Abstract
For the anterior segment surgeon, the implantation of Boston type 1 keratoprosthesis is a multistep process that begins with careful patient selection. Success depends on thorough preoperative evaluation, detailed surgical planning, and frequent postoperative follow-up. New practice patterns have emerged for each of these phases as the international experience with keratoprosthesis grows. This review details special considerations that can improve outcomes and also allow surgeons to consider its use in challenging patient populations at each step.
Introduction
The Boston type 1 keratoprosthesis (Kpro) has undergone significant advances in prosthetic design and surgical technique in the last decade. These changes have been accompanied by rising numbers of Kpro implantation for expanding etiologies, and even the adoption of the Kpro as a primary procedure for selected indications. Internationally, single and multicenter groups have published short- and intermediate-term Kpro outcomes with attention to specific conditions. These results underscore the critical importance of careful patient selection and perioperative planning. We will review special considerations regarding patient selection, preoperative evaluation, surgical planning, and postoperative follow-up for Boston type 1 Kpro.
Patient selection
The success of the device begins with appropriate patient selection for Kpro implantation. For a potential candidate, the surgeon should consider the etiology of vision loss, visual acuity of both the operative and fellow eye, lid position and ocular surface status, and concomitant ocular and systemic diseases. Patient reliability is an important factor, as compliance with medications and follow-up is critical for device retention. Similarly, patient expectations must be assessed at the outset.
Outcomes of the Boston type 1 Kpro have been associated with the preoperative diagnosis (). Studies suggest patients with Stevens–Johnson syndrome and ocular cicatricial pemphigoid fare worse than those with non-cicatrizing etiologies, such as repeated graft failure for infections or dystrophies, with chemical burns and aniridia falling somewhere in the middle of the spectrum.Citation1,Citation2 The Boston type 1 keratoprosthesis Study Group identified three risk factors for Kpro device loss, including autoimmune etiology (eg, ocular cicatricial pemphigoid, Stevens–Johnson syndrome), ocular surface exposure requiring tarsorrhaphy, and increased number of prior failed penetrating keratoplasties.Citation3 Given the poor prognosis of a traditional penetrating keratoplasty in some conditions, such as those with limbal stem cell deficiency, Kpro as a primary procedure may be considered in certain patients.Citation4
Table 1 Table summarizing published Kpro outcomes based on the preoperative diagnosis
Device survival is shortened in patients with poor ocular surface stability, whether from cicatrizing disease or limbal stem cell dysfunction (LSCD), with increased rates of complications. Preoperative ocular surface disease, particularly in Stevens–Johnson syndrome and ocular cicatricial pemphigoid, is an identified risk factor for endophthalmitis following Kpro placement.Citation5 Similarly, increased rates of sterile keratolysis are reported in patients with underlying autoimmune disease and retroprosthetic membranes (RPMs).Citation6 RPM development, in turn, is associated with aniridia and infectious keratitis as the indication for Kpro placement.Citation7
Although autoimmune diseases were initially considered a relative contraindication due to the heightened risk of complications, recent improvements in postoperative management have allowed increased confidence in the placement of Kpro in very select patients with reasonable tear production and controlled ocular surface inflammation.Citation8,Citation9 However, the surgeon should proceed with caution, armed with a tailored management plan, after discussing the associated risks with the patient.Citation8,Citation9
Regardless of the specific indication, a detailed discussion of the procedure should be carried out with all patients prior to surgery, including the risks of device extrusion, endophthalmitis, and glaucoma progression, as well as realistic expectations of visual rehabilitation. Of particular importance is that the patient understands the commitment to frequent postoperative visits, as well as the necessity for indefinite postoperative prophylactic topical medications.
Preoperative evaluation
A thorough office examination is a key step in successful Kpro placement, including an assessment of visual potential. The World Health Organization criteria stipulates that a patient be monocular or functionally blind in both eyes to be considered eligible for a Boston Kpro; however, many surgeons now offer Kpro surgery despite relatively good vision in the contralateral eye. This evolution in practice pattern is largely due to improving rates of postoperative visual function, restoration of binocularity, and enhanced cosmesis.Citation10,Citation11 A recent study evaluated patient responses to the National Eye Institute Visual Function Questionnaire 25 (NEI VFQ-25) following Kpro implantation, and found a significant improvement in quality-of-life measures even in those patients with vision better than 20/200 in their nonsurgical eye.Citation12 To optimize outcomes, patients should possess certain essential characteristics, including adequate lid anatomy, good blink function, controlled ocular surface inflammation, and a sufficiently moist ocular surface.
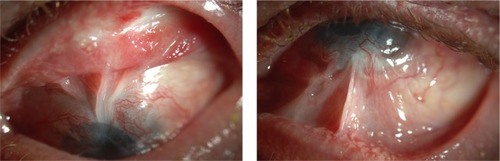
Fornices should be evaluated and able to accommodate a large bandage contact lens after surgery. Long-term use of a bandage lens postoperatively can prevent drying of the ocular surface, dellen formation, and corneal melts, and absence of a lens is an independent risk factor for postoperative complications of infections and corneal melts.Citation13 Thus, some patients may benefit from fornix reconstruction as a first stage procedure in preparation for implantation of the Boston Kpro (). Oculoplastic consultation may also be indicated in patients with poor lid closure or lagophthalmos to minimize exposure.
Figure 1 Forniceal scarring documented in a patient during a preoperative evaluation for keratoprosthesis.
A careful corneal and anterior segment examination must be performed. As discussed previously, ocular surface stability is integral to device survival.Citation5 Tear production can be measured with Schirmer testing, and goblet cell and meibomian gland function evaluated to assess all three layers of the tear film. Limbal stem cell function is also critical, and often compromised in patients with Stevens–Johnson syndrome, aniridia, chemical burns, and ocular cicatricial pemphigoid. Clinical findings of LSCD include peripheral corneal vascularization, advancement of conjunctival epithelium onto the cornea, and the presence of goblet cells on the corneal surface.Citation14 If LSCD is suspected, strategies for epithelial healing must be part of the surgical plan. Conjunctival scarring should be noted, as it may affect contact lens fit or placement of a planned tube shunt.
A thorough history can help the surgeon determine the possibility of significant optic nerve dysfunction, retinal pathology, or dense amblyopia. B-scan ultrasonography should be performed to evaluate retinal anatomy if the posterior segment cannot be visualized. It is critical to assess the adequacy of intraocular pressure (IOP) control preoperatively to determine the need for additional glaucoma procedures. A more complete picture can be established by collecting multiple IOP measurements, the number and duration of topical antihypertensives, and history of any prior filtering procedures or glaucoma lasers. Physicians may attempt to quantify preexisting optic nerve damage, but optic nerve head optical coherence tomography (OCT) and automated visual field testing are often precluded by poor preoperative vision and a limited view to the posterior segment. Significant optic nerve cupping can sometimes be seen on B-scan ultrasound. A confrontation visual field should be done to ensure a patient has light perception with projection. Peripheral anterior synechiae and angle anatomy can be evaluated by anterior segment OCT (AS-OCT), if available.
Lens’ status must be determined to order the Boston type 1 Kpro with the appropriate refractive power. The Boston Kpro is available in either an aphakic or pseudophakic version, and surgeons should consider preoperatively which of these will be implanted. If a patient is phakic, a lensectomy must be performed, and an aphakic Kpro is implanted or an intraocular lens with a pseudophakic model according to the surgeon’s preference. As in standard cataract surgery, the goal should be total preservation of the posterior capsule. If the patient is pseudophakic, implant stability and chamber depth should be assessed and can be evaluated with AS-OCT or ultrasound biomicroscopy. Stable posterior chamber lenses may be left in place; however, anterior chamber or unstable lenses should be explanted. If an aphakic Kpro is planned, the axial length of the eye should be measured by a scan. The Boston type 1 Kpro is available in 0.5 mm increments of axial length ().Citation15
Surgical planning
The Kpro surgeon and patient benefit from access to a multispecialist team familiar with Kpro implantation and follow-up, as these patients commonly need care for glaucoma, oculoplastic, and retinal issues. Often, several procedures can be combined to allow the best chance for visual recovery, device retention, and IOP control.
Adnexal and lid anomalies noted during the preoperative evaluation may prompt an oculoplastic referral. Procedures can be carried out during or prior to Kpro implantation, in efforts to minimize postoperative inflammation. Several strategies are available to promote epithelial healing in patients with LSCD or a dry, inflamed ocular surface. Persistent epithelial defects, a frequent complication in LSCD patients, must be taken seriously as they are associated with sterile keratolysis and device extrusion.Citation16 Temporary or permanent partial tarsorrhaphies may be considered at the time of Kpro implantation. Ocular surface toxicity from the postoperative drop regimen can be minimized by using preservative-free formulations and limiting the number of topical medications. Punctal plugs or cautery may improve ocular surface moisture. An adequate contact lens fit is necessary, and a contact lens specialist familiar with keratoprostheses is invaluable, as often multiple fittings or a custom lens may be required.Citation17 Scleral or hybrid lenses may be useful in challenging patients in whom other measures fail.
Uncontrolled IOP or the use of maximum medical antihypertensive therapy preoperatively should alert the clinician to consider a simultaneous glaucoma procedure, such as a tube shunt, at the time of Kpro implantation. In addition, glaucoma drainage device implantation can be also considered for aniridic patients without a history of glaucoma given that their high risk of glaucoma development is further increased by Kpro implantation. Tubes may be implanted either in the anterior chamber or ciliary sulcus without vitrectomy, or as a pars plana tube with vitrectomy ().Citation18,Citation19 Conjunctival scarring, crowded anterior chambers with iridocorneal adhesions, and the need for a good bandage contact lens fit postoperatively are considerations in deciding the position of tube placement. Reported outcomes from these combined procedures have been successful in controlling IOP with low risk of complications.Citation20 Similarly, patients who have significant retinal history or an unclear retinal status given poor view to the posterior segment preoperatively may benefit from concurrent vitrectomy.Citation21
The back plate is available in either Polymethyl methacrylate (PMMA) or titanium in the snap-on version. Originally the back plate was made of PMMA, but titanium, a newer option, can be machined to be thinner than its PMMA counterparts, potentially reducing the risk of RPM formation and anterior chamber congestion.Citation22 Titanium is nonferromagnetic and compatible with magnetic resonance imaging, and studies demonstrate that the back plate can be colored to achieve better cosmesis via electrochemical anodization.Citation23 Potential disadvantages of titanium are the metallic artifact created on AS-OCT that makes visualization of the angle anatomy more difficult, unlike PMMA, and a worse cosmetic appearance.
Two size options exist for the diameter of the back plate: 7 or 8.5 mm. In patients with small corneas, short axial lengths or a crowded anterior chamber, the surgeon may request the 7 mm back plate for better sizing. The newer click-on Boston type 1 Kpro is only available with an 8.5 mm titanium back plate, and does not require a separate locking ring.
Postoperative management
Implantation is only one aspect of Kpro management, and close, lifetime follow-up is critical for device survival. Patients must understand preoperatively that a commitment to frequent monitoring and drop maintenance is linked to implant success. More than a decade of international experience with postoperative complications offers insight into their prevention and management. In addition, improved imaging techniques provide a better understanding of the interactions between the device and host anatomy.
Behlau et al demonstrated that daily, topical antibacterial prophylaxis in KPro patients significantly reduces the risk of endophthalmitis. In the USA, the rate declined by 75% following the widespread adoption of long-term daily antibiotic use.Citation24 Regimens differ among Kpro surgeons, but agents should cover for Gram-positive organisms, the most common culprit, and Gram-negative microbes. Massachusetts Eye and Ear Infirmary (MEEI) has recommended daily dosing of compounded vancomycin (concentration of 14 mg/mL) plus a fourth-generation fluoroquinolone, to be increased to twice daily in patients with autoimmune disease. The fluoroquinolone may be substituted for another antibiotic, such as trimethoprim/polymyxin B, and long-term vancomycin use may be limited to cases with autoimmune disease, chemical burns, and only eyes.Citation25 Cost, frequency of dosing, and patient compliance may influence drop selection.Citation24, Citation26 Fungal keratitis and endophthalmitis remain rare, and no consensus exists regarding routine fungal prophylaxis. In climates in which fungal keratitis is endemic, MEEI recommends short cycles of an antifungal agent. Natamycin 5% or compounded amphotericin 0.15% may be given twice daily for 1 week every 2–3 months.Citation27 An additional option to augment antimicrobial preventive care is to instill one drop of 5% povidone iodine at each visit.Citation28
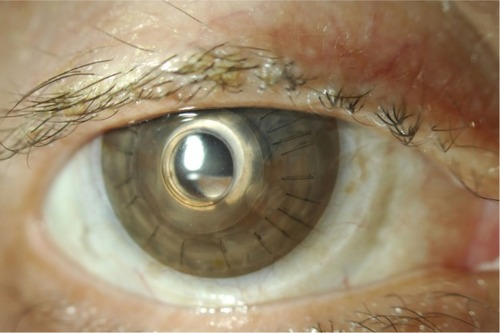
The value of a long-term bandage contact lens in Kpro patients is manifold. As discussed earlier, a contact lens protects the corneal surface surrounding the anterior implant from desiccation, epithelial breakdown, and melt.Citation29 These complications, in turn, place the patient at risk of infectious keratitis, sterile keratolysis with implant extrusion, and endophthalmitis. In addition, a lens often improves patient comfort, cosmesis, and glare, as well as addresses any refractive errors. Tinted contact lenses may be effectively used to reduce light scatter and glare ().Citation30 Bandage lenses, however, are not without complications. Protein deposits and inflammatory biofilms may develop, and lenses must be routinely changed. No consensus exists as to the ideal wear schedule of bandage contact lenses in Kpro. Typically, contact lenses are exchanged every 3–4 months, but may be discarded at different intervals depending on individual patient characteristics.Citation28
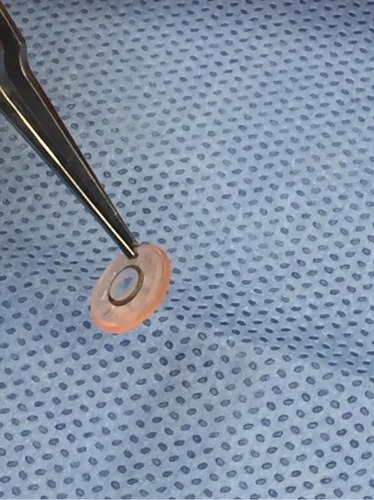
Figure 4 Intraoperative photo of assembled keratoprosthesis prior to implantation, including optic, trephinated corneal graft, and back plate.
Advances in the imaging techniques of AS-OCT and ultrasound biomicroscopy allow in vivo imaging of the Boston type 1 Kpro. Spectral domain, high-resolution AS-OCT can demonstrate the extent and thickness of RPMs, epithelium over the anterior Kpro plate, periprosthetic gaps, thinning in the corneal graft, angle closure, peripheral anterior synechiae, and associated anterior chamber shallowing.Citation31,Citation32 Often, these entities may not be easily appreciated on clinical exam. An imaging protocol consisting of cross-sectional scans of the device and anterior chamber over 360° allows serial comparison over time.Citation33 In patients with persistent epithelial defects, AS-OCT can assess for corneal graft thinning and imminent back plate exposure. In some patients, pooling of fluorescein may be seen beneath the anterior optic, suggestive of a gap between the corneal carrier and posterior surface of the device front plate.Citation34 AS-OCT may be helpful to distinguish corneal thinning as the cause versus asymmetrical seating of the device.
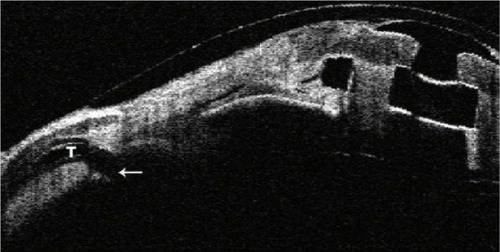
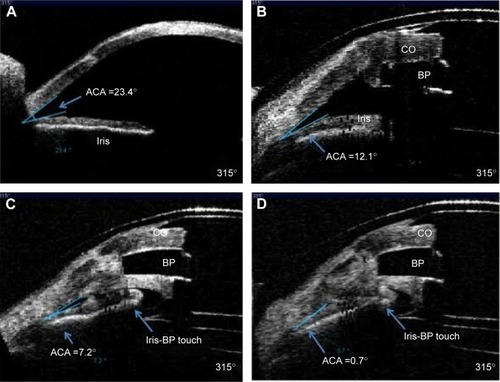
Development or progression of glaucoma is one of the leading causes of vision loss following Kpro implantation, and its detection may be difficult.Citation35 Progressive closing of the angle on AS-OCT has been demonstrated in the majority of Kpro patients in several studies ().Citation33,Citation36 The correlation between angle closure and increased IOP is still unclear and the subject of further study, but it may represent one mechanism of progression in these patients. Serial optic nerve OCT and optic disc photos as well as functional testing with visual fields are recommended at intervals shorter than those utilized for the follow-up of other forms of glaucoma. Posterior segment OCT can monitor for cystoid macular edema, an often under-recognized but treatable complication in this group of patients. As the AS-OCT cannot visualize posterior to the pigmented iris tissue, ultrasound biomicroscopy may be used to image glaucoma tube shunts, posterior chamber intraocular lens implants, and other structures behind the iris plane.Citation37
Figure 5 Progressive closure of the angle in a single patient following keratoprosthesis implantation.
Abbreviations: ACA, Anterior chamber angle; CO, Cornea; BP, Backplate.
Conclusion
In conclusion, careful preoperative evaluation, surgical planning, and postoperative management improve outcomes of the Boston type 1 Kpro implantation. Treatment plans should be tailored for each patient based on their presenting diagnosis and clinical features. Multidisciplinary care, involving glaucoma, retinal, oculoplastic, and contact lens specialists, is often required. Finally, the patient is a key member of the team, and must be committed to long-term care of their prosthetic.
Disclosure
The authors report no conflicts of interest in this work.
References
- KhanBDudenhoeferEJDohlmanCHKeratoprosthesis: an updateCurr Opin Ophthalmol200112428228711507341
- GreinerMALiJYMannisMJLonger-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the University of California, DavisOphthalmology201111881543155021397948
- CiolinoJBBelinMWTodaniAAl-ArfajKRudniskyCJBoston Keratoprosthesis Type 1 Study GroupRetention of the Boston keratoprosthesis type 1: multicenter study resultsOphthalmology201312061195120023499061
- KangJJde la CruzJCortinaMSVisual outcomes of Boston kerato-prosthesis implantation as the primary penetrating corneal procedureCornea201231121436144022367042
- NouriMTeradaHAlfonsoECFosterCSDurandMLDohlmanCHEndophthalmitis after keratoprosthesis: incidence, bacterial causes, and risk factorsArch Ophthalmol2001119448448911296013
- SivaramanKRHouJHAllemannNde la CruzJCortinaMSRetroprosthetic membrane and risk of sterile keratolysis in patients with type I Boston KeratoprosthesisAm J Ophthalmol2013155581482223352344
- RudniskyCJBelinMWTodaniABoston Type 1 Keratoprosthesis Study GroupRisk factors for the development of retroprosthetic membranes with Boston keratoprosthesis type 1: multicenter study resultsOphthalmology2012119595195522361316
- ColbyKAKooEBExpanding indications for the Boston keratoprosthesisCurr Opin Ophthalmol20112226727321537184
- SayeghRRAngLPFosterCSDohlmanCHThe Boston keratoprosthesis in Stevens-Johnson syndromeAm J Ophthalmol200814543844418207122
- AldaveAJKamalKMVoRCYuFThe Boston type I keratoprosthesis: improving outcomes and expanding indicationsOphthalmology2009116464065119243830
- PinelesSLEla-DalmanNRosenbaumALBinocular visual function in patients with Boston type I keratoprosthesesCornea2010291397140020847681
- CortinaMSHallakJVision-related quality-of-life assessment using NEI VFQ-25 in patients after Boston keratoprosthesis implantationCornea201534216016425411934
- KammerdienerLLSpeiserJLAquavellaJVProtective effect of soft contact lenses after Boston keratoprosthesisBr J Ophthalmol2015014
- RamaeshKRamaeshTDuttonGNDhillonBEvolving concepts on the pathogenic mechanisms of aniridia related keratopathyInt J Biochem Cell Biol20053754755715618012
- Massachusetts Eye and Ear InfirmaryBoston Keratoprosthesis update Available from: http://www.masseyeandear.org/~/media/testupload/files/2010-kpro-newsletter.pdf?la=enAccessed December 21, 2015
- SejpalKYuFAldaveAJThe Boston keratoprosthesis in the management of corneal limbal stem cell deficiencyCornea201130111187119421885964
- NauACDrexlerSDhaliwalDKMahFRajuLDeschlerEContact lens fitting and long-term management for the Boston keratoprosthesisEye Contact Lens201440318518924508771
- VajaranantTSBlairMPMcMahonTWilenskyJTde la CruzJSpecial considerations for pars plana tube-shunt placement in Boston type 1 keratoprosthesisArch Ophthalmol2010128111480148221060051
- LawSKHuangJSNassiriNTechnique of combined glaucoma tube shunt and keratoprosthesis implantationJ Glaucoma201423850150725275831
- HuhESArefAAVajaranantTSde la CruzJChauFYCortinaMSOutcomes of pars plana glaucoma drainage implant in Boston type 1 keratoprosthesis surgeryJ Glaucoma2014231e39e4424370810
- KiangLSippelKCStarrCEVitreoretinal surgery in the setting of permanent keratoprosthesisArch Ophthalmol2012130448749222491917
- TodaniACiolinoJBAmentJDTitanium back plate for a PMMA keratoprosthesis: clinical outcomesGraefes Arch Clin Exp Ophthalmol2011249101515151821519940
- PaschalisEChodoshJSpurr-MichaudSIn vitro and in vivo assessment of titanium surface modification for coloring the backplate of the Boston keratoprosthesisInvest Ophthalmol Vis Sci20135463863387323661373
- BehlauIMartinKVMartinJNInfectious endophthalmitis in Boston keratoprosthesis: incidence and preventionActa Ophthalmol2014927e546e55524460594
- BOSTON KPro newsAntimicrobial prophylaxis for life: as important as everFall2011 Number 8. Available from: http://www.masseye-andear.org/~/media/testupload/files/2011-kpro-newsletter.pdf?la=enAccessed December 21, 2015
- DurandMLDohlmanCHSuccessful prevention of bacterial endophthalmitis in eyes with the Boston keratoprosthesisCornea200928889690119654525
- BOSTON KPro newsBoston KPro: Past Successes and Future Challenges92015 Number 11. Available from: http://www.masseyeandear.org/~/media/testupload/files/kpronewsletter2015web.pdf?la=enAccessed December 21, 2015
- AmentJDPinedaRLawsonBBelauIDohlmanCHThe Boston Keratoprosthesis International Protocol Version 26152009
- ThomasMShorterEJoslinCEMcMahonTJCortinaMSContact Lens Use in Patients With Boston Keratoprosthesis Type 1: Fitting, Management, and ComplicationsEye Contact Lens201541633434026020487
- SayeghRRAvena DiazLVargas-MartínFWebbRHDohlmanCHPeliEOptical functional properties of the Boston KeratoprosthesisInvest Ophthalmol Vis Sci201051285786319815733
- KangJJAllemannNCruz JdeLCortinaMSSerial Analysis of Anterior Chamber Depth and Angle Status Using Anterior Segment Optical Coherence Tomography After Boston KeratoprosthesisCornea201332101369137423974896
- ShapiroBL1CortésDEChinEKHigh-resolution spectral domain anterior segment optical coherence tomography in type 1 Boston keratoprosthesisCornea201332795195523591146
- QianCXHassanalySHarissi-DagherMAnterior segment optical coherence tomography in the long-term follow-up and detection of glaucoma in Boston type I keratoprosthesisOphthalmology2015122231732525264027
- FernandezAGRadcliffeNMSippelKCBoston type I keratoprosthesis-donor cornea interface evaluated by high-definition spectral-domain anterior segment optical coherence tomographyClin Ophthalmol201261355135922969280
- KamyarRWeizerJSde PaulaFHGlaucoma associated with Boston type I keratoprosthesisCornea201231213413922134402
- PanarelliJFKoASidotiPAGarciaJPBanittMRAngle closure after Boston keratoprosthesisJ Glaucoma201322972572922595935
- GarciaJPJrde la CruzJRosenRBBuxtonDFImaging implanted keratoprostheses with anterior-segment optical coherence tomography and ultrasound biomicroscopyCornea200827218018818216573