Abstract
Background
The purpose of this study was to prospectively evaluate and compare intraocular pressure (IOP) reduction efficacy and safety between bimatoprost and latanoprost-timolol fixed combination (LTFC) in Japanese patients with open-angle glaucoma.
Methods
In this prospective, randomized, non-masked study, after enrolling 70 eyes of 70 Japanese open-angle glaucoma patients who had used latanoprost monotherapy for more than 4 weeks, the subjects were randomly divided into a bimatoprost group or an LTFC group. Both groups were switched from latanoprost to bimatoprost or LTFC for 12 weeks. IOP, conjunctival injection score, corneal epitheliopathy score (area density classification; AD score), tear film break-up time, heart rate, and blood pressure were evaluated at 0, 4, and 12 weeks after switching. The paired t-test and Mann–Whitney U-test were used for the statistical analysis.
Results
After 13 of the 70 patients dropped out, 57 were analyzed for IOP reduction and safety. There was a significant decrease in mean IOP at 4 weeks compared with week 0 in both groups (both P<0.0001). Comparisons between the two groups showed no statistically significant differences. The conjunctival injection score was higher in the bimatoprost group than in the LTFC group at 12 weeks (P=0.0091). There were no statistically significant differences between the two drugs in relation to AD score, tear film break-up time, heart rate, and blood pressure.
Conclusion
Bimatoprost and LTFC exhibited similar efficacy for reduction of IOP. Safety results indicated that only the conjunctival injection score at 12 weeks was higher in the bimatoprost group compared with the LTFC group.
Introduction
Glaucoma is one of the most devastating diseases worldwide,Citation1,Citation2 as is the main cause of blindness in adults. Studies have shown that intraocular pressure (IOP) is the most important risk factor for glaucoma and its progression.Citation3,Citation4 Since reduction of IOP is reportedly the most effective treatment for glaucoma,Citation5,Citation6 topical antiglaucoma medications are usually chosen as the primary treatment. Although topical β-blockers have been the primary drugs of choice in the past, prostaglandin analogs are now playing an important role in the medical treatment of the disease. In many cases, these new drugs have halted or reduced the progression of glaucoma.Citation5,Citation7–Citation9 The guidelines of the European Glaucoma Society and Japanese Glaucoma SocietyCitation10 have recommended that if the first-choice monotherapy is not effective by itself, it is preferable to switch to another drug before giving consideration to any drug combination. Furthermore, when use of a prostaglandin analog alone cannot achieve glaucoma control, another prostaglandin or, perhaps, a second drug is added.
Bimatoprost 0.03% (LUMIGAN®, Senju Pharmaceutical Co Ltd, Osaka, Japan) is a synthetic prostamide analog. It has been reported that bimatoprost acts on a prostamide-specific receptor that is different from normal prostaglandin receptors, which suggests a new mechanism of action.Citation11 The IOP-lowering properties of bimatoprost are achieved by enhancing both the trabecular meshwork and uveoscleral outflow without diminishing the production of aqueous humor.Citation12 Several studies have reported that bimatoprost exhibits a greater IOP-lowering effect than latanoprost 0.005% (Xalatan®, Pfizer Inc, Tokyo, Japan).Citation12–Citation15 Among the possible drug combinations that can be used when adding a second drug, utilization of a prostaglandin analog with a β-blocker has been shown to be one of the most effective and widely prescribed combinations.Citation16 In Japan, the fixed combination of latanoprost 0.005% and timolol maleate 0.5% (LTFC, Xalacom®, Pfizer Inc) has recently become available. Among the several countries that have conducted trials for this drug, some have reported finding no significant advantage compared with latanoprost monotherapy,Citation17,Citation18 whereas others have reported that LTFC was as effective as concomitant administration of the individual components.Citation19–Citation21 However, to date, there have been no reported clinical trials on the IOP reduction efficacy and safety between bimatoprost and LTFC in Japanese patients with glaucoma.
The current study was designed to prospectively evaluate and compare the IOP reduction efficacy and safety using bimatoprost and LTFC in Japanese open-angle glaucoma patients who had not controlled by latanoprost monotherapy.
Subjects and methods
In this prospective, randomized, non-masked study, we examined 70 eyes of 70 Japanese open-angle glaucoma patients (42 females and 28 males, mean age 66.9±12.9 years) who had received latanoprost monotherapy for more than 4 weeks in at least one eye. Patients were randomly divided into a bimatoprost group or an LTFC group. Both groups were switched from latanoprost to either bimatoprost or LTFC for 12 weeks. This study was approved by the institutional review board of Kyoto Prefectural University of Medicine, Kyoto, Japan. All experimental procedures were conducted in accordance with the tenets set forth in the Declaration of Helsinki. Written informed consent was obtained from all patients. Patients who were treated by latanoprost monotherapy at the Kyoto Prefectural University of Medicine and Oike-Ikeda Eye Clinic and could not achieve IOP control or had progressive glaucomatous changes in their visual field were enrolled in the study between 2011 and 2013. All subjects involved in the study were Japanese. The diagnostic criteria for normal-tension glaucoma (NTG) were: (1) normal iridocorneal open angle, (2) no evidence of high IOP (IOP ≤21 mmHg), (3) glaucomatous changes in the visual field with optic nerve cupping, and (4) absence of other optic neuropathies. For the diagnosis of primary open-angle glaucoma (POAG), while numbers (1), (3), and (4) of the earlier listed criteria were the same, patients had to have a maximum IOP >21 mmHg. All NTG and POAG diagnoses were made according to the guidelines of the Japan Glaucoma Society and European Glaucoma Society. Patients with a past history of other treatments, such as laser surgery or glaucoma surgery, were excluded from the study. We also excluded patients who had diseases that are not suitable for β-blocker eyedrops, such as asthma, hyperreactive airway disease, and heart disease.
In all patients, IOP, conjunctival injection score (grade: 0–3), corneal epitheliopathy score (area density classification; AD score), tear film break-up time (BUT), systolic and diastolic blood pressure (BP), and heart rate (HR) were evaluated at 0, 4±1, and 12±2 weeks after switching. Since each patient came to the hospital at the same time for these three measurements, we were able to measure all parameters at the same time of day. IOP was measured by a Goldmann applanation tonometer. If both eyes were available, we used the right eye data only. At every visit, the mean BUT score was calculated from three measurements determined using a stopwatch, and conjunctival status were evaluated from slit-lamp images. Three glaucoma specialists conducted the study.
The following parameters were compared between the two groups: mean IOP values, mean IOP changes, injection score, AD score, BUT, HR, systolic BP, and diastolic BP. Mean IOP changes were compared between the two groups at different time points.
Statistical analysis
Fisher’s exact test was used to compare the number of patients in the two groups who had responded to therapy and exhibited an IOP reduction. We also compared the ratio of patients in the two groups who exhibited no IOP changes, increased IOP, or reduced IOP. Patients who had changes that were within a ±10% IOP reduction were classified as exhibiting no changes. The paired Student’s t-test was used to compare parameter values within each of the two groups, while the Mann–Whitney U-test was used for statistical comparison of values between the two groups. A P-value of <0.05 was considered to be statistically significant. These analyses were performed using scientific biostatistics graphing software (GraphPad Prism® version 6.0.3, GraphPad Software Inc, La Jolla, CA, USA). For the sample size calculations, the significance level was set to 5% and the power to 80%. The 35-patients-per-group number was calculated for a Δ of 1.7 mmHg and standard deviation of 2.5 mmHg.
Results
Patient characteristics
Thirteen of the 70 patients dropped out of the study, resulting in 57 (33 females and 24 males, mean age 64.7±12.9 years) being available for analysis of IOP reduction and safety. The primary reason why patients dropped out of the study was poor compliance (five patients in the LTFC group). Side effects led to four patients in the bimatoprost group (itching and blurred vision) and two patients in the LTFC group (coughing and palpitations, pain in the eyes) also dropping out of the study. Another two patients were withdrawn because they were discovered to be taking oral steroid medications.
Of the 57 analyzed patients, 30 were assigned to the bimatoprost group (19 females and eleven males, mean age 66.4±12.4 years) and 27 were assigned to the LTFC group (14 females and 13 males, mean age 62.9±13.5 years). The bimatoprost group consisted of five POAG patients and 22 NTG patients, while the LTFC group consisted of six POAG patients and 24 NTG patients. There were no statistically significant differences found between groups for mean age (P=0.3001) or sex (P=0.4295) ratio.
Mean IOP change in the bimatoprost and LTFC groups
At 0, 4, and 12 weeks, the mean IOPs of the bimatoprost (30 eyes) and LTFC (27 eyes) groups were 13.2, 11.6, and 11.6 mmHg, and 13.3, 11.5, and 11.6 mmHg, respectively. In both groups, there was already a significant decrease in mean IOP at 4 weeks compared with week 0 (P<0.0001 in both groups; ). There were no IOP differences at 12 weeks compared with the values at 4 weeks in both groups (P=0.8498 for the bimatoprost group and P=0.8427 for the LTFC group; ). IOP comparisons between the two groups additionally showed no significant differences at 4 or 12 weeks (P=0.6743 and P=0.9143; , ).
Figure 1 Mean IOP change from baseline (week 0) in the bimatoprost and LTFC groups.
Abbreviations: IOP, intraocular pressure; LTFC, latanoprost-timolol fixed combination; ns, non-significant.
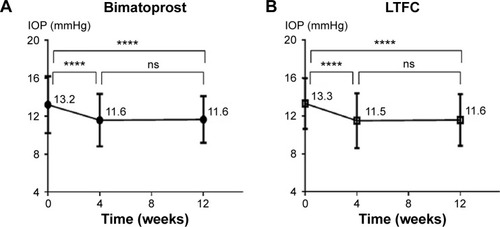
Figure 2 Mean IOP change from baseline (week 0). No significant difference between the two groups was observed at week 4 (P=0.4052) or at week 12 (P=0.6968).
Abbreviations: IOP, intraocular pressure; LTFC, latanoprost-timolol fixed combination.
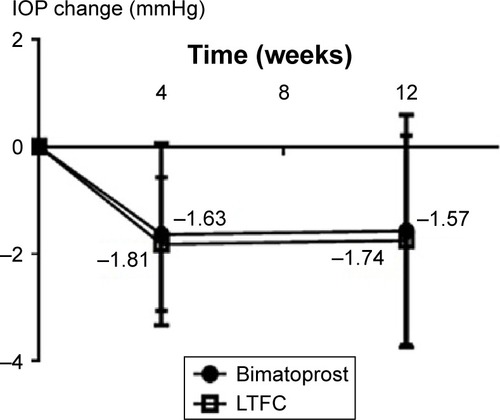
Table 1 Intraocular pressure levels in the LTFC and bimatoprost groups at baseline, week 4, and week 12
We also compared the two groups for the ratio of patients with no IOP changes, with an increased IOP, and with a reduced IOP. There were no significant differences in any of the ratios between any of the groups analyzed ().
Table 2 Percentage of patients whose mean IOP increased, decreased, or stayed the same compared with baseline after switching from latanoprost monotherapy at week 12
Side effects observed in the LTFC and bimatoprost groups
The conjunctival injection scores were 0.6±0.5, 0.9±0.7, and 1.2±0.8 for the bimatoprost group and 0.7±0.5, 0.7±0.6, and 0.6±0.5 for the LTFC group at 0, 4, and 12 weeks, respectively. The conjunctival injection score at 12 weeks was higher in the bimatoprost group vs the LTFC group (P=0.0091; ). There were no significant differences in either of the groups for the conjunctival injection score at 4 weeks (P=0.4032; ).
Table 3 Side effects in the LTFC and bimatoprost groups at baseline, week 4, and week 12
The corneal AD scores (total score of area and density grade) at baseline were 1.9±1.5 for the bimatoprost group and 1.6±1.4 for the LTFC group. The scores were 1.5±1.4 and 1.6±1.4 for the bimatoprost group and 1.6±1.4 and 1.7±1.4 for the LTFC group at 4 and 12 weeks, respectively. At baseline, BUT was 6.0±4.4 seconds in the bimatoprost group and was 5.2±4.0 seconds in the LTFC group. The values were 6.0±4.7 and 5.4±4.3 seconds in the bimatoprost group and 5.0±4.5 and 5.2±3.9 seconds in the LTFC group at 4 and 12 weeks, respectively. There were no significant differences between the two drugs in relation to AD score or BUT ().
At 0, 4, and 12 weeks, the mean systolic/diastolic BP in the bimatoprost group and LTFC group was 127/73, 127/74, and 129/75 mmHg, respectively, and 130/75, 128/76, and 129/74 mmHg. There were no significant differences in systolic BP or diastolic BP at 4 and 12 weeks compared with the values at week 0 in either group. BP comparisons between the two groups also showed no significant differences at 4 or 12 weeks ().
At 0, 4, and 12 weeks, mean HR in the bimatoprost and LTFC groups was 80, 79, and 77 beats per minute, respectively, and 77, 76, and 77 beats per minute. There were no significant differences in HR at 4 and 12 weeks compared with week 0 in either group. In addition, there were no significant differences in HR comparisons between the two groups at 4 and 12 weeks (P=0.3719 and P=0.8803; ).
Discussion
In this randomized clinical study, 70 Japanese open-angle glaucoma patients uncontrolled IOP being treated by latanoprost only were changed to a new therapy of either bimatoprost or LTFC. While bimatoprost and LTFC have previously been reported to have the same efficacy for lowering IOP in some studies,Citation22–Citation24 another study reported that LTFC was superior to bimatoprost in reducing IOP.Citation25 Our study, which is the first randomized prospective clinical trial of these two treatments in the Japanese population, showed that both treatments were effective in controlling IOP starting from baseline. It has also been reported that bimatoprost has a greater IOP-lowering effect than latanoprost,Citation12–Citation15 and that LTFC reduces the IOP levels to a greater degree than latanoprost monotherapyCitation26–Citation30 in both the Japanese population and in other races. We found similar results in our current study.
However, some patients in the current study did not exhibit any IOP reduction after being changed to either bimatoprost or LTFC. Since we only enrolled patients who responded to latanoprost, it is possible that patients who exhibited no reduction in their IOP after being changed to LTFC could have been nonresponders or poor responders to β-blockers. It has also been reported that LTFC was more effective than latanoprost monotherapy, but less efficacious than unfixed combinations of latanoprost-timolol.Citation31 In these patients, the β-blocker had been previously used twice a day while LTFC was used only once a day, so LTFC might be less effective if we used latanoprost once a day plus β-blockers twice a day.
On the other hand, there were also 13 patients (43.3%) who did not exhibit a reduced IOP after being changed to bimatoprost, indicating that the effect of bimatoprost is almost equal to that of latanoprost. One patient (3.3%) who exhibited an increased IOP after being changed to bimatoprost was thought to be a nonresponder to bimatoprost. The IOP-lowering effect of bimatoprost was reported to be equal to that of latanoprost in a previous report.Citation32 We found similar results in some of our patients in the current study. Thus, the IOP-lowering effect of bimatoprost could differ from patient to patient. Some patients did not exhibit a reduced IOP after switching to bimatoprost or LTFC; however, most of the patients exhibited minor IOP reduction. Moreover, IOP was considerably reduced in some patients after switching to bimatoprost and LTFC, so we found significant differences in IOP after switching from latanoprost.
While it appears that bimatoprost has great potential with regard to its IOP-lowering effect, it has been reported to cause more conjunctival hyperemia than has been seen in patients treated with latanoprostCitation15,Citation33 or LTFC.Citation23,Citation24 In our study, we found that the conjunctival injection score at 12 weeks was higher in the bimatoprost group vs the LTFC group, which mirrors the results of the previous reports.
Since LTFC contains β-blocker eyedrops, the possibility exists that it could affect both HR and BP. β-blocker eyedrops can be directly absorbed into the systemic circulation, which occurs mainly through the nasopharyngeal mucosa. However, this has yet to be definitively clarified, as some previous papers have reported that eyedrops containing a β-blocker affected the HR but not the BP,Citation25,Citation34 while other studies have reported that these eyedrops did not affect either HR or BP.Citation35,Citation36 Our current study could not find any differences in HR or BP between the bimatoprost and LTFC groups. HR and BP changes can also be affected by several other parameters, such as age and duration of β-blocker eyedrop use. Since β-blocker eyedrops cannot be used in patients with heart or pulmonary disease, we excluded these patients from our study. Thus, this exclusion could have affected our results. It has recently been reported that no differences were found in 24-hour IOP, systolic BP, or diastolic BP between latanoprost and bimatoprost in NTG patients.Citation37 In that study, they also investigated diastolic ocular perfusion pressure (DOPP), and the mean 24-hour DOPP for latanoprost was increased from baseline for latanoprost, but not for bimatoprost. In this study, we did not investigate DOPP; however, it would be interesting to investigate.
It has also been reported that β-blocker eyedrops can have adverse effects on the ocular surface epithelium or tear function.Citation38–Citation40 Our current study could not find any differences in either the AD scores or the BUT. LTFC eyedrops contain a β-blocker (timolol). Since we used LTFC once a day and timolol is usually used twice a day, this could have affected our results. In a previous report, significant differences were found at 20 weeks after starting β-blocker eyedrops.Citation29 We only observed our patients for 12 weeks, and if we had continued our investigations for a longer time period, we may have found differences between the two groups.
The results of the current study indicated no differences in IOP reduction in the bimatoprost and LTFC groups, suggesting that both treatments can be considered for use in patients with uncontrolled IOP after latanoprost monotherapy. However, since both types of eyedrop are associated with various side effects, the type of eyedrop used needs to be carefully chosen in accordance with each individual case. Furthermore, as patients with pulmonary or heart disease can encounter severe side effects after administration of β-blocker eyedrops, the past history should be carefully examined before choosing the type of eyedrop to use.
Some patients are not aware that heart or pulmonary disease is a contraindication to β-blocker use when the symptoms of those patients are mild. Moreover, as the possibility exists that these diseases can emerge with age, it should always be considered that there is a possible risk of side effects in patients even when they report that they do not have these diseases. Moreover, since there is an increase in the percentage of patients with pulmonary or heart disease with age, it might be best to choose bimatoprost rather than LTFC when treating elderly patients.
Out of the total of 70 patients, 13 patients dropped out before finishing this study. Reasons for dropping out included not being able to visit the hospital at the appointed time or because they found it impossible to use the eyedrops according to the set schedule. The fact that so many subjects dropped out raises the possibility that compliance issues could be a major factor that needs to be taken into consideration when setting up treatments. We also found that side effects led to four patients in the bimatoprost group (itching and blurred vision) and two patients in the LTFC group (coughing and palpitations, pain in their eyes) dropping out of the study. Even though the number of patients who dropped out due to side effects was larger in the bimatoprost group than in the LTFC group in this study, bimatoprost was a better treatment for some patients because bimatoprost did not contain a β-blocker and was able to effectively control their IOP.
Since more conjunctival injections are required for bimatoprost vs LTFC, cosmetic problems are of particular concern in women and young people, and it is recommended that LTFC be used in patients who express a concern about these potential cosmetic problems. Therefore, when choosing eyedrops to treat patients, one must carefully consider not only the patient’s age, sex, and physical condition, but also the degree of compliance expected.
It should be noted that this study did have some limitations. First, we only compared the efficacies of bimatoprost and LTFC in patients who had been previously treated with latanoprost. Furthermore, this study did not simply compare the IOP-lowering effects of the two drugs.
Another limitation was that there were four times as many NTG patients vs POAG patients in this study. Using a Japanese population, the findings of a previous study revealed that there were many more NTG patients than POAG patients.Citation41 We had to further decrease the IOP in the NTG patients when one drug could not halt or reduce the progression of visual field loss if the IOP was in the low teen values because the baseline was low. In a fixed combination study, it was reported that there was a strong linear relationship between the mean baseline IOP and the treatment-induced mean reduction in IOP.Citation42 In that study, since most of the patients were NTG patients with the characteristic Japanese glaucoma type, no significant differences were found between the two groups because the IOP reduction was low due to the low baseline IOP. We plan on enrolling more POAG patients to compare the IOP reduction between bimatoprost and LTFC in POAG patients in a future investigation.
An additional limitation was that there was no washout period when we switched from latanoprost to bimatoprost or LTFC. In this study, some patients’ visual field loss progressed with the disease, so there was not enough time to have a washout period. However, we evaluated IOP and other parameters at 12 weeks after switching from latanoprost, which should be a sufficient amount of time to eliminate the influence of latanoprost.
A further limitation was that although we compared IOP and BP at the same time in each patient, we were not able to measure IOP and BP at the same time in all patients. Further, we had compliance issues. There might be a possibility that the IOP reduction after switching to bimatoprost or LTFC was partly due to the fact that patient compliance was better after switching from latanoprost, ie, the Hawthorne effect.
We also had additional limitations. Although this study was randomized, it was not masked, and it was conducted not only in a university hospital but also in a private clinic, so it was difficult to prescribe eyedrops in a masked manner. Another limitation was that although 70 patients were enrolled, only 57 patients were ultimately analyzed due to the number of dropouts. In the future, we plan to enroll a greater number of patients for analysis. Finally, we only observed patients for 12 weeks in the current study. Thus, further investigations with a longer follow-up period will need to be undertaken.
Conclusion
Bimatoprost and LTFC have similar efficacy for the reduction of IOP. Safety comparisons between the two drugs showed that only the conjunctival injection score at 12 weeks was higher in the bimatoprost group vs the LTFC group.
Disclosure
YI, KM and MU have received financial support from Senju Pharmaceutical Co Ltd. The authors have no other conflicts of interest in this work.
References
- ResnikoffSPascoliniDEtya’aleDGlobal data on visual impairment in the year 2002Bull World Health Organ2004821184485115640920
- QuigleyHABromanATThe number of people with glaucoma worldwide in 2010 and 2020Br J Ophthalmol200690326226716488940
- LeskeMCConnellAMWuSYHymanLGSchachatAPRisk factors for open-angle glaucoma. The Barbados Eye StudyArch Ophthalmol199511379189247605285
- ColemanALMigliorSRisk factors for glaucoma onset and progressionSurv Ophthalmol200853Suppl 1S3S1019038621
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol2002120670171312049574
- HeijlALeskeMCBengtssonBHusseinMMeasuring visual field progression in the Early Manifest Glaucoma TrialActa Ophthalmol Scand200381328629312780410
- LichterPRMuschDCGillespieBWInterim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgeryOphthalmology2001108111943195311713061
- AGIS InvestigatorsThe Advanced Glaucoma Intervention Study (AGIS): 9. Comparison of glaucoma outcomes in black and white patients within treatment groupsAm J Ophthalmol2001132331132011530042
- HeijlALeskeMCBengtssonBHymanLHusseinMReduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma TrialArch Ophthalmol2002120101268127912365904
- The Japan Glaucoma Society Guidelines for Glaucoma (3rd Edition)Nihon Ganka Gakkai Zasshi20121161346 Japanese22352070
- SharifNAWilliamsGWKellyCRBimatoprost and its free acid are prostaglandin FP receptor agonistsEur J Pharmacol20014322–321121311740958
- BrubakerRFMechanism of action of bimatoprost (Lumigan)Surv Ophthalmol200145Suppl 4S347S35111434937
- GandolfiSSimmonsSTSturmRChenKVanDenburghAMThree-month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertensionAdv Ther200118311012111571823
- HigginbothamEJSchumanJSGoldbergIOne-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertensionArch Ophthalmol2002120101286129312365906
- SatoSHirookaKBabaTEfficacy and safety of switching from topical latanoprost to bimatoprost in patients with normal-tension glaucomaJ Ocul Pharmacol Ther201127549950221790301
- VenturaMPSahebNESolariHPCost considerations of the new fixed combinations for glaucoma medical therapyJ Clin Pharm Ther200530325125415896242
- HigginbothamEJFeldmanRStilesMDubinerHLatanoprost and timolol combination therapy vs monotherapy: one-year randomized trialArch Ophthalmol2002120791592212096962
- MagachoLReisRShettyRKSantosLCAvilaMPEfficacy of latanoprost or fixed-combination latanoprost-timolol in patients switched from a combination of timolol and a nonprostaglandin medicationOphthalmology2006113344244516458964
- KonstasAGBanyaiLBlaskKDIntraocular pressure and safety in glaucoma patients switching to latanoprost/timolol maleate fixed combination from mono- and adjunctive therapiesJ Ocul Pharmacol Ther200420537538215650512
- DiestelhorstMLarssonLIA 12-week, randomized, double-masked, multicenter study of the fixed combination of latanoprost and timolol in the evening versus the individual componentsOphthalmology20061131707616263174
- InoueKOkayamRHigaROcular hypotensive effects and safety over 3 months of switching from an unfixed combination to latanoprost 0.005%/timolol maleate 0.5% fixed combinationJ Ocul Pharmacol Ther201127658158722011049
- ManniGCentofantiMParravanoMOddoneFBucciMGA 6-month randomized clinical trial of bimatoprost 0.03% versus the association of timolol 0.5% and latanoprost 0.005% in glaucomatous patientsGraefes Arch Clin Exp Ophthalmol2004242976777015241611
- RossettiLKarabatsasCHTopouzisFComparison of the effects of bimatoprost and a fixed combination of latanoprost and timolol on circadian intraocular pressureOphthalmology2007114122244225117459480
- MesciCAydinNErbilHHTwenty-four-hour intraocular pressure control with latanoprost-timolol-fixed combination versus bimatoprost in patients who switched from timololJ Glaucoma201120847748121048508
- FacioACReisASVidalKSA comparison of bimatoprost 0.03% versus the fixed-combination of latanoprost 0.005% and timolol 0.5% in adult patients with elevated intraocular pressure: an eight-week, randomized, open-label trialJ Ocul Pharmacol Ther200925544745119860553
- InoueKFujimotoTHigaREfficacy and safety of a switch to latanoprost 0.005% + timolol maleate 0.5% fixed combination eyedrops from latanoprost 0.005% monotherapyClin Ophthalmol2012677177522693419
- VarmaRHwangLJGrundenJWBeanGWUsing diurnal intraocular pressure fluctuation to assess the efficacy of fixed-combination latanoprost/timolol versus latanoprost or timolol monotherapyBr J Ophthalmol2010941808419692375
- PalmbergPKimEEKwokKKTresslerCSA 12-week, randomized, double-masked study of fixed combination latanoprost/timolol versus latanoprost or timolol monotherapyEur J Ophthalmol201020470871820099236
- LazaridouMNMontgomeryDMHoWOJaberooDChanges in intraocular pressure following a switch from latanoprost monotherapy to latanoprost/timolol fixed combination therapy in patients with primary open-angle glaucoma or ocular hypertension: results from a clinical practice databaseCurr Med Res Opin200824102725272818713491
- KonstasAGBoboridisKTzetziDTwenty-four-hour control with latanoprost-timolol-fixed combination therapy vs latanoprost therapyArch Ophthalmol2005123789890216009829
- QuarantaLBiagioliERivaIProstaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysisJ Ocul Pharmacol Ther201329438238923231442
- HuangHLSunXHXiaoMComparison of intraocular pressure reducing effects of three prostaglandin eyedrops in open-angle glaucomaZhonghua Yan Ke Za Zhi2011472109113 Chinese21426839
- CassonRJLiuLGrahamSLEfficacy and safety of bimatoprost as replacement for latanoprost in patients with glaucoma or ocular hypertension: a uniocular switch studyJ Glaucoma200918858258819826386
- KashiwagiKEfficacy and safety of switching to travoprost/timolol fixed-combination therapy from latanoprost monotherapyJpn J Ophthalmol201256433934522581454
- ChiselitaDAntohiIMedvichiRDanielescuCComparative analysis of the efficacy and safety of latanoprost, travoprost and the fixed combination timolol-dorzolamide; a prospective, randomized, masked, cross-over design studyOftalmologia20054933945 Romanian16408674
- MoisseievEKurtzSLazarMShemeshGIntraocular pressure reduction using a fixed combination of timolol maleate 0.5% and brimonidine tartrate 0.2% administered three times dailyClin Ophthalmol201371269127323836956
- QuarantaLPizzolanteTRivaITwenty-four-hour intraocular pressure and blood pressure levels with bimatoprost versus latanoprost in patients with normal-tension glaucomaBr J Ophthalmol20089291227123118586898
- ShimazakiJHanadaKYagiYChanges in ocular surface caused by antiglaucomatous eyedrops: prospective, randomised study for the comparison of 0.5% timolol v 0.12% unoprostoneBr J Ophthalmol200084111250125411049949
- OhtsukiMYokoiNMoriKAdverse effects of beta-blocker eye drops on the ocular surfaceNihon Ganka Gakkai Zasshi2001105314915411280872
- ValenteCIesterMCorsiERolandoMSymptoms and signs of tear film dysfunction in glaucomatous patientsJ Ocul Pharmacol Ther201127328128521557633
- IwaseASuzukiYAraieMThe prevalence of primary open-angle glaucoma in Japanese: the Tajimi StudyOphthalmology200411191641164815350316
- HolloGVuorinenJTuominenJFixed-dose combination of tafluprost and timolol in the treatment of open-angle glaucoma and ocular hypertension: comparison with other fixed-combination productsAdv Ther201431993294425213118
