Abstract
Purpose
To evaluate the durability of fixed bimonthly dosing of intravitreal aflibercept for neovascular age-related macular degeneration.
Methods
Records of 16 patients were retrospectively reviewed. Patients received three initial 2.0 mg monthly doses of aflibercept then 8-weekly doses according to the product label. Best-corrected visual acuity (Early Treatment Diabetic Retinopathy Study [ETDRS] letters), central macular thickness, fluid on optical coherence tomography, and pigment epithelial detachment (PED) were measured.
Results
Prior to starting aflibercept, 13 patients had subretinal fluid (SRF), five had intraretinal fluid (IRF), four had PED, and baseline visual acuity (VA) was 62 approximate ETDRS letters. Following the monthly dosing, seven patients had no improvement or decreased VA, ten patients still had SRF/IRF, and PED had worsened in one patient. At Visit 4, an average of 6.8 weeks after Visit 3, VA had decreased in seven patients, SRF/IRF had increased in 12 patients, and PED had returned in all patients who initially responded. Based on the presence of fluid after the initial monthly injections, 12 patients could not be extended to fixed bimonthly dosing.
Conclusion
This study adds to the growing body of evidence on the need for flexible dosing schedules for the personalized treatment of neovascular age-related macular degeneration.
Introduction
Therapies that target vascular endothelial growth factor (VEGF) have revolutionized the treatment of neovascular (or wet) age-related macular degeneration (nAMD) and have become the standard of care for this condition.Citation1
Ranibizumab (Lucentis®; Novartis Pharma AG, Basel, Switzerland, and Genentech, Inc., South San Francisco, CA, USA), a recombinant, humanized anti-VEGF Fab fragment that neutralizes all forms of VEGF-A,Citation2 was approved for intravitreal use to treat nAMD by the Food and Drug Administration (FDA) in 2006.Citation3 According to the European summary of product characteristics, patients can be given monthly injections until maximal visual acuity (VA) is achieved, followed by flexible dosing, where treatment and monitoring intervals are based on disease activity as assessed by VA and/or anatomical parameters visualized using optical coherence tomography (OCT) or other appropriate imaging modalities.Citation3
Bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA, USA, a member of the Roche group) is also used to treat nAMD; however, this drug is not licensed for nAMD, and its use is off-label. Bevacizumab is a humanized monoclonal anti-VEGF antibody that was initially designed as an anti-angiogenesis inhibitor to treat metastatic colorectal cancer.Citation4,Citation5
Aflibercept (Eylea®; Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA, and Bayer AG, Berlin, Germany) is a soluble recombinant fusion protein, which is made up of portions of the human VEGF receptor fused to the Fc portion of human IgG1,Citation6 and binds to VEGF-A, VEGF-B, and placental growth factor.Citation7 In 2011, aflibercept was approved by the FDA for nAMD,Citation8 with the current approved treatment regimen for aflibercept consisting of three initial 2 mg monthly injections followed by 8-weekly dosing.Citation8,Citation9
When faced with a patient with nAMD, the ophthalmologist therefore has three potential anti-VEGF therapies to choose from: ranibizumab, bevacizumab, and aflibercept. The decision on which anti-VEGF to treat patients with is multifactorial and factors include availability, dosing schedule, recent trial data on efficacy and safety, and cost.Citation10 Based on the efficacy data from the VIEW trial, we decided to start fixed bimonthly aflibercept treatment in some of our patients in accordance with the product label. This study reports our early experience with these patients and was conducted to assess whether the response to fixed 8-weekly aflibercept treatment was beneficial in all treatment-experienced patients with nAMD.
Patients and methods
Study design
This was a consecutive, retrospective, observational study to assess visual outcomes in treatment-experienced patients with nAMD who were treated with aflibercept in accordance with the drug label. The study was conducted in a single center in the USA between December 2011 and November 2013. To be included in the study, patients must have been diagnosed with nAMD and received three consecutive monthly loading doses of aflibercept 2.0 mg (Visits 1–3), in accordance with the product label, and a follow-up visit with an average of 6.8 weeks after the monthly dosing (Visit 4). In addition, all the patients must have received one or more previous treatments of ranibizumab 0.5 mg or bevacizumab 1.25 mg. Patients who did not receive the three loading doses of aflibercept were excluded from the study.
Diagnosis
Neovascular AMD was diagnosed by fundoscopy, OCT, and fluorescein angiography.
Outcome measures
Patient demographics
Demographic data were collected and included age, sex, ethnicity, treated eye, number of previous ranibizumab and/or bevacizumab injections, time between injections, and response to treatment as determined by OCT and best-corrected visual acuity (BCVA).
OCT outcomes
Central macular thickness (CMT) was recorded at baseline and at each visit. The presence of pigment epithelial detachment (PED) or intra- or subretinal fluid (IRF and SRF, respectively) on OCT was also recorded at baseline and at each visit.
VA
BCVA was assessed using the Snellen scale at each visit. VA data were converted to approximate Early Treatment Diabetic Retinopathy Study (ETDRS) letters using the method reported by Gregori et al.Citation11
Ethics statement
Due to the retrospective nature of this study, it received exempt approval from the Western Institutional Review Board. Accordingly, patient information was anonymized and de-identified before analysis. Research was carried out in accordance with the Declaration of Helsinki.
Results
A total of 16 eyes from 16 patients met the inclusion criteria. Baseline patient demographics are shown in . The mean age of the patients was 75 years (range: 63–88 years). All of the patients were Caucasian, and the majority (12; 75%) was women. Equal numbers of right and left eyes were treated. The patients received a mean of 9.4 injections (range: 2–19) of either bevacizumab, ranibizumab, or a combination of both before aflibercept treatment. Following the initial three monthly aflibercept injections, the patients received a follow-up assessment with an average of 6.8 weeks after the third injection.
Table 1 Patient demographics
BCVA
The mean BCVA at baseline (Visit 1) was 62 approximate ETDRS letters (range: 0–85 approximate ETDRS letters) (). At the end of the monthly dosing period, nine patients (56%) had improved BCVA and seven patients (44%) had stable or decreased BCVA. Mean BCVA at month 3 was 67 approximate ETDRS letters. However, between Visit 3 and Visit 4, seven patients (44%) had decreased VA, and the mean BCVA at Visit 4 was 63 approximate ETDRS letters. This meant that there was an overall mean loss of four approximate ETDRS letters between Visit 3 and Visit 4.
Table 2 Changes in BCVA in 16 patients treated with fixed aflibercept dosingTable Footnotea
CMT
summarizes the CMT measurements across the study period. CMT at baseline ranged from 244 to 571 μm, with a mean of 367 μm. Following the three initial monthly doses, there were improvements in CMT in 13 patients (87%). The mean reduction in CMT from baseline to Visit 3 was 76 μm. Between Visit 3 and Visit 4, there was a mean increase in CMT of 54.4 μm (range: −32–383 μm).
Table 3 Changes in CMT (μm) and fluid observations in 16 patients treated with fixed aflibercept dosingTable Footnotea
PED
PED was observed in four patients at baseline (Visit 1). Following the initial monthly dosing, PED improved in three patients and enlarged in one patient. However, by Visit 4, PED was apparent in all the four patients ().
Table 4 PED measurements (μm) at Visits 1–4
IRF and SRF
All 16 patients presented with fluid at baseline (Visit 1) on OCT ( and ). IRF, including intraretinal cysts, was present in five patients (31%), and SRF was present in 13 patients (81%). Following the initial three monthly aflibercept injections, ten patients (77%) had improved SRF and two out of five patients (40%) had improved IRF. At the fourth visit, SRF had increased in nine out of 13 patients (69%), existing IRF was increased in three out of five patients (60%), and new IRF had developed in one out of 16 patients (6.3%). Fluid remained in 12 out of 16 patients (75%).
Figure 1 Optical coherence tomography images from eye no 8.
Abbreviations: no, number; CMT, central macular thickness.
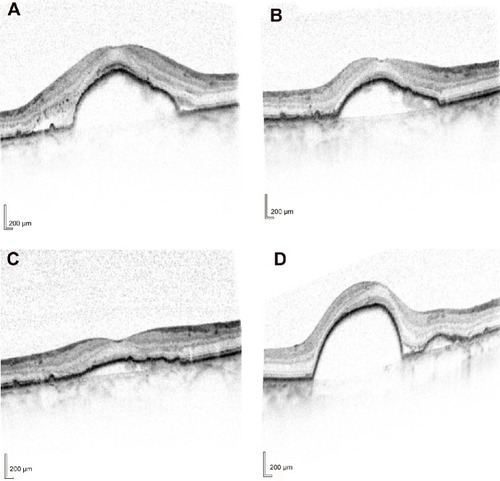
Based on the presence of IRF and SRF described earlier, the majority of patients (75%) had recurrent fluid and could not be extended to dosing every 8 weeks ().
Discussion
This study reports our clinical experience using fixed bimonthly aflibercept in treatment-experienced patients with nAMD. Overall, there were anatomical (SRF and/or IRF) and visual improvements following the initial three monthly aflibercept injections in 12 patients (75%) and nine patients (56%), respectively; however, when the dosing interval was extended, BCVA decreased in seven patients (44%) and fluid increased in 12 patients (75%). Only four patients (25%) could be extended to dosing every 8 weeks based on fluid on OCT. These findings suggest that the fixed 8-weekly dosing schedule may not be optimal for all treatment-experienced patients in clinical practice. This is an important consideration for ophthalmologists as the aflibercept product label mandates 8-weekly dosing in the first year of treatment for nAMD.Citation8
While 8-weekly injections have several potential benefits over monthly injections and monitoring, including fewer visits for patients and fewer ocular injections, a careful balance must be struck to maintain any gains achieved with monthly dosing. Data from the pivotal VIEW trials showed that 8-weekly aflibercept dosing following three initial monthly injections was noninferior to monthly ranibizumab dosing at week 52.Citation12 In addition, at week 52, patients treated with 8-weekly aflibercept had reductions in central retinal thickness that were similar to the reductions experienced by patients treated with monthly ranibizumab, although fluctuations were observed in the 8-weekly aflibercept treatment group.Citation12 In line with the VIEW data, a recent, small (n=26), single-arm, prospective study of fixed 8-weekly dosing of aflibercept in treatment-experienced patients showed functional anatomical improvements with aflibercept after 6 months.Citation13
Based on the VIEW study data described above and the approved product label,Citation8,Citation12 we expected a maintenance or improvement of visual and anatomical outcomes with this fixed dosing schedule; however, this is not what we observed. The results of our study, which included patients from our clinic who received aflibercept treatment in the expectation of achieving an 8-weekly dosing schedule, suggest that the duration of action of aflibercept may be <8 weeks in a meaningful proportion of patients. This aligns with the fluctuations in central retinal thickness observed in the 8-weekly aflibercept group in the VIEW study.Citation12
Several other small-scale retrospective studies and chart reviews investigating aflibercept treatment in real-life clinical practice have also recently been performed; however, the results from these studies vary.Citation14–Citation20 Collectively however, the results provide ophthalmologists with valuable information to consider when deciding the best treatment options for their patients.
Limitations of our study include the single-arm retrospective design, the relatively small number of cases, the lack of controls, and the fact that our observations are limited to the visits after only four injections of aflibercept. However, our data do suggest that patients respond differently to treatment and fixed dosing may therefore be suboptimal, leaving some patients under- or overtreated. If this is the case, there may be a subsequent impact on health care providers expecting to provide aflibercept according to a fixed 8-weekly regimen if many patients require more frequent injections (ie, <8 weeks apart) in terms of clinic organization and visit scheduling, as well as potential implications for the comparative cost-effectiveness of aflibercept and other anti-VEGF agents. Our findings should therefore be further investigated in studies of aflibercept use in clinical practice and randomized controlled trials of different aflibercept dosing schedules. Existing data from recent studies with anti-VEGF agents suggest the benefits of flexible dosing regimens. The efficacy of a pro re nata (PRN; as needed) regimen with monthly monitoring has been confirmed in the HARBOR trial, where patients required a mean of 7.7 injections during the first year of treatment, including the initial three injections,Citation21 while the CATT study illustrated that equivalent improvements in VA were observed with both monthly and PRN ranibizumab dosing after 2 years of treatment.Citation22 Furthermore, treat-and-extend regimens that have the potential to reduce clinic burden are increasingly being investigated, with a recent prospective study achieving visual outcomes after 12 and 24 months that were comparable with the pivotal ranibizumab trials (ANCHOR and MARINA), but with fewer injections and clinic visits.Citation23
Overall, our data add to the growing body of evidence indicating that fixed-dose regimens are not suitable for all patients with nAMD and highlight a need for flexible dosing schedules with treatment and monitoring intervals based on disease activity.
Acknowledgments
Dr Khanani would like to thank Fishawack Communications Ltd. for medical writing services.
Disclosure
Medical writing assistance was sponsored by Novartis. The author has been a speaker and consultant for Novartis. The author reports no other conflicts of interest in this work.
References
- LimLSMitchellPSeddonJMHolzFGWongTYAge-related macular degenerationLancet201237998271728173822559899
- RosenfeldPJBrownDMHeierJSMARINA Study GroupRanibizumab for neovascular age-related macular degenerationN Engl J Med2006355141419143117021318
- Novartis PharmaAGLucentis European summary of product characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000715/WC500043546.pdfAccessed November 2014
- AbouammohMSharmaSRanibizumab versus bevacizumab for the treatment of neovascular age-related macular degenerationCurr Opin Ophthalmol201122315215821483262
- FerraraNHillanKJGerberHPNovotnyWDiscovery and development of bevacizumab, an anti-VEGF antibody for treating cancerNat Rev Drug Discov20043539140015136787
- SemeraroFMorescalchiFDuseSParmeggianiFGambicortiECostagliolaCAflibercept in wet AMD: specific role and optimal useDrug Des Devel Ther20137711722
- PapadopoulosNMartinJRuanQBinding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab and bevacizumabAngiogenesis201215217118522302382
- Eylea® (aflibercept) Injection [Prescribing Information]New York, NYRegeneron Pharmaceuticals Inc2012
- Bayer PharmaAGEylea European summary of product characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002392/WC500135815.pdfAccessed November 2014
- StewartMWClinical and differential utility of VEGF inhibitors in wet age-related macular degeneration: focus on afliberceptClin Ophthalmol201261175118622973088
- GregoriNZFeuerWRosenfeldPJNovel method for analyzing Snel-len visual acuity measurementsRetina20103071046105020559157
- HeierJSBrownDMChongVVIEW 1 and VIEW 2 Study GroupsIntravitreal aflibercept (VEGF trap-eye) in wet age-related macular degenerationOphthalmology2012119122537254823084240
- SinghRPSrivastavaSEhlersJPBediRSchachatAPKaiserPKA single-arm, investigator-initiated study of the efficacy, safety and tolerability of intravitreal aflibercept injection in subjects with exudative age-related macular degeneration, previously treated with ranibizumab or bevacizumab: 6-month interim analysisBr J Ophthalmol201498suppl 1i22i2724836866
- BakallBFolkJCBoldtHCAflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumabAm J Ophthalmol201315611522.e123706500
- ChoHShahCPWeberMHeierJSAflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumabBr J Ophthalmol20139781032103523766432
- FerronePJAnwarFNaysanJEarly initial clinical experience with intravitreal aflibercept for wet age-related macular degenerationBr J Ophthalmol201498suppl 1i17i2124795335
- HeussenFMShaoQOuyangYJoussenAMMullerBClinical outcomes after switching treatment from intravitreal ranibizumab to aflibercept in neovascular age-related macular degenerationGraefes Arch Clin Exp Ophthalmol2014252690991524362854
- HoVYYehSOlsenTWShort-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitorsAm J Ophthalmol201315612328.e223664153
- KumarNMarsigliaMMrejenSVisual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degenerationRetina20133381605161223549101
- MessengerWBCampbellJPFaridiAInjection frequency and anatomic outcomes 1 year following conversion to aflibercept in patients with neovascular age-related macular degenerationBr J Ophthalmol20149891205120724795334
- BusbeeBGHoACBrownDMHARBOR Study GroupTwelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degenerationOphthalmology201312051046105623352196
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research GroupMartinDFMaguireMGRanibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year resultsOphthalmology201211971388139822555112
- AbediFWickremasingheSIslamAFInglisKMGuymerRHAnti-VEGF treatment in neovascular age-related macular degeneration. A treat-and-extend protocol over 2 yearsRetina20143481531153824637667
