Abstract
Purpose
To describe the peripheral autofluorescence images and clinical features of patients with retinal dystrophy who showed radial fundus autofluorescence (FAF) at the posterior pole.
Methods
The authors retrospectively reviewed pooled wide-field FAF images of 711 patients with retinal dystrophy and 56 family members.
Results
Eleven eyes of seven women exhibited radial FAF at the posterior pole. Wide-field FAF showed extension of the radial pattern to the periphery in all eyes except one. One woman showed radial hyper-FAF only in the periphery, not at the posterior pole. These eight individuals were X-linked retinitis pigmentosa patients or carriers. The tapetal-like reflex was not observed in their color fundus photographs. The peripheral visual field showed wedge-shaped restriction in some individuals.
Conclusion
Wide-field FAF imaging can depict radial FAF not only at the posterior pole but also in the periphery in X-linked retinitis pigmentosa carriers. The authors therefore agree with previous reports that radial FAF may be a hallmark of X-linked retinitis pigmentosa.
Introduction
Retinitis pigmentosa (RP) is an inherited retinal dystrophy and a major cause of visual impairment in developed countries. RP is conventionally diagnosed on the basis of night blindness or visual field defects and the results of funduscopic examination and electroretinography. However, recent advances in technology, such as optical coherence tomography and fundus autofluorescence (FAF) imaging, have elucidated the morphology of the RP-affected retina and its correlation with visual function.Citation1–Citation7
FAF imaging was performed in patients with various macular diseases in the 1990s by using a scanning laser ophthalmoscope.Citation8–Citation12 Patients with RP exhibited a ring of hyperautofluorescence at the fovea that was associated with central foveal function measured using pattern electroretinography.Citation13 In another study, peripheral hypoauto-fluorescence depicted by wide-field FAF imaging was correlated with the visual field area measured using the Goldmann perimeter.Citation14 This abnormal FAF in patients with RP usually assumes a ring shape, which is compatible with the symptom of concentric visual field restriction. Meanwhile, Wegscheider et alCitation15 found radial FAF at the posterior pole in carriers belonging to two families with X-linked RP.
The authors also observed radial FAF in some patients; therefore, in this study, the authors investigated the rate of radial FAF observed on pooled wide-field FAF images of patients with retinal dystrophy, and described the clinical features of the individuals who exhibited radial FAF.
Methods
All procedures conformed to the tenets of the Declaration of Helsinki. The study was approved by the Institutional Review Board/Ethics Committee of the Kyoto University Graduate School of Medicine.
The authors retrospectively reviewed bilateral wide-field FAF images obtained from 711 patients with retinal dystrophy (362 male and 349 female patients) and 56 family members (29 male and 27 female participants) who visited the retinal degeneration service at Kyoto University Hospital from March 2012 through November 2014. The patients were diagnosed with nonsyndromic/syndromic RP (n=540), cone–rod dystrophy (n=66), Stargardt disease (n=29), other macular dystrophies (n=15), Bietti’s crystalline dystrophy (n=19), fundus albipunctatus (n=8), X-linked retinoschisis (n=8), choroideremia (n=11), and Leber congenital amaurosis (n=15) on the basis of the history, funduscopic examination, electroretinography, fluorescein angiography, and genotyping findings. Of the 767 individuals, 5 male and 8 female participants were diagnosed as RPGR RP patients and carriers, respectively. The patients with noninherited retinal dystrophy such as age-related macular degeneration, acute zonal occult outer retinopathy, and cancer-associated retinopathy were excluded. The family members of the probands underwent wide-field FAF imaging and provided blood samples for cosegregation in the authors’ previous genotype screening study.Citation16
The wide-field FAF images were obtained, as previously reported, using the Optos 200Tx imaging system (Optos PLC, Dunfermline, United Kingdom). The pupils were dilated with 0.5% tropicamide and 0.5% phenylephrine in all patients, but not family members.
The authors evaluated the presence of radial FAF at the posterior pole by using representative FAF images illustrated by Wegscheider et alCitation15 (their ). Positive radial FAF was confirmed by three authors (KO, AO, and SM).
The peripheral appearance of wide-field FAF, symptoms, genotype, and other clinical findings were described for all patients with radial FAF through a review of their clinical records.
Results
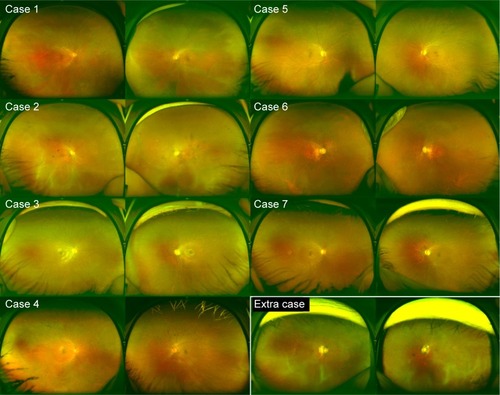
Of the 767 individuals, 7 women (11 eyes) exhibited radial FAF at the posterior pole (), and this was found to extend to the periphery on wide-field FAF images in 6 of the 7 women (10 of the 11 eyes) (). The radial FAF was unilateral in three patients. One additional individual displayed radial FAF only at the periphery. These eight individuals were X-linked RP family members (). None of the other dystrophies was associated with radial FAF. The characteristics of the cases are described hereafter and summarized in .
Figure 1 Radial FAF at the posterior pole.
Abbreviation: FAF, fundus autofluorescence.
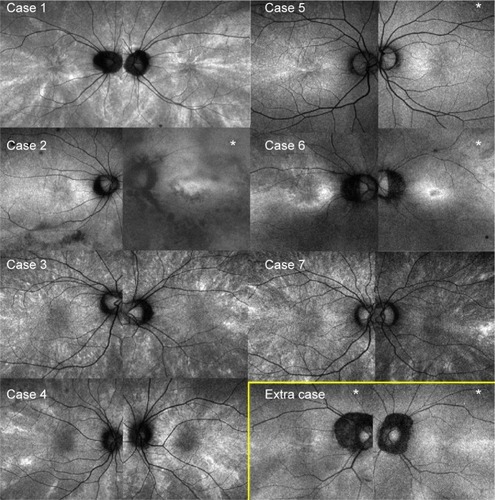
Figure 2 Peripheral radial FAF.
Abbreviation: FAF, fundus autofluorescence.
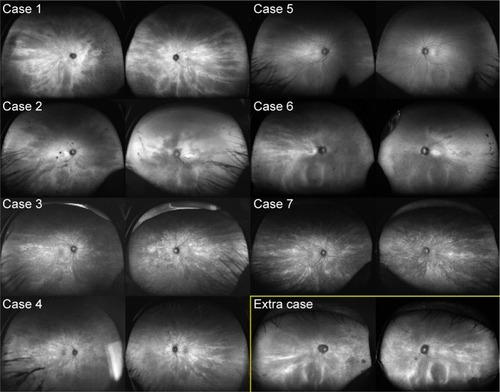
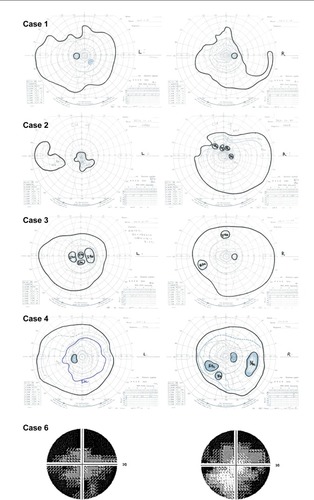
Figure 3 Visual fields of patients with radial FAF.
Abbreviation: FAF, fundus autofluorescence.
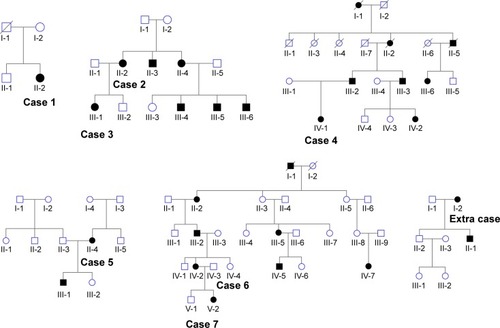
Table 1 Characteristics of the cases with radial pattern fundus autofluorescence
Case 1
A 38-year-old woman presented with temporal visual field defects in the right eye and night blindness since the age of 30. No one in her family was diagnosed with RP, but her deceased father had blindness at least in the right eye. Her fundus showed mild bone spicule pigmentation and atrophy of the retinal pigment epithelium (RPE) in the midperiphery. Marked decrease of amplitudes on electroretinography suggested RP. Genotype screening with next-generation sequencing showed a novel heterozygous deletion (c.1860_1861del) of the RPGR gene. Goldmann perimetry showed a temporal scotoma in the right eye and wedge-shaped visual field restriction in both eyes, which were atypical of RP (). Hypoautofluorescence in the nasal region and radial FAF in the periphery corresponded to her symptoms and wedge-shaped visual field restriction, respectively.
Case 2
A 40-year-old woman with amblyopia since childhood, represented by a refractive error of −11.25 diopters in the left eye and −8.25 diopters in the right eye, was diagnosed with a visual field defect in the left eye at 30 years of age. Her brother was diagnosed with RP, and next-generation sequencing revealed a novel missense mutation (c.922G > C) in exon 8 of the RPGR gene. The patient also exhibited this heterozygous mutation. Her fundus showed RPE atrophy around the vascular arcade and optic nerve disc, although bone spicule pigmentation was not observed. Electroretinography showed an extinguished pattern in the left eye and a more preserved pattern in the right eye. Although radial FAF was observed in the right eye, the image of her left eye showed a ring of hypo-FAF in the macular region and patchy atrophy around the vascular arcade, typical of RP. Wedge-shaped visual field restriction was observed in her right eye, while concentric visual field restriction and temporal islands were observed in her left eye.
Case 3
An 11-year-old girl, the daughter of the patient in Case 2, exhibited the same heterozygous mutation (c.922G > C) in the RPGR gene. Interestingly, she also showed amblyopia in the left eye. Although wide-field FAF images showed a radial pattern in both eyes, electroretinography revealed higher amplitude in the right eye than in the left eye. Her fundus showed mild RPE atrophy without bone spicule pigmentation. Goldmann perimetry revealed a peripheral island scotoma of V/4e or III/4e isopter in the right eye and a ring or central scotoma of I/4e isopter in the left eye.
Case 4
The individual in case 4 was a 22-year-old woman who had visited Kyoto University Hospital without any symptoms at 12 years of age because her father was blinded by RP. Her family history suggested an X-linked inheritance pattern. Next-generation sequencing confirmed the previously found missense variant (c.785C > G, rs138018739) in the RPGR gene, but this variant was judged as benign because of its relatively high prevalence in the Japanese population according to the authors’ filtering process (http://www.genome.med.kyoto-u.ac.jp/SnpDB/index.html). Her fundus showed bone spicule pigmentation and RPE atrophy in the midperiphery. The amplitudes for both eyes were 50% preserved according to the International Society for Clinical Electrophysiology of Vision protocol, and radial FAF showed a similar pattern in both eyes. A peripheral island scotoma of III/4e or I/4e isopter in the right eye and wedge-shaped visual field restriction of I/3e isopter in the left eye were identified using Goldmann perimetry.
Case 5
The individual in case 5 was a 42-year-old asymptomatic woman whose son was diagnosed with RP. She was examined for segregation of the mutation. Both the patient and her son had known deletion of exon 8 (c.894_895del) in the RPGR gene.Citation16 Her color fundus photograph appeared normal, although radial FAF at the posterior pole was observed in the right eye. Her peripheral FAF images appeared normal.
Case 6
The individual in case 6 was a 43-year-old woman with night blindness since childhood, whose father was diagnosed with RP. Her family history revealed an X-linked inheritance pattern. Genotype screening had not yet been performed. Her fundus showed myopic changes without bone spicule pigmentation. Radial FAF at the posterior pole was observed only in the right eye. Peripheral FAF images showed radial FAF in the temporal region and diffuse hypo-FAF in the nasal region. In the left eye, a ring of hyper-FAF was observed in the macular region, typical of RP. The Humphrey visual field test (30-2) showed concentric restrictions in both eyes. Electroretinography was not performed.
Case 7
An 11-year-old girl, the daughter of the patient in case 6, was diagnosed with amblyopia in the left eye, represented by a refractive error of −7.75 diopters in the left eye and −2.5 diopters in the right eye. Her eyes appeared normal in color fundus photographs; however, radial FAF was observed. A visual field test and electroretinography were not performed. Genotype screening had not yet been performed.
Extra case
This participant was a 60-year-old woman whose son was diagnosed with RP. She was examined to allow segregation of the mutation. She and her son both exhibited a heterozygous indel (c.1087_1088insGTAG) in exon 10 of the RPGR gene.Citation16 Although color fundus photographs showed only a myopic fundus and FAF at the posterior pole did not show a radial pattern, the periphery showed radial hyper-FAF. She experienced no visual disturbances in daily life for over six decades.
Discussion
The current study showed, for the first time to the authors’ knowledge, peripheral FAF on wide-field images of patients with radial FAF at the posterior pole. The radial FAF extended to the periphery in all patients except for the patient in case 5. Furthermore, the peripheral radial FAF corresponded to wedge-shaped restriction of the visual field in cases 1, 2, and 4.
In the study cohort, radial FAF at the posterior pole was only observed in seven women belonging to five families with X-linked RP. RPGR gene mutations were segregated in three of the five families. One of the remaining two families was not tested, while the causative mutation was not identified in another family even though targeted exome sequencing was performed in a previous study by the authors.Citation16 Five of the eight female participants in the study cohort with radial FAF showed RPGR mutations. Together with the findings of previous studies,Citation15,Citation17 these results strongly suggest an association between radial FAF and X-linked RP. It will be interesting to determine whether carriers of other X-linked RP genes also exhibit radial FAF, although there are no reports in the literature and no individuals in the study cohort.
Carriers of RPGR gene-associated RP show mild to severe phenotypes.Citation18 The tapetal-like reflex in the fundus is one of the features observed in carriers.Citation18 Although color fundus photographs obtained using the Topcon nonmydriatic camera or Optos () were reviewed, this reflex was not observed in the seven women with radial FAF. Wegscheider et alCitation15 described a discrete tapetoretinal reflex in a carrier with clear radial FAF and an intense tapetoretinal reflex in a carrier without radial FAF. Acton et alCitation17 also demonstrated that the tapetal-like reflex that was well depicted on color fundus photographs or 488 nm reflectance was at a location different from that of radial FAF.
Figure 5 Family history of patients with radial FAF.
Abbreviation: FAF, fundus autofluorescence.
Radial FAF has been explained as the result of X-chromosome inactivation and peripheral migration of mosaic stem cells.Citation15 The unilateral radial FAF in cases 2, 5, and 6 or amblyopia in cases 2, 3, and 7 may have been derived from random inactivation in each organ. However, the studies cited by Wegscheider did not show peripheral migration of stem cells.Citation19,Citation20 Reese et alCitation19,Citation20 described the term radial not in relation to the periphery, but as a transverse pattern through the retina. The whole-mounted retina of a transgenic mouse did not show radial dispersion of transgenic-active columns.Citation19,Citation20 The reason for this radial pattern needs to be elucidated.
This study has some limitations. First, it was a retrospective, small case study. Second, the definition of radial FAF was ambiguous because previously reported images were used. Third, the lack of molecular genetics in two families led to an undetermined association between radial FAF and the RPGR gene. However, no evidence of radial FAF in patients with other dystrophies suggests the specificity of radial FAF to X-linked RP. The presence of asymptomatic carriers leads to confused opinions about the inheritance pattern in clinical settings.Citation21,Citation22 In addition to the tapetal-like reflex, the detection of radial FAF would be helpful for the diagnosis of carriers of X-linked RP. Accurate diagnosis of X-linked inheritance mode reduces the time and cost of genetic testing. Furthermore, radial FAF in the periphery, but not at the posterior pole, was found in one patient. Disease progression might make the radial FAF at the posterior pole unclear. In this regard, Optos can be considered useful for screening because wide-field FAF images can be obtained even in the nonmydriatic state and in under 1 second.
In conclusion, wide-field FAF imaging can depict radial FAF not only at the posterior pole but also in the periphery in X-linked RP patients and carriers. The present study results concur with previous reports that radial FAF may be a hallmark of X-linked RP and present a potentially useful diagnostic technique for this condition.
Acknowledgments
This work was partly supported by the Innovative Techno-Hub for Integrated Medical Bio-Imaging of the Project for Developing Innovation Systems from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Disclosure
The authors report no conflicts of interest in this work.
References
- InuiEOishiAOishiMTomographic comparison of cone-rod and rod-cone retinal dystrophiesGraefes Arch Clin Exp Ophthalmol201425271065106924441883
- MakiyamaYOotoSHangaiMMacular cone abnormalities in retinitis pigmentosa with preserved central vision using adaptive optics scanning laser ophthalmoscopyPLoS One2013811e7944724260224
- OginoKOtaniAOishiAKurimotoMSekiyaTYoshimuraNConcentric division of 10 degrees visual field tests in retinitis pigmentosaJpn J Ophthalmol201357326827423443900
- OishiANakamuraHTatsumiIOptical coherence tomographic pattern and focal electroretinogram in patients with retinitis pigmentosaEye (Lond)200923229930318344968
- OishiAOginoKNakagawaSLongitudinal analysis of the peripapillary retinal nerve fiber layer thinning in patients with retinitis pigmentosaEye (Lond)201327559760423519274
- OishiAOtaniASasaharaMPhotoreceptor integrity and visual acuity in cystoid macular oedema associated with retinitis pigmentosaEye (Lond)20092361411141618724276
- OishiAOtaniASasaharaMRetinal nerve fiber layer thickness in patients with retinitis pigmentosaEye (Lond)200923356156618344951
- von RuckmannAFitzkeFWGregorZJFundus autofluorescence in patients with macular holes imaged with a laser scanning ophthalmoscopeBr J Ophthalmol19988243463519640179
- von RuckmannAFitzkeFWBirdACIn vivo fundus autofluorescence in macular dystrophiesArch Ophthalmol199711556096159152128
- von RuckmannAFitzkeFWBirdACDistribution of fundus autofluorescence with a scanning laser ophthalmoscopeBr J Ophthalmol19957954074127612549
- SolbachUKeilhauerCKnabbenHWolfSImaging of retinal autofluorescence in patients with age-related macular degenerationRetina19971753853899355185
- KitagawaKNishidaSOguraYIn vivo quantitation of autofluorescence in human retinal pigment epitheliumOphthalmologica19891992–31161212587019
- RobsonAGEl-AmirABaileyCPattern ERG correlates of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuityInvest Ophthalmol Vis Sci20034483544355012882805
- OishiAOginoKMakiyamaYNakagawaSKurimotoMYoshimuraNWide-field fundus autofluorescence imaging of retinitis pigmentosaOphthalmology201312091827183423631947
- WegscheiderEPreisingMNLorenzBFundus autofluorescence in carriers of X-linked recessive retinitis pigmentosa associated with mutations in RPGR, and correlation with electrophysiological and psychophysical dataGraefes Arch Clin Exp Ophthalmol2004242650151115173948
- OishiMOishiAGotohNComprehensive molecular diagnosis of a large cohort of Japanese retinitis pigmentosa and Usher syndrome patients by next-generation sequencingInvest Ophthalmol Vis Sci201455117369737525324289
- ActonJHGreenbergJPGreensteinVCEvaluation of multimodal imaging in carriers of X-linked retinitis pigmentosaExp Eye Res2013113414823669302
- BirdACX-linked retinitis pigmentosaBr J Ophthalmol19755941771991138842
- ReeseBEHarveyARTanSSRadial and tangential dispersion patterns in the mouse retina are cell-class specificProc Natl Acad Sci U S A1995927249424987708672
- ReeseBENecessaryBDTamPPFaulkner-JonesBTanSSClonal expansion and cell dispersion in the developing mouse retinaEur J Neurosci19991182965297810457191
- BranhamKOthmanMBrummMMutations in RPGR and RP2 account for 15% of males with simplex retinal degenerative diseaseInvest Ophthalmol Vis Sci201253138232823723150612
- ChurchillJDBowneSJSullivanLSMutations in the X-linked retinitis pigmentosa genes RPGR and RP2 found in 8.5% of families with a provisional diagnosis of autosomal dominant retinitis pigmentosaInvest Ophthalmol Vis Sci20135421411141623372056