we observed. After accounting for the estimated effect of the three confounders, the estimated adjusted effect of the intervention remained statistically significant.
Future research could further enhance our understanding of postcataract endophthalmitis. National endophthalmitis registries, as in SwedenCitation10 and Taiwan,Citation5 indicate trends in rates, with large enough sample sizes to ensure statistical and clinical significance. Annual reporting of the US Medicare endophthalmitis rate would help American surgeons compare their rates to international rates and trends. Large health care systems, such as Kaiser PermanenteCitation11 and the United States Veterans Administration,Citation57 could establish uniform standards for prophylaxis and monitor known risk factors such as posterior capsule rupture and IOL type. Then, they could report endophthalmitis rates as interventions are made, providing prospective evidence for their efficacy. Studies showing resistance rates of endophthalmitis pathogens should give the MIC90 values,Citation18,Citation19 together with percentage resistance,Citation16,Citation17,Citation30 because topical and intracameral antibiotics attain much higher concentrations than the break points for systemic antibiotics.Citation31 When intracameral antibiotics are used, the added risk reduction due to topical antibiotics is probably small.Citation9–Citation11 Because US surgeons commonly use fourth-generation fluoroquinolones for topical endophthalmitis prophylaxis,Citation13 emerging resistance to these agents should continue to be monitored.Citation18,Citation30 Surgeons concerned about emerging fluoroquinolone resistance may choose to switch to more effective, older-generation antibiotics, such as trimethoprim–polymyxin B,Citation31 as we did.
In summary, we experienced a significant reduction in postcataract endophthalmitis when we switched from plain BSS to the vancomycin/BSS irrigating solution for hydrating incisions and pressurizing eyes, while maintaining our standard prophylactic measures of betadine antisepsis of the eyelids and ocular surface, antibiotic-soaked pledgets, topical outpatient antibiotics, and vancomycin irrigation. The pharmacokinetics profile indicates that the switch in fluids for hydration and irrigation was necessary for effective endophthalmitis prophylaxis with vancomycin irrigation. Diluting compounded vancomycin in the irrigating bottle may offer significant safety advantages relative to direct intracameral injection of compounded drugs. Switching from majority use of fourth-generation fluoroquinolone outpatient topical antibiotics to uniform use of polymyxin B/trimethoprim was not associated with any cases of endophthalmitis. Cataract surgeons who irrigate with vancomycin should consider hydrating incisions and pressurizing eyes with the irrigating solution, instead of plain BSS.
Author contributions
LPS reviewed literature, developed intervention protocols, reviewed the data, performed data analysis, drafted the manuscript and edited the final copy. MAS performed investigation of cases before baseline, developed intervention protocols, trained staff in interventions, extracted and reviewed data, edited the draft manuscript, and approved the final copy.
Based on these contributions, both authors comply with the guidelines for authorship.
Acknowledgments
The authors thank Robin L Meinberg, Department of Infection Control, Kaiser Permanente Colorado Region, for her expert data collection. Salary support for the individuals performing this project (including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript) was provided by the Colorado Permanente Medical Group and the Kaiser Permanente Colorado Region. This work was presented in part at the European Ophthalmology Society Annual Meeting, Copenhagen, Denmark, June 2013.
Disclosure
The authors report no conflicts of interest in this work.
References
- Endophthalmitis Vitrectomy Study GroupResults of the endophthalmitis vitrectomy studyArch Ophthalmol199511312147914967487614
- KeayLGowerEWCassardSDTielschJMScheinODPostcataract surgery endophthalmitis in the United States: analysis of the complete 2003 to 2004 Medicare database of cataract surgeriesOphthalmology2012119591492222297029
- GolloglyHEHodgeDOSt SauverJLErieJCIncreasing incidence of cataract surgery: population-based studyJ Cataract Refract Surg20133991383138923820302
- United States Census DepartmentUS and World Population Clock2015 Available from: www.census.gov/popclockAccessed January 16, 2015
- FangYTChienLNNgYYAssociation of hospital and surgeon operation volume with the incidence of postoperative endophthalmitis: Taiwan experienceEye (Lond)200620890090716113636
- CaoHZhangLLiLLoSRisk factors for acute endophthalmitis following cataract surgery: a systematic review and meta-analysisPLoS One201388e7173123990980
- WallinTParkerJJinYKefalopoulosGOlsonRJCohort study of 27 cases of endophthalmitis at a single institutionJ Cataract Refract Surg200531473574115899450
- SpeakerMGMenikoffJAProphylaxis of endophthalmitis with topical povidone-iodineOphthalmology19919812176917751775308
- ESCRS Endophthalmitis Study GroupProphylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factorsJ Cataract Refract Surg200733697898817531690
- FrilingELundstromMSteneviUMontanPSix-year incidence of endophthalmitis after cataract surgery: Swedish national studyJ Cataract Refract Surg2013391152123245359
- ShorsteinNHWinthropKLHerrintonLJDecreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye departmentJ Cataract Refract Surg201339181423036356
- AnijeetDPalimarPPeckarCIntracameral vancomycin following cataract surgery: an eleven-year studyClin Ophthalmol2010432132620463800
- LeamingDResults of the 2012 ASCRS Cataract Surgery Survey2015 Available from: www.analeyz.com/NEWAnaleyz%20ASCRS%202012.htmAccessed February 6, 2015
- GillsJPFilters and antibiotics in irrigating solution for cataract surgery (Letter)J Cataract Refract Surg19911753851861260
- SotoAMMendivilMPThe effect of topical povidone-iodine, intraocular vancomycin, or both on aqueous humor cultures at the time of cataract surgeryAm J Ophthalmol2001131329330011239859
- HanDPWisniewskiSRWilsonLAEndophthalmitis Vitrectomy Study GroupSpectrum and susceptibilities of microbiologic isolates in the endophthalmitis vitrectomy studyAm J Ophthalmol199612211178659579
- RecchiaFMBusbeeBGPearlmanRBCarvalho-RecchiaCAHoAHChanging trends in the microbiologic aspects of postcataract endophthalmitisArch Ophthalmol2005123334134615767476
- HarperTMillerDFlynnHWJrIn vitro efficacy and pharmacodynamic indices for antibiotics against coagulase-negative Staphylococcus endophthalmitis isolatesOphthalmology2007114587187517383732
- SealDReischlUBehrAESCRS Endophthalmitis Study GroupESCRS Endophthalmitis Study Group. Laboratory diagnosis of endophthalmitis: comparison of microbiology and molecular methods in the European Society of Cataract and Refractive Surgeons multi-center study and susceptibility testingJ Cataract Refract Surg20083491439145018721702
- LowdinEOdenholtICarsOIn vitro studies of pharmacodynamic properties of vancomycin against Staphylococcus aureus and Staphylococcus epidermidisAntimicrob Agents Chemother19984210273927449756787
- KodjikianLRenaudFNRoquesCIn vitro influence of vancomycin on adhesion of a Staphylococcus epidermidis strain encoding intercellular adhesion locus ica to intraocular lensesJ Cataract Refract Surg20053151050105815975477
- FerroJFde PablosMLogronoMJGuisasolaLAizpuruFPostoperative contamination after using vancomycin and gentamicin during phacoemulsificationArch Ophthalmol199711521651709046249
- FeysJSalvanet-BouccaraAEmondJPDublanchetAVancomycin prophylaxis and intraocular contamination during intraocular surgeryJ Cataract Refract Surg19972388948979292675
- GritzDCCevallosAVSmolinGWhitcherJPIntraocular supplementation of intraocular irrigating solutions: an in vitro model of antibacterial actionOphthalmology19961038120412098764788
- LibrePEDella-LattaPChinNXIntracameral antibiotic agents for endophthalmitis prophylaxis: a pharmacokinetic modelJ Cataract Refract Surg20032991791179414522303
- MurphyCCNicholsonSQuahSABatterburyMNealTKayeSBPharmacokinetics of vancomycin following intracameral bolus injection in patients undergoing phacoemulsification cataract surgeryBr J Ophthalmol200791101350135317389745
- GimbelHVThe Case for Intracameral Vancomycin Cataract and Refractive Surgery Today20057375 Available from: http://crstoday.com/2005/02/0205_F9_Gimbel.html/Accessed February 6, 2015
- LehmannOThompsonJJWhiteLOKeysMFCampbellMJHalf-life of intracameral gentamicin after phacoemulsificationJ Cataract Refract Surg19972368838889292673
- GillsJPConsultation sectionJ Cataract Refract Surg200430816161617
- SchimelAMMillerDFlynnHWJrEvolving fluoroquinolone resistance among coagulase-negative Staphylococcus isolates causing endophthalmitisArch Ophthalmol201213012161723229711
- AsbellPAColbyKADengSOcular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolatesAm J Ophthalmol2008145695195818374299
- BlandJMAltmanDGStatistics notes: the odds ratioBMJ20003207247146810827061
- ArshinoffSABastianelliPAIncidence of postoperative endophthalmitis after immediate sequential bilateral cataract surgeryJ Cataract Refract Surg201137122105211422108106
- SchimelAMAlfonsoECFlynnHWJrEndophthalmitis prophylaxis for cataract surgery: are intracameral antibiotics necessary?JAMA Ophthalmol2014132111269127025125316
- ChangDFBraga-MeleRMamalisNASCRS Cataract Clinical Committee. Prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2007 ASCRS member surveyJ Cataract Refract Surg2007331801180517889779
- BarryPAdoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgeryJ Cataract Refract Surg201440113814224355725
- OlaviPOcular toxicity in cataract surgery because of inaccurate preparation and erroneous use of 50 mg/mL intracameral cefuroximeActa Ophthalmol2012902e153e15421470387
- DelyferMNRougierMBLeoniSOcular toxicity after intracameral injection of very high doses of cefuroxime during cataract surgeryJ Cataract Refract Surg201137227127821241909
- QuerishiFClarkDMacular infarction after inadvertent intracameral cefuroximeJ Cataract Refract Surg20113761168116921596262
- FryLVancomycin dilution error (Letter)J Cataract Refract Surg2005318167416129316
- GoldbergRAFlynnHWJrMillerDGonzalezSIsomRFStreptococcus endophthalmitis outbreak after intravitreal injection of bevacizumab: one-year outcomes and investigative resultsOphthalmology201312071448145323453511
- MamalisNEdelhauserHFDawsonDGChewJLe BoyerRMWernerLToxic anterior segment syndromeJ Cataract Refract Surg200632232433316565012
- LiesegangTJIntracameral antibiotics: questions for the United States based on prospective studiesJ Cataract Refract Surg200834350550918299079
- Braga-MeleRChangDFHendersonBAMamalisNTalley-RostovAVasavadaAASCRS Clinical Cataract Committee. Intracameral antibiotics: safety, efficacy, and preparationJ Cataract Refract Surg201440122134214225465691
- VilladaJRVicenteUJavaloyJAlióJLSevere anaphylactic reaction after intracameral antibiotic administration during cataract surgeryJ Cataract Refract Surg200531362062115811754
- MoisseievELevingerEAnaphylactic reaction following intracameral cefuroxime injection during cataract surgeryJ Cataract Refract Surg20133991432143423850230
- MontanPWejdeGSetterquistHRylanderMZetterstromCProphylactic intracameral cefuroxime: evaluation of safety and kinetics in cataract surgeryJ Cataract Refract Surg200228698298712036640
- BallJLBarrettGDProspective randomized controlled trial of the effect of intracameral vancomycin and gentamicin on macular retinal thickness and visual function following cataract surgeryJ Cataract Refract Surg200632578979416765796
- Axer-SiegelRStiebel-KalishHRosenblattIStrassmannEYassurYWeinbergerDCystoid macular edema after cataract surgery with intraocular vancomycinOphthalmology199910691660166410485531
- Task Force AAO-CDCThe Prophylactic use of Vancomycin for Intraocular Surgery Quality of Care Publications. Number 515San Francisco, CAAmerican Academy of Ophthalmology1999
- GordonYJVancomycin prophylaxis and emerging resistance: are ophthalmologists the villains? The heroes?Am J Ophthalmol2001131337137611239872
- DrlicaKThe mutant selection window and antimicrobial resistanceJ Antimicrob Chemother2003521111712805267
- SeppalaHAl-JuhaishMJarvinenHLaitinenRHouvinenPEffect of prophylactic antibiotics on antimicrobial resistance of viridans streptococci in the normal flora of cataract surgery patientsJ Cataract Refract Surg200430230731515030817
- SharifiEPorcoTCNaseriACost-effectiveness analysis of intracameral cefuroxime use for prophylaxis of endophthalmitis after cataract surgeryOphthalmology2009116101887189619560825
- Massachusetts Executive Office of Health and Human ServicesMass Health Drug List, Table 34: Antibiotics: Ophthalmic2015 Available from: https://masshealthdruglist.ehs.state.ma.us/MHDL/pubtheradetail.do?id=34Accessed January 24, 2015
- HabibMMandalKBunceCVFraserSGThe relation of volume with outcome in phacoemulsification surgeryBr J Ophthalmol200488564364615090416
- GreenbergPBTsengVLWuWCPrevalence and predictors of ocular complications associated with cataract surgery in United States veteransOphthalmology2011118350751421035868
