Abstract
Glaucoma is the second leading cause of blindness worldwide and is most notably characterized by progressive optic nerve atrophy and advancing loss of retinal ganglion cells (RGCs). The main concomitant factor is the elevated intraocular pressure (IOP). Existing treatments are focused generally on lowering IOP. However, both RGC loss and optic nerve atrophy can independently occur with IOP at normal levels. In recent years, there has been substantial progress in the development of neuroprotective therapies for glaucoma in order to restore vital visual function. The present review intends to offer a brief insight into conventional glaucoma treatments and discuss exciting current developments of mostly preclinical data in novel neuroprotective strategies for glaucoma that include recent advances in noninvasive diagnostics going beyond IOP maintenance for an enhanced global view. Such strategies now target RGC loss and optic nerve damage, opening a critical therapeutic window for preventative monitoring and treatment.
Introduction
Glaucoma is the second leading cause of blindness worldwide,Citation1 with an estimated 8.4 million people suffering from blindness in 2010 alone, accounting for 21% of total blindness in the world today, and it is estimated that the number of those with glaucoma worldwide will rise to 79.6 million by the year 2020.Citation2 Glaucoma is considered a progressive neurodegenerative eye disorder with varied symptoms most notably characterized in earlier stages by the degeneration and loss of retinal ganglion cells (RGCs) and their axons, leading to optic neuropathy and visual field loss. This occurs through the loss of function and death of RGC through initially the process of programmed cell death (apoptosis). In later stages of the disease, progression includes neuronal loss in the lateral geniculate nucleus and visual cortex.Citation3 Elevated intraocular pressure (IOP) is considered to be one of the key factors in glaucoma; however, elevated IOP is typically followed by the appearance of structural damage at the optic nerve head, including neuroretinal rim thinning, cupping of the optic disc, and sectoral retinal nerve fiber layer (RNFL) thinning, indicating the pathological progression of the disease within the retina as a whole.Citation4
The best known classification of glaucoma relies on the anatomy of the anterior chamber and the drainage pathway (open and narrow angles). It is usually categorized as primary or secondary, based on several combined pathologies including comorbid conditions such as infection, mechanical injury, or neovascularization that often affect a single eye alone. In addition, primary open-angle glaucoma (POAG) can present with high and low IOPs, with the latter termed “normal tension glaucoma” representing 40% of patients with POAG.Citation5 Furthermore, there is increasing recognition that even significant lowering of IOP may be insufficient to prevent visual loss in some patients. Therefore, alternative treatments besides lowering IOP, especially via prevention of neuronal cell loss, are now being investigated with an emphasis on neuroprotection to slow down the progression of glaucoma. Most data cited are obtained from preclinical studies.
The present review will 1) give a brief overview of the current treatments aiming to maintain IOP, 2) discuss neuroprotective and neuroregenerative strategies targeting RGC loss and the prevention of optic nerve damage, and 3) introduce new advances in diagnostic technologies.
Current and existing medication for glaucoma
The therapeutic aim for glaucoma treatment remains the prevention of vision loss by predominantly decreasing the risk factors of the disease. At present, the only effective treatment is the lowering of IOP.Citation6,Citation7 Currently available treatment modalities for glaucoma focus on the lowering of IOP through various strategies often in tandem, including decreasing the production of aqueous humor and/or increasing the drainage through the trabecular meshwork (TM) and/or enhancing uveoscleral outflow. A significant reduction of IOP (20%–40%) can limit progressive loss in the visual field.Citation8 To date, there are numerous drugs that control IOP, which are most commonly in the form of topically applied eyedrops – a convenient noninvasive method of administration. These topical drugs decrease the production of aqueous humor and facilitate drainage through the TM, increasing uveoscleral outlflow.Citation9 There are mainly five categories: β-blockers, carbonic anhydrase inhibitors, prostaglandin analogs, sympathomimetic drugs, and parasympathomimetic drugs.Citation7,Citation9 In addition, some fixed combination therapies are also given to some patients for effective IOP control when they do not respond to one pure form of medication. To date, fixed combination therapies for glaucoma mainly include prostaglandin analogs/β-blockers, carbonic anhydrase inhibitors/β-blockers, and α2-adrenergic agonists/β-blockers and carbonic anhydrase inhibitors/α2-adrenergic agonists. All the therapies were approved by the US Food and Drug Administration (FDA) in 2013.Citation9 It is also worth mentioning that as an exception, triple fixed combination of prostaglandin analogs/α2-adrenergic agonists/β-blockers is available in Mexico.Citation9 However, it was found elucidated that the currently available fixed combination of dorzolamide/dimolol and brimonidine/timolol could reduce IOP more effectively with reduced ocular allergy than their component used separately as monotherapy.Citation10
Neuroprotection therapies for glaucoma
Although there are clinically effective IOP reduction treatments, there has been a growing realization that alternative treatments are needed to also target the prevention of RGC loss as patients with glaucoma have been seen to continue to lose visual function even after successful IOP lowering.
Neuroprotection, by definition, is “the relative preservation of neuronal structure and/or function”.Citation11 In addition to reduction of IOP, neuroprotective therapies have the potential to halt RGC loss through promoting cell survivalCitation12 and preventing further structural and functional damage of the optic nerve to preserve vision in those showing glaucomatous signs.Citation4,Citation13
Though the primary mechanisms of glaucoma have not yet been fully elucidated, several key processes have been identified, such as mechanical compression,Citation14 ischemia,Citation15 oxidative stress,Citation16 neurotropic growth factor deprivation,Citation17 intracellular calcium toxicity,Citation18 activation of autoimmunity,Citation19 and glutamate neurotoxicity.Citation20 In recent years, researchers have investigated a number of compounds that can protect RGCs via targeting the aforementioned pathways.
Glutamate toxicity-blocking agents
Excitotoxicity in neurodegenerative diseases can occur by stimulation of N-methyl-d-aspartic acid receptors (NMDARs), the receptor for the amino acid transmitter glutamate.Citation21 Glutamate is an essential excitatory neurotransmitter released by bipolar cells onto RGCs in the vertebrate retinaCitation22 and has been shown to induce excitotoxicity in glaucoma.Citation23 By the stimulation of NMDARs, extensive levels of Ca2+ enter the cells, activating phospholipases, endonucleases, proteases, etc, thereby leading to the apoptotic and/or necrotic cell death in neurons.Citation24 It was demonstrated that the glutamate concentration was elevated chronically in the inner eye both in patients with glaucoma and in glaucoma animal models.Citation25,Citation26 This then caused the overactivation of the glutamate receptor, leading to neuron damage.Citation9 Thus, antiexcitotoxic agents such as NMDAR antagonists, glutamate release inhibitors, and calcium channel blockers could all potentially be employed to inhibit excitotoxicity with the intent of halting related neuronal cells loss.
“Memantine” is the best known antagonist of NMDARs and is approved by the FDA for the treatment of Alzheimer’s disease following a multinational clinical trial in 2009.Citation27 However, it was not found to be effective in a clinical trial in Alzheimer’s disease (NCT00235716).Citation28 In addition, despite some evidence of its efficacy preclinically,Citation29 a clinical trial of Memantine against chronic glaucoma (NCT00168350) finished in 2010 with results yet unpublished.
Ca2+ channel blockers have been suggested to be neuroprotective in glaucoma.Citation30 Topical 2% flunarizine was confirmed to significantly ameliorate ischemic retinal damage in rabbit models.Citation31 Iganidipnie, nimodipine, and lomerizine were applied to cultured rat RGCs under hypoxic conditions, evidencing a significant increase in RGCs’ viability.Citation30 Clinically, orally administered nilvadipine slightly slowed down visual field degeneration and maintained the optic disc rim of patients with normal low-tension IOP open-angle glaucoma in a 3-year study.Citation32
Neurotrophic factors
Among other roles, neurotrophic factors (NTFs) are involved in the maintenance and enhancement of neuronal cell survival and contribute to their differentiation, growth, and regeneration. They are essential for neuronal development within the nervous system. In theory, a therapeutic approach could involve the direct supply of exogenous neurotrophins or through the upregulation of endogenous expression of neurotrophins.
Brain-derived neurotrophic factor (BDNF) is secreted into the synaptic cleft, binding to surface tropomyosin receptor kinases B, which are then transported retrogradely to neuronal cells to regulate their activities.Citation33 BDNF was reported to rescue RGCs after optic nerve axotomy,Citation34 with exogenously applied BDNF proving neuroprotective on RGCs in moderately chronic hypertensive rat models.Citation35 Leucine rich repeat and lg domain containing 1 (LINGO-1) is specific to the central nervous system and functions as a negative regulator of axonal regeneration and neuronal survival. Fu et al demonstrated that combing LINGO-1 antagonist with BDNF could provide long-term protection for RGCs in a chronic ocular hypertension (OHT) models, solving the problem of the neuroprotective limitations of BDNF downregulating the tropomyosin receptor kinases B receptor.Citation36
Ciliary neurotropic factor (CNTF),Citation37,Citation38 nerve growth factor (NGF),Citation39 and glial cell-line-derived neurotropic factorCitation40 have all been identified as neuroprotective factors for RGCs. Among them, CNTF, delivered by an encapsulated cell technology implant, has been used in a Phase II clinical trial against geographic atrophy and has been proved to slow the progression of vision loss.Citation41 A Phase I clinical trial for CNTF implant in patients with POAG (NCT01408472) completed in October 2014 is yet to be published. A significant reduction in RCG loss has been demonstrated with topical NGF application in rat glaucoma models at 7 weeks. Its efficacy is directly associated with the inhibition of cell death by apoptosis.Citation42 Lentiviral vector-mediated retinal gene transfer of pigment epithelium-derived factor was achieved through subretinal injection in both transient OHT and N-methyl-d-aspartic acid-induced models, indicating pigment epithelium-derived factor had led to neuroprotective effects rescuing RGCs.Citation43
Brimonidine (BMD) is a selective α2-adrenergic receptor agonist that is used clinically to lower IOP by decreasing the production of aqueous humor and facilitating its outflow via the TM.Citation44 Neuroprotective efficacy of BMD has been found in several animal experimental disease models, such as optic nerve injuryCitation45 and OHT models,Citation46 with both convincing histological evidence and functional protection. BMD has been found to prevent progressive RGC loss and thinning of the inner retina layer and visual impairment in a normal tension glaucoma murine model. This was achieved through excitotoxic inhibition of upregulated phosphorylated NR2B, stimulation of production of NGF, BDNF, and basic fibroblast growth factor in multiple pathways.Citation47 In recent years, there have been several ongoing clinical trials for BMD. In a 4-year double-masked, randomized, and multicenter clinical trial testing the efficacy of monotherapy with BMD tartrate 0.2% versus timolol maleate 0.5% eyedrops (NCT00317577), the BMD group had a significantly lower rate of visual field progression, attributed to an IOP–independent protective effect of BMD or conversely, a potentially harmful effect of timolol.Citation48,Citation49
To date, some identified limitations regarding neurotrophins include potential instability of eyedrops, the necessity of repeated intravitreal administration, the long-term or short lasting effects, and the mode of delivery (invasive vs noninvasive).Citation50,Citation51 Therefore, further novel approaches, such as gene therapy or stem cell therapy, need to be comprehensively investigated and developed to make neurotrophin application a more realistic therapy for glaucoma.
Oxidative stress suppression
Reactive oxygen species are chemically reactive molecules, including oxygen ions and peroxides, generated in the metabolism of oxygen and cleared by antioxidants. Their accumulation induces oxidative stress imbalance, which overburdens the cell’s ability to readily clear the intermediates and repair the subsequent damage. It could also lead to damage of cellular components, such as DNA, fatty acids in lipids, and amino acids in protein. During the past 2 decades, oxidative stress was shown to be involved in the pathogenesis of glaucoma,Citation16,Citation52 especially in POAG.Citation53
Aminoguanidine, an inhibitor of NOS-2, is believed to reduce reactive oxygen species production and showed significant prevention of RGC loss in rat glaucoma models, offering a hint that this well-tolerated pharmacological agent could be a reasonable candidate for neuroprotective therapy in glaucoma.Citation54 Vitamin E, a scavenger of peroxyl radicals,Citation9 has been indicated as protective of RGCs. Its daily supplementation in patients was found to be associated in decreasing the rate of primary glaucomatous progression.Citation55–Citation57 Conversely, a higher rate of RGC death in vitamin E deficiency was linked to elevated lipid peroxidation.Citation58 Recent studies showed that vitamin E-modified silicone-hydrogel contact lenses can also help to increase the sustained release of such drugs, making the effect of the therapy last longer than the wear duration.Citation59,Citation60
Ginkgo biloba extract is another compound of interest, mainly containing antioxidants such as flavonoids and cyanidine,Citation16,Citation61 which exert significant protective effects against free radical and lipid peroxidation.Citation62 They can be used as a neuroprotective therapy for RGC survival against glaucomaCitation63 though the underlying mechanism is not established.
Coenzyme Q10 (CoQ10), an important potent antioxidant, was previously proved to be an effective neuroprotectant in Parkinson’s and Huntington’s diseases.Citation64 Furthermore, it was suggested that the intraocular administration of CoQ10 minimizes glutamate release and protects RGCs against ischemia-induced injury.Citation65 Additionally, CoQ10 was also shown to protect RGCs both in vitro and in vivo against oxidative stress.Citation66 Recently, topical CoQ10 has been shown to positively affect retinal function in patients with POAG.Citation67
Immune modulatory treatment
Recent research has confirmed that cellular interactions play an important role in the immune system’s regulation of glaucoma.Citation68 These include the stress-induced immune response, innate immune cells, autoreactive T-cells, dysfunctional cross-talk between neurons and glia, and overproduction of proinflammatory cytokines.Citation57–Citation73 One of the key proinflammatory cytokines, tumor necrosis factor-alpha (TNF-α), is secreted by damaged glial cells and through the binding of TNF-receptor-1 (TNF-R1) contributes to apoptotic RGC death.Citation74 Dysfunction of immunoregulation of RGC-related T-cells is implicated in glaucoma.Citation68,Citation75
Agmatine, an anti-inflammatory agent, was shown in vitro to inhibit TNF-α production in hypoxic RGC conditions.Citation76 Agmatine was also found to effectively lower IOP via topical administration and rescue RGCs in chronic ocular hypertensive rat models.Citation77 Etanercept, a TNF-α blocker, is commonly used clinically for other indications. It was evidenced to inhibit microglial response, preventing axonal degeneration and subsequent RGC loss in a rat glaucoma models.Citation78
The primary task of microglia cells, astrocytes, and Müller cells in the retina is to provide support, create an interface between RGCs and surrounding blood vessels, and help maintain the ion homeostasis. They are also able to remove excess glutamate and prevent excitotoxicity.Citation9,Citation79 In addition, they participate in several inflammatory processes, such as the production of inflammatory mediators, cytokines, and chemokines, which trigger their activity in glaucoma.Citation66,Citation80,Citation81 It is believed that further understanding of glial changes at the molecular level may provide innovative potential treatments that seek to selectively inhibit neuronal damage due to unwanted glial activation response in glaucoma.Citation69
RGC apoptosis may also be mediated by Fas or TNF-receptors, initiated by the efflux of cytochrome c from the mitochondria.Citation24 It was demonstrated that glaucoma was associated with increased RGC apoptosis.Citation82,Citation83 TNF-α inhibitors could also be used as neuroprotective agents in line with immunomodulatory treatments as discussed previously in this paper: for example, TNF-α inhibitors could be utilized along with other agents such as Baculoviral IAP repeat-containing protein-4, a potent caspase inhibitor, or a modified viral (adeno-associated Virus) vector, which was seen to significantly protect against optic nerve axon deterioration in chronic OHT rat models.Citation84
Stem cell therapy
Stem cells are not only able to self-renew but are also characterized by their ability to differentiate into various cell types.Citation85 The use of stem cells in neuroprotection and neuroregeneration within glaucoma has been a burgeoning area of research and interest.
Due to the unique properties of stem cells, there are two potential avenues where stem cells could be applied in the treatment of glaucomatous damage. The most promising and powerful therapeutic potential of stem cells lies in their ability to differentiate into new cell types and to effect tissue regeneration. It is conceivable that stem cells may offer a replacement for lost RGCs and optic nerves in glaucoma.Citation86,Citation87 Stem cell therapies could target TM cells and RGCs, as TM cells are one of the prime culprits in the increased resistance to the aqueous outflow leading to elevated IOP. The main aqueous outflow pathway of the eye includes a series of channels, such as the TM, Schlemm’s canal, collector channels, and the episcleral venous system. In glaucoma, a reduction of TM cells is frequently observed.Citation88 Therefore, using stem cells to replace or repair TM cells becomes an attractive and plausible approach for IOP management. Isolated and characterized stem cells from human TM proved that they were able to differentiate into TM cells and exhibit phagocytic function, which was expected to have the potential to functionally maintain the aqueous outlow.Citation89,Citation90 Mesenchymal stem cells (MSCs) were demonstrated to be capable of migrating predominantly to the area of tissue damage, decreasing IOP, and restoring aqueous humor drainage, as well as stimulating proliferation of nestin-expressing ocular progenitor cells in the ciliary body.Citation91 Emre et al intravitreally transplanted bone marrow-derived and adipose tissue-derived MSCs to an OHT model and demonstrated that RGC numbers significantly improved in stem cell-treated OHT groups, with the number of cells expressing proinflammatory cytokins (interferon-gamma and TNF-α) decreasing in the MSC-transferred group.Citation12
Embryonic stem cells have the potential to self-renew and retain pluripotency to generate all specialized cell types, including cells damaged in retinal neuroregeneration.Citation92,Citation93 RGC bodies are located in the retina, while the terminal axons extend into the brain, making stem cell transplantation into the inner membrane for retinal integration difficult. Cell lineage restricted mouse pluripotent stem cells converted into retinal ganglion-like cells, which exhibited long synapses, and were unable to integrate into the normal retina after transplantation.Citation95 A safe and efficient therapeutic application of retinal progenitor cells is still in process due to the complexity of their cellular components and their transient state, along with the challenge of the retina’s multilayered architecture, but it is given full attention in experimental research nowadays.
Schwann cells (SCs) are important glial cells within the peripheral nervous system and are able to de-differentiate into progenitor cells under the condition of axonal injury, which could potentially serve as a way to replace damaged axons and provide an environment suitable for neuronal survival and axonal regrowth.Citation96,Citation97 Guo et al compared SC delivery to an injured optic nerve sheath with intravitreal SC delivery. SC delivery to an injured optic nerve sheath resulted in delayed but long-lasting effects on TGC protection, significantly increasing RGC survival through targeting secondary rather than primary degeneration.Citation98
Though stem cells transplantation methods and strategies for the retina still have a long journey of development ahead, they promise a fundamental prospective neuroprotective approach for structural and functional recovery in the treatment of glaucoma. It is clear that stem cells possess multiple protective characteristics that may be capable of alleviating disease progression and promoting cell survival through various mechanisms, and we have also pointed out previously that neurotrophic factor deprivation has been implicated as one of the underlying pathophysiology of RGC death in glaucoma.Citation33 Thus, it is hypothesized that transplantation of certain types of stem cells may activate multiple endogenous neuroprotective pathways via secretion of various factors.Citation96 Stem cells could be applied as an intraocular slow-release device for multiple bioactive factors to achieve RGC neuroprotection, which could potentially solve the problem of the short-lived effect of neurotrophins via a single administration. To this aim, the Sadan group investigated culturing MSCs in a defined environment, which upregulated the secretion of a variety of NTFs.Citation97 This approach revealed a neuroprotective impact on several neurodegenerative models, such as Parkinson’s diseaseCitation99 and optic nerve transection.Citation100 Flachsbarth et al genetically modified neural stem (NS) cells by intravitreally grafting them into intravitreal optic nerve crush mice models. The results were encouraging. NS cells were shown to stably express CNTF and significantly attenuate the loss of axotomized RGCs over a 4-month period.Citation101 A Phase I clinical trial for genetically modified CNTF-secreting retinal pigment epithelium cell implant was finished last year (NCT01408472), though results are still awaited.
Diagnostic technologies of detection in glaucoma
Due to the irreversible blindness of glaucomatous damage, treatment strategies are insufficient, requiring an early and thorough diagnostic approach in tandem to lower the risk of visual impairment progression, opening a critical neuroprotective window before the damage becomes irreversible. Adequate and efficient monitoring and detection for the progression of the disease is essential.
Conventional examination of glaucoma
Other than the commonly used detection of elevated IOP, traditional tests for diagnosing glaucoma largely depend on pathological structural change observed in the retina along with functional tests. Assessment of structural changes of the optic nerve head and detection of visual field defects are fundamental in establishing the diagnosis and evaluating the progression of glaucoma.Citation102 However, in plenty of cases, visual field loss develops unnoticed, accompanied by a substantial and unrecoverable loss of RGCs.Citation103,Citation104 Visual field testing is performed via standard automated perimetry (SAP) that has been used as the golden standard for managing glaucoma.Citation101 However, previous studies have demonstrated that 25%–35% of RGCs are lost, before any visual field defect can be detected through SAP.Citation105,Citation106
Therefore, developing tools for early identification and diagnosis of structural damage of the optic nerve head and RNFL along with RGC loss is a pressing need within glaucoma. Along such lines, Alencar and Medeiros developed an approach to estimate RGC counts from structural and functional measurements, providing a single combined metric of structure and function.Citation101 The method combined estimates of RGC counts obtained from OCT RNFL thickness with those obtained from SAP. Advances in spectral domain optical coherence tomography also appear to be very promising, although none of these technologies have been validated in their own right for glaucoma diagnosis.Citation107,Citation108
Detection of apoptosing retinal cell technology
As irreversible glaucomatous vision loss can be slowed or halted, the search for an early diagnostic tool for glaucoma is now at a peak. Apoptosis is a form of programmed cell death that is implicated in both pathological and physiological processes, which plays essential roles in several neurodegenerative diseases.Citation109 RGC apoptosis has been demonstrated to be one of the most critical in ophthalmological study as it occurs through the whole etiological process of glaucoma.Citation72,Citation82,Citation110 In early apoptosis, externalization and exposure of phosphatidylserine (PS) occur at the cell membrane surface, causing asymmetry within the phospholipid membrane of the cells.Citation110,Citation111 The annexin family is known to be able to bind to negatively charged PS with high affinity in a Ca2+-dependent manner.Citation112,Citation113 Fluorescently conjugated annexin V has been used for detection of apoptosis in a variety of studies. Technetium-99m (99m Tc)-labeled annexin V first enabled apoptosis to be detected in vivo. In recent years, this in vivo technique has been performed in various clinical trials, including post-heart transplantation,Citation114 acute myocardial infarction,Citation115 breast cancer,Citation116 and rectal cancer.Citation117
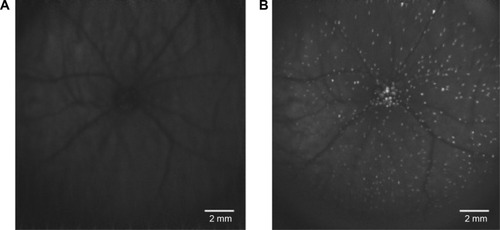
Detection of apoptosing retinal cell (DARC) provides a real-time in vivo imaging technology, using the transparent nature of the eye to view apoptotic RGCs via a nonradioactive method, allowing direct microscopic observation of cellular processes in the retina. DARC is based on the visualization of fluorescently labeled annexin V-positive cells using an ophthalmoscope such as the confocal laser scanning ophthalmoscope, with originally an argon laser of 488 nm necessary to excite the administered annexin V-bound fluorophore. Fluorescence was then detected by a solid-state photodetector with a wide band-pass filter and a short-wavelength cutoff of a 521 nm filter; finally, the signal was digitized by a frame grabber and stored on the computer.Citation118,Citation119
During the last decade, DARC has been refined and successfully applied in various animal studies to assess RGC apoptosis in glaucomatous rat models and Alzheimer’s disease models. Furthermore, DARC has been performed at various time points (hours, days, and months) with histological validation of RGCs labeling. The results have indicated and confirmed that RGC apoptosis happens in early stages of glaucoma.Citation83,Citation118,Citation120,Citation121 DARC has also been used in the evaluation of neuroprotective agents in glaucoma, such as beta amyloid targeting therapy,Citation122 topical-administrated CoQ10,Citation120 glutamate receptor agonists,Citation121 and retinal photocoagulative treatments.Citation123 As previously noted, the limitation of existing glaucoma diagnostic devices are that patients could only be identified when significant structural changes have already occurred and damage had progressed past the point of effective intervention. The most immediate advantageous benefit of DARC is to monitor progression and evaluate neuroprotective efficacy in real-time (). DARC is currently undergoing a Phase I clinical trial in glaucoma (NCT02394613).
Figure 1 The DARC in vivo retinal image.
Abbreviations: DARC, detection of apoptosing retinal cell; RGCs, retinal ganglion cells.
Conclusion
Existing available glaucoma diagnostics, medications, and therapies need further advancement, especially if visual loss of any kind is to be eliminated.Citation124 Diagnostics are now taking into consideration a variety of structural and pathological developments within the retina to identify the early onset and progression of glaucoma with a critical focus on RGC loss and degeneration moving beyond conventional IOP measurement diagnostic alone. With this in mind, the early and accurate identification of glaucoma, providing a longer, critical therapeutic window is fundamental to reduce and halt progression. This can be achieved through neuroprotection and neuroenhancement as the next exciting step into the future of glaucoma treatment with an emphasis on early prevention.
Disclosure
MF Cordeiro is a named inventor on a patent on DARC technology owned by UCL. The authors report no other conflicts of interest in this work.
References
- KingmanSGlaucoma is second leading cause of blindness globallyBull World Health Organ2004821188788815640929
- QuigleyHABromanATThe number of people with glaucoma worldwide in 2010 and 2020Br J Ophthalmol200690326226716488940
- KaushikSPandavSSRamJNeuroprotection in glaucomaJ Postgrad Med20034919012865582
- ChangEEGoldbergJLGlaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancementOphthalmology2012119597998622349567
- DielemansIVingerlingJRWolfsRCWHofmanAGrobbeeDEde JongPTVMThe prevalence of primary open-angle glaucoma in a population-based study in the Netherlands: the Rotterdam studyOphthalmology199410111185118557800368
- LeskeMCWuSYHennisABESs Study GroupRisk factors for incident open-angle glaucoma: the Barbados eye studiesOphthalmology20081151859317629563
- BagnisAPapadiaMScottoRTraversoCECurrent and emerging medical therapies in the treatment of glaucomaExpert Opin Emerg Drugs201116229330721406029
- HeijlABengtssonBHymanLLeskeMCEarly Manifest Glaucoma Trial GroupNatural history of open-angle glaucomaOphthalmology2009116122271227619854514
- KolkoMPresent and new treatment strategies in the management of glaucomaOpen Ophthalmol J201598910026069521
- HigginbothamEJConsiderations in glaucoma therapy: fixed combinations versus their component medicationsClin Ophthalmol2010411920169043
- CassonRJChidlowGEbneterAWoodJPCrowstonJGoldbergITranslational neuroprotection research in glaucoma: a review of definitions and principlesClin Exp Ophthalmol2012404350357
- EmreEYükselNDuruksuGNeuroprotective effects of intravitreally transplanted adipose tissue and bone marrow–derived mesenchymal stem cells in an experimental ocular hypertension modelCytotherapy201517554355925618560
- SenaDFRamchandKLindsleyKNeuroprotection for treatment of glaucoma in adultsCochrane Database Syst Rev20102CD00653920166085
- O’NeillECMackeyDAConnellPPHewittAWDanesh-MeyerHVCrowstonJGThe optic nerve head in hereditary optic neuropathiesNat Rev Neurol20095527728719488085
- OsborneNNUgarteMChaoMNeuroprotection in relation to retinal ischemia and relevance to glaucomaSurv Ophthalmol199943S102S12810416754
- IzzottiABagnisASaccàSCThe role of oxidative stress in glaucomaMutat Res2006612210511416413223
- QuigleyHAMcKinnonSJZackDJRetrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in ratsInvestig Ophthalmol Vis Sci200041113460346611006239
- D’hondtCSrinivasSPVereeckeJHimpensBAdenosine opposes thrombin-induced inhibition of intercellular calcium wave in corneal endothelial cellsInvest Ophthalmol Vis Sci20074841518152717389480
- KuehnMHKimCYOstojicJRetinal synthesis and deposition of complement components induced by ocular hypertensionExp Eye Res200683362062816677633
- DreyerEBA proposed role for excitotoxicity in glaucomaJ Glaucoma19987162679493118
- MichaelisEKMolecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and agingProg Neurobiol19985443694159522394
- MasseySCRedburnDATransmitter circuits in the vertebrate retinaProg Neurobiol198728155962881324
- DkhissiOChanutEWasowiczMRetinal TUNEL-positive cells and high glutamate levels in vitreous humor of mutant quail with a glaucoma-like disorderInvestig Ophthalmol Vis Sci19994099099410102297
- SongWHuangPZhangCNeuroprotective therapies for glaucomaDrug Des Devel Ther2015914691479
- DreyerEBZurakowskiDSchumerRAPodosSMLiptonSAElevated glutamate levels in the vitreous body of humans and monkeys with glaucomaArch Ophthalmol199611432993058600890
- BrooksDEGarciaGADreyerEBZurakowskiDFranco-BourlandREVitreous body glutamate concentration in dogs with glaucomaAm J Vet Res19975888648679256971
- OsborneNNRecent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandonedActa Ophthalmol200987445045419141144
- DyskenMWSanoMAsthanaSEffect of vitamin E and meman-tine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trialJAMA20143111334424381967
- GabeltBTRasmussenCATektasOYStructure/function studies and the effects of memantine in monkeys with experimental glaucomaInvestig Ophthalmol Vis Sci20125342368237622427549
- MayamaCCalcium channels and their blockers in intraocular pressure and glaucomaEur J Pharmacol20137399610524291107
- TakahashiKLamTTEdwardDPBuchiERTsoMOMProtective effects of flunarizine on ischemic injury in the rat retinaArch Ophthalmol199211068628701596236
- KosekiNAraieMTomidokoroAA placebo-controlled 3-year study of a calcium blocker on visual field and ocular circulation in glaucoma with low-normal pressureOphthalmology2008115112049205718672290
- PooMNeurotrophins as synaptic modulatorsNat Rev Neurosci200121243211253356
- YanQWangJMathesonCRUrichJLGlial cell line – derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: comparison to and combination with brain-derived neurotrophic factor (BDNF)J Neurobiol199938338239010022580
- KoM-LHuD-NRitchRSharmaSCChenC-FPatterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of ratsNeurosci Lett2001305213914211376903
- FuQLLiXYipHKCombined effect of brain-derived neurotrophic factor and LINGO-1 fusion protein on long-term survival of retinal ganglion cells in chronic glaucomaNeuroscience2009162237538219422885
- Parrilla-ReverterGAgudoMSobrado-CalvoPSalinas-NavarroMVillegas-PérezMPVidal-SanzMEffects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo studyExp Eye Res2009891324119268467
- JiJZElyamanWYipHKCNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathwayEur J Neurosci200419226527214725620
- ChengLSapiehaPKittlerováPHauswirthWWDi PoloATrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivoJ Neurosci200222103977398612019317
- Checa-CasalenguaPJiangCBravo-OsunaIRetinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedureJ Control Release201115619210021704662
- ZhangKHopkinsJJHeierJSCiliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degenerationProc Natl Acad Sci U S A2011108156241624521444807
- LambiaseAAloeLCentofantiMExperimental and clinical evidence of neuroprotection by nerve growth factor eye drops: implications for glaucomaProc Natl Acad Sci U S A200910632134691347419805021
- MiyazakiMIkedaYYonemitsuYPigment epithelium-derived factor gene therapy targeting retinal ganglion cell injuries: neuroprotection against loss of function in two animal modelsHum Gene Ther201022555956521175295
- AkmanACetinkayaAAkovaYAErtanAComparison of additional intraocular pressure-lowering effects of latanoprost vs brimonidine in primary open-angle glaucoma patients with intraocular pressure uncontrolled by timolol – dorzolamide combinationEye200519214515115184958
- Levkovitch-VerbinHHarris-CerrutiCGronerYWheelerLASchwartzMYolesERGC death in mice after optic nerve crush injury: oxidative stress and neuroprotectionInvestig Ophthalmol Vis Sci200041134169417411095611
- WheelerLAGilDWWoldeMussieERole of alpha-2 adrenergic receptors in neuroprotection and glaucomaSurv Ophthalmol200145S290S29411377451
- SembaKNamekataKKimuraAHaradaCMitamuraYHaradaTBrimonidine prevents neurodegeneration in a mouse model of normal tension glaucomaCell Death Dis201457e134125032864
- KrupinTLiebmannJMGreenfieldDSRitchRGardinerSA randomized trial of brimonidine versus timolol in preserving visual function: results from the low-pressure glaucoma treatment studyAm J Ophthalmol2011151467168121257146
- De MoraesCGLiebmannJMGreenfieldDSGardinerSKRitchRKrupinTRisk factors for visual field progression in the low-pressure glaucoma treatment studyAm J Ophthalmol2012154470271122835512
- KolomeyerAMZarbinMATrophic factors in the pathogenesis and therapy for retinal degenerative diseasesSurv Ophthalmol201459213416524417953
- CuencaNFernández-SánchezLCampelloLCellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseasesProg Retin Eye Res20144315925016980
- IzzottiASaccàSCCartigliaCDe FloraSOxidative deoxyribo-nucleic acid damage in the eyes of glaucoma patientsAm J Med2003114863864612798451
- SaccàSCPascottoACamicionePCaprisPIzzottiAOxidative DNA damage in human trabecular meshwork and its correlation with intraocular pressure and visual field in primary open angle glaucomaArch Ophthalmol200512345846315824217
- NeufeldAHPharmacologic neuroprotection with an inhibitor of nitric oxide synthase for the treatment of glaucomaBrain Res Bull200462645545915036557
- BirichTVBirichTAMarchenkoLNRemizonovaDNFedylovASVitamin E in the complex treatment of patients with primary glaucomaVestn Oftalmol1985102210133716072
- CelliniMCaramazzaNMangiaficoPPossatiGLCaramazzaRFatty acid use in glaucomatous optic neuropathy treatmentActa Ophthalmol Scand199876S2274142
- BuschiniEFeaAMLaviaCARecent developments in the management of dry age-related macular degenerationClin Ophthalmol2015956357425878491
- KoM-LPengP-HHsuS-YChenC-FDietary deficiency of vitamin E aggravates retinal ganglion cell death in experimental glaucoma of ratsCurr Eye Res201035984284920795867
- HsuKHde la JaraPLAriyavidanaARelease of betaine and dexpanthenol from vitamin E modified silicone-hydrogel contact lensesCurr Eye Res201440326727324833321
- HsuK-HCarbiaBEPlummerCChauhanADual drug delivery from vitamin E loaded contact lenses for glaucoma therapyEur J Pharm Biopharm20159431232126071799
- DeFeudisFVGinkgo Biloba Extract (EGb 761): Pharmacological Activities and Clinical ApplicationsParisElsevier1991
- Detry-MorelMProspects in the medical treatment of glaucomatous neuropathy. Basics of neuroprotectionJ Fr Ophtalmol199922112210221206
- Cybulska-HeinrichAKMozaffariehMFlammerJGinkgo biloba: an adjuvant therapy for progressive normal and high tension glaucomaMol Vis20121839022355250
- BealMFCoenzyme Q10 administration and its potential for treatment of neurodegenerative diseasesBiofactors199992–426126610416039
- NucciCTartaglioneRCerulliARetinal damage caused by high intraocular pressure – induced transient ischemia is prevented by coenzyme Q10 in ratInt Rev Neurobiol20078239740617678974
- NakajimaYInokuchiYNishiMShimazawaMOtsuboKHaraHCoenzyme Q 10 protects retinal cells against oxidative stress in vitro and in vivoBrain Res2008122622623318598676
- ParisiVCentofantiMGandolfiSEffects of coenzyme Q10 in conjunction with vitamin E on retinal-evoked and cortical-evoked responses in patients with open-angle glaucomaJ Glaucoma201423639140425079307
- TezelGImmune regulation toward immunomodulation for neuroprotection in glaucomaCurr Opin Pharmacol2013131233123084793
- TezelGThe immune response in glaucoma: a perspective on the roles of oxidative stressExp Eye Res201193217818620709058
- WaxMBTezelGImmunoregulation of retinal ganglion cell fate in glaucomaExp Eye Res200988482583019233171
- TezelGThe role of glia, mitochondria, and the immune system in glaucomaInvest Ophthalmol Vis Sci20095031001101219244206
- NaskarRWissingMThanosSDetection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucomaInvestig Ophthalmol Vis Sci20024392962296812202516
- TezelGLiLYPatilRVWaxMBTNF- and TNF-receptor-1 in the retina of normal and glaucomatous eyesInvestig Ophthalmol Vis Sci20014281787179411431443
- McKinnonSJThe cell and molecular biology of glaucoma: common neurodegenerative pathways and relevance to glaucomaInvest Ophthalmol Vis Sci20125352485248722562847
- WaxMBTezelGYangJInduced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fasligandJ Neurosci20082846120851209619005073
- HongSParkKKimCYSeongGJAgmatine inhibits hypoxia-induced TNF-alpha release from cultured retinal ganglion cellsBiocell200832220120518825914
- HongSKimCYLeeWSShimJYeomHYSeongGJOcular hypotensive effects of topically administered agmatine in a chronic ocular hypertensive rat modelExp Eye Res20109019710319782071
- RohMZhangYMurakamiYEtanercept, a widely used inhibitor of tumor necrosis factor-α (TNF-α), prevents retinal ganglion cell loss in a rat model of glaucomaPLoS One201277e4006522802951
- BringmannAGroscheAPannickeTReichenbachAGABA and glutamate uptake and metabolism in retinal glial (Müller) cellsFront Endocrinol2013448
- WangLCioffiGACullGDongJFortuneBImmunohistologic evidence for retinal glial cell changes in human glaucomaInvest Ophthalmol Vis Sci20024341088109411923250
- NeufeldAHMicroglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucomaArch Ophthalmol199911781050105610448748
- QuigleyHANickellsRWKerriganLAPeaseMEThibaultDJZackDJRetinal ganglion cell death in experimental glaucoma and after axotomy occurs byInvest Ophthalmol1995364774786
- CordeiroMFGuoLCoxonKMImaging multiple phases of neurodegeneration: a novel approach to assessing cell death in vivoCell Death Dis201011e321364622
- McKinnonSJLehmanDMTahzibNGBaculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma modelMol Ther20025678078712027563
- MorrisonSJKimbleJAsymmetric and symmetric stem-cell divisions in development and cancerNature200644170971068107416810241
- JohnsonTVBullNDMartinKRStem cell therapy for glaucoma: possibilities and practicalitiesExpert Rev Ophthalmol20116216517421686079
- BullNDMartinKRUsing stem cells to mend the retina in ocular diseaseRegen Med20094685586419903004
- LitonPBChallaPStinnettSLunaCEpsteinDLGonzalezPCellular senescence in the glaucomatous outflow pathwayExp Gerontol200540874574816051457
- DuYRohDSMannMMFunderburghMLFunderburghJLSchumanJSMultipotent stem cells from trabecular meshwork become phagocytic TM cellsInvest Ophthalmol Vis Sci20125331566157522297497
- DuYYunHYangESchumanJSStem cells from trabecular mesh-work home to TM tissue in vivoInvestig Ophthalmol Vis Sci20135421450145923341019
- Manuguerra-GagnéRBoulosPRAmmarATransplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitmentStem Cells20133161136114823495088
- MeyerJSKatzMLMaruniakJAKirkMDEmbryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptorsStem Cells200624227428316123383
- VuglerALawrenceJWalshJEmbryonic stem cells and retinal repairMech Dev20071241180782917881192
- ChenMChenQSunXGeneration of retinal ganglion – like cells from reprogrammed mouse fibroblastsInvest Ophthalmol Vis Sci201051115970597820484577
- JagathaBDivyaMSSanalkumarRIn vitro differentiation of retinal ganglion-like cells from embryonic stem cell derived neural progenitorsBiochem Biophys Res Commun2009380223023519167364
- BullNDJohnsonTVMartinKRÃStem cells for neuroprotection in glaucomaProg Brain Res20081730851151918929131
- SadanOShemeshNCohenYMelamedEOffenDAdult neurotrophic factor-secreting stem cells: a potential novel therapy for neurodegenerative diseasesIsr Med Assoc J200911420120419603590
- GuoLDavisBNizariSDirect optic nerve sheath (DONS) application of Schwann cells prolongs retinal ganglion cell survival in vivoCell Death Dis2014510e146025321467
- SadanOBahat-StromzaMBarhumYProtective effects of neurotrophic factor-secreting cells in a 6-OHDA rat model of Parkinson diseaseStem Cells Dev20091881179119019243240
- Levkovitch-VerbinHSadanOVanderSIntravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transectionInvest Ophthalmol Vis Sci201051126394640020926814
- FlachsbarthKKruszewskiKJungGNeural stem cell-based intraocular administration of ciliary neurotrophic factor attenuates the loss of axotomized ganglion cells in adult miceInvest Ophthalmol Vis Sci201455117029703925270193
- SchwartzMYolesENeuroprotection: a new treatment modality for glaucoma?Curr Opin Ophthalmol200011210711110848215
- MedeirosFAAlencarLMZangwillLMSamplePAWeinrebRNThe relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucomaOphthalmology200911661125113319376584
- HarwerthRSCarter-DawsonLSmithELBarnesGHoltWFCrawfordMLJNeural losses correlated with visual losses in clinical perimetryInvest Ophthalmol Vis Sci20044593152316015326134
- Kerrigan-BaumrindLAQuigleyHAPeaseMEKerriganDFMitchellRSNumber of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same personsInvest Ophthalmol Vis Sci200041374174810711689
- MedeirosFALisboaRWeinrebRNLiebmannJMGirkinCZangwillLMRetinal ganglion cell count estimates associated with early development of visual field defects in glaucomaOphthalmology2013120473674423246120
- BusselIIWollsteinGSchumanJSOCT for glaucoma diagnosis, screening and detection of glaucoma progressionBr J Ophthalmol201398suppl 2ii15ii1924357497
- El ChehabHDelbarreMMaréchalMNew neuroretinal rim analysis with spectral domain optical coherence tomography, spectralis (Heidelberg Engineering, Germany). Preliminary studyJ Fr Ophtalmol2015381465225575418
- FriedlanderRMApoptosis and caspases in neurodegenerative diseasesN Engl J Med2003348141365137512672865
- BizrahMDakinSCGuoLA semi-automated technique for labeling and counting of apoptosing retinal cellsBMC Bioinformatics201415116924902592
- van EngelandMNielandLJRamaekersFCSchutteBReutelingspergerCPAnnexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposureCytometry1998311199450519
- CarrollADMoyenCVan KesterenPTookeFBatteyNHBrownleeCCa2+, annexins, and GTP modulate exocytosis from maize root cap protoplastsPlant Cell1998108126712769707528
- MeersPMealyTCalcium-dependent annexin V binding to phospholipids: stoichiometry, specificity, and the role of negative chargeBiochemistry1993324311711117218218240
- BlankenbergFGKatsikisPDTaitJFIn vivo detection and imaging of phosphatidylserine expression during programmed cell deathProc Natl Acad Sci U S A19989511634963549600968
- FlotatsACarrióINon-invasive in vivo imaging of myocardial apoptosis and necrosisEur J Nucl Med Mol Imaging200330461563012638039
- YangDJAzhdariniaAWuPIn vivo and in vitro measurement of apoptosis in breast cancer cells using 99mTc-EC-annexin VCancer Biother Radiopharm2001161738311279800
- HorisbergerKErbenPStröbelPAnnexin and survivin in locally advanced rectal cancer: indicators of resistance to preoperative chemoradiotherapy?Onkologie2010338–943944420838059
- CordeiroMFGuoLLuongVReal-time imaging of single nerve cell apoptosis in retinal neurodegenerationProc Natl Acad Sci U S A200410136133521335615340151
- CordeiroMFMigdalCBloomPFitzkeFWMossSEImaging apoptosis in the eyeEye (Lond)201125554555321436846
- GuoLMossSEAlexanderRAAliRRFitzkeFWCordeiroMFRetinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrixInvest Ophthalmol Vis Sci200546117515623771
- GuoLSaltTEMaassAAssessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivoInvest Ophthalmol Vis Sci200647262616431960
- GuoLSaltTELuongVTargeting amyloidbeta in glaucoma treatmentProc Natl Acad Sci U S A200710433134441344917684098
- Schmitz-ValckenbergSGuoLMaassAReal-time in vivo imaging of retinal cell apoptosis after laser exposureInvest Ophthalmol Vis Sci2008496277318281610
- Prevention of blindness and visual impairment: Priority eye diseasesWorld Health Organization Available from: http://www.who.int/blindness/causes/priority/en/index9.htmlAccessed October 16, 2015
