Abstract
Purpose
The aim of the study was to assess the outcomes of severe retinopathy of prematurity (ROP) in zone I or posterior zone II treated with intravitreal ranibizumab (IVR) as monotherapy or combined treatment with laser photocoagulation.
Methods
This is a retrospective study analyzing clinical records of the included patients. Patients were divided into two groups: group 1 included patients who received only IVR treatment; and group 2 was subdivided into group 2A – including patients with IVR as initial treatment and complementary laser photocoagulation if retinal neovascularization or plus disease did not regress, and group 2B – including patients with initial laser photocoagulation and IVR as rescue therapy. Favorable outcomes were regression of the retinal neovascularization and plus disease, meaning control of the disease. Unfavorable outcomes were progression to stages 4 and 5 of ROP.
Results
Fifty-seven eyes were included in the study. Mean birth weight and gestational age were 1,281±254 g and 29.5±2.1 weeks, respectively. Group 1 comprised of 16 eyes, with favorable outcomes in 14 eyes (87.5%). Group 2 comprised of 41 eyes, with favorable outcomes in 29 eyes (70.7%), in a mean follow-up period of 12.8 months.
Conclusion
IVR was effective to treat severe cases of ROP as a primary or a combined treatment. Forty-three of the 57 treated eyes (75.4%) achieved regression of ROP and favorable outcomes.
Introduction
Aggressive posterior retinopathy of prematurity (AP-ROP) is an uncommon, severe, and rapidly progressing presentation of ROP.Citation1
AP-ROP, when not promptly diagnosed and treated, can progress to retinal detachment and blindness (stage 4 or 5 of ROP).Citation2,Citation3 AP-ROP, usually, occurs in more immature or sicker babies with extremely low birth weight (BW) as described in North America or Western European countries, as well as in bigger babies in Latin American, East European, or Asian countries.Citation4
Laser photocoagulation remains the gold standard for the treatment of severe ROP in threshold or in type 1 prethreshold diseases according to the randomized trials Cryo-ROP and ETROP,Citation5,Citation6 but the anatomical and visual results of laser or cryotherapy in patients with severe ROP affecting zone I or posterior zone II are usually unfavorable.Citation2,Citation7–Citation11
The role of vascular endothelial growth factor (VEGF) in the pathogenesis of ROP is well defined.Citation12–Citation15 Currently, bevacizumab is the most studied drug for ROP treatment with encouraging results and very few ocular- or systemic-related complications.Citation16–Citation20 A prospective, randomized, multicenter trial (BEAT-ROP) assessed the efficacy of bevacizumab monotherapy in the treatment of premature patients with ROP in zone I or posterior zone II compared with conventional laser therapy. The study concluded that intravitreal bevacizumab monotherapy showed a significant benefit for zone I but not for zone II disease, as compared with laser photocoagulation.Citation21
There are pharmacokinetic differences between bevacizumab and ranibizumab, which may be important for safety in premature infants undergoing organogenesis. Despite the advantages related to ranibizumab when compared with bevacizumab for use in preterm infants with very low or with extremely low BW, there are a few studies on this drug for the treatment of severe cases of ROP.Citation22,Citation23
This study aims at analyzing the clinical records of patients with AP-ROP in zone I and posterior zone II and severe ROP 3 plus in posterior zone II treated with intravitreal ranibizumab (IVR) as monotherapy or as a combined treatment and reports the results of the treatment.
Methods
Study design
This is an institutional-based retrospective study conducted from July 2009 to June 2012 at the University Hospital of Maracaibo, Venezuela.
Patients
The study included all preterm infants with AP-ROP, defined according to the International Classification of ROP revisited from 2005,Citation1 as well as preterm infants with severe ROP 3 plus who were treated with IVR in the Institution. There were no exclusion criteria.
Ophthalmological examination and interventions
All patients were evaluated preoperatively by binocular indirect ophthalmoscopy and by fundus retinal documentation with the RetCam® Imaging System (Clarity Medical Systems, Pleasanton, CA, USA). BW, gestational age (GA), classification of ROP in stages, zones, extension and severity, postconceptional age (PCA) at presentation, and PCA at treatment were registered.
Anti-VEGF therapy with IVR was performed in both eyes simultaneously. The injections of 0.25 mg (0.025 mL) of ranibizumab were injected at 1.5 mm posterior to the corneal limbus. A disposable 1 mL syringe with a 30 G needle was used to treat all eyes. Preoperatively, Betadine was applied for cutaneous asepsis in all patients. All the injections were administered in the surgical room under topical anesthesia with proparacaine eye drops. After administering IVR injection, eye drops of antibiotic and steroids were used every 6 hours for 1 week.
Diode laser photocoagulation (Solitaire; Ellex Medical Pty Ltd®, Adelaide, SA, Australia), 0.15 seconds, and 110 mW power, was applied in all quadrants (360°) from the posterior avascular retina to the ora serrata in a near confluent pattern (laser burns of less than one-half burn width apart). Laser photocoagulation was performed in the surgical room under general anesthesia or sedation.
Treatment criteria for initial IVR therapy were ROP location in zone I or posterior zone II and severity of ROP (severity of plus disease, pupillary rigidity and lack of pupillary dilation, or occurrence of vitreous hemorrhage not allowing laser photocoagulation). PCA at presentation was also considered for appropriate therapeutic approach.
Patients were included in one of the two groups: group 1 included patients who received only one IVR injection; and group 2, subdivided into groups 2A and 2B, included patients who received combined treatment with only one IVR injection and laser photocoagulation (group 2A received IVR as initial treatment followed by laser photocoagulation after 3 weeks if retinal neovascularization or plus disease did not regress; and group 2B, received initial laser photocoagulation and IVR as rescue therapy after failure of the laser treatment defined by the presence of persistent plus disease, progression of retinal neovascularization, or persistence of vitreous hemorrhage).
Outcomes evaluated and follow-up
Favorable outcomes were considered regression of the AP-ROP after treatment (regression of retinal neovascularization and plus disease, meaning control of the disease), and unfavorable outcomes were progression to stages 4 and 5 of ROP. Follow-up evaluations were performed at 24 hours, 48 hours, 1 week, 2 weeks, 1 month, 2 months, 3 months, 6 months, 12 months, or more after treatment.
Ocular or systemic adverse effects registered
Short- and long-term (after 1-year follow-up) ocular adverse effects were registered as signs of intraocular inflammation or endophthalmitis, intraocular pressure elevation, and secondary cataract formation. Systemic adverse effects were closely monitored during perioperative and postoperative periods in the Neonatal Intensive Care Unit, including continuous monitoring of blood pressure, oxygen saturation, and heart rates. Respiratory and cardiologic frequencies, and urinary or allergic side effects (rash or bronchospasm), were also monitored.
Ethical aspects
The Research Ethics Committee of the University Hospital of Maracaibo approved the study protocol, and it was consistent with the principles of the Declaration of Helsinki, 1995 (revised in Edinburgh in 2000). The parents or their representatives have signed a consent form before the treatment.
Results
Twenty-nine patients (57 eyes) were included in the study. AP-ROP in zone I or in posterior zone II was diagnosed in 26 patients (51 eyes) and severe ROP 3 plus (threshold ROP) in posterior zone II was diagnosed in three patients (six eyes). Seventeen patients were female. Mean GA at birth was 29.4±2.2 weeks (range: 25–33 weeks), BW was 1,272±255 g (range: 900–1,900 g), PCA at ROP diagnosis was 36±2.7 weeks, and PCA at treatment was 37.7±2.6 weeks ().
Table 1 Demographic characteristics of the included patients and PCA at treatment
Group 1 comprised 16 eyes from eight patients (patients treated only once with IVR); and group 2 comprised 41 eyes from 21 patients (patients who received combined treatment with IVR and laser photocoagulation). In group 1, a total of 14 eyes (seven patients) achieved favorable outcomes after the use of only one IVR injection in each eye (87.5%). It was remarkable that all the eyes that achieved favorable outcomes in group 1 remained with peripheral avascular retina up to 6 months after treatment (). Two eyes of the same patient in group 1 had unfavorable outcomes with progression to ROP stage 4B in one eye and ROP stage 5 in the fellow eye after 3 weeks ().
Figure 1 Image before IVR monotherapy (A); 6 days after IVR monotherapy (B); and after 6 months of IVR monotherapy (C).
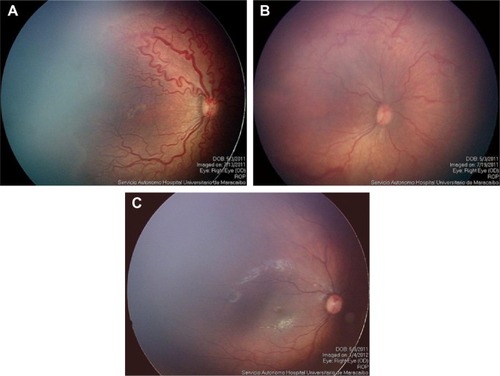
Table 2 Results of treatment according to groups
In group 2, 29 eyes (15 patients) achieved favorable outcomes after combined treatment (70.7%). Twelve eyes in group 2 (29.3%) developed progression of ROP to stage 4 or 5 with unfavorable outcomes, after a mean of 6 weeks. Thirteen eyes (group 2A) received IVR as initial treatment, and after plus disease reactivation, they were treated with laser photocoagulation in zones II and III, but not in zone I. There were no unfavorable outcomes in patients included in group 2A. Twenty-eight eyes (group 2B) received laser photocoagulation as initial treatment and IVR was used after laser failure. Twelve eyes of group 2B had unfavorable outcomes (42.8%; ).
The mean follow-up period was 12.8 months. No short- or long-term ocular or systemic adverse effects were registered in this cohort of 29 treated patients.
Discussion
The characteristic features of AP-ROP are its posterior location (zone I or posterior zone II), prominence of plus disease with flat neovascularization, retinal hemorrhages at the junction between the vascularized and avascular retina, intraretinal shunting without ridge tissue formation, and ill-defined nature of the retinopathy without progression through classic stages 1–3 of ROP.Citation1–Citation3
Bevacizumab is a full-length antibody that contains the fraction of neonatal Fc (FcRn) receptor while ranibizumab does not contain this particular factor.Citation24 Earlier studies have reported that the trans-retinal penetration varies with the molecular weight of the diffusing agent and the blood–brain barrier contains the FcRn immunoglobulin G (IgG) receptor/transporter that is necessary to permit the passage of the intravitreal drug through the retinal barrier achieving extraocular penetration.Citation24,Citation25 The authors suggest that bevacizumab escapes from the vitreous into the systemic circulation and reduces the systemic VEGF concentrations in ROP infants.Citation26,Citation27 Ranibizumab does not contain the fraction of FcRn, and it is supposed that IVR does not achieve significant extraocular concentration after use. Ranibizumab, as a smaller molecule, represents greater activity with less half-life in the vitreous as compared with the bevacizumab (2.88 days versus 4.32 days, respectively).Citation28,Citation29
This study reports a cohort of 29 preterm infants with mean BW and GA of 1,272±255 g (range: 900–1,900 g) and 29.4±2.2 weeks (range: 25–33 weeks), respectively. These characteristics of BW and GA are representative for cohorts of patients originated from the Latin American countries, which comprised bigger and more mature babies developing severe ROP than the cohorts of patients related in USA, Canada, or Western European countries, usually restricted to smaller and more immature babies developing severe ROP. In developing countries, missed ROP screening, lack of awareness of risk factors for ROP, and unmonitored supplemental oxygen in many less equipped Neonatal Intensive Care Units are some of the causative factors for this difference.Citation4,Citation30,Citation31 The optimal time for treatment is very important for ROP outcomes. Most of the patients included in the study were transferred from different institutions for ROP treatment; mean PCA at ROP diagnosis was 36±2.7 weeks and at treatment was 37.7±2.6 weeks. However, PCA at treatment may influence outcomes.Citation32 Group 1 underwent treatment at a mean PCA of 36.6 weeks and group 2 at 38.2 weeks. It appears that better results observed in group 1 might be influenced by this variable.
Laser therapy is effective for the treatment of severe forms of ROP, as prethreshold type 1 ROP, threshold ROP, and in ∼50% of the patients treated with AP-ROP,Citation2,Citation4,Citation33 but laser always damages the peripheral retina. Anti-VEGF therapy does not produce peripheral retinal ablation and may help to maintain a normal retinal function.Citation34 However, there are many concerns with the anti-VEGF therapy in such at-risk population regarding the possibility of long-term systemic complications because organogenesis is occurring during the same period when ROP should be treated.Citation35,Citation36
Serum concentrations of bevacizumab and VEGF were reported by Sato et al in infants who were treated with 0.5–1.0 mg of intravitreal bevacizumab for severe ROP. The authors suggested that bevacizumab escapes from the vitreous into the systemic circulation and reduces the VEGF concentrations in ROP infants.Citation27 Ranibizumab is a different molecule, and it is supposed that this drug does not achieve significant extraocular concentration after the intravitreal use. In animal models, no ranibizumab was detected in the serum or on the fellow untreated eye, whereas small amounts of intravitreal bevacizumab have been detected in the serum and in the fellow uninjected eye.Citation28,Citation29 Ranibizumab, as a smaller molecule, represents greater activity with less half-life in the vitreous as compared with bevacizumab.Citation28,Citation29 In adults, Carneiro et al reported similar efficacy of ranibizumab and bevacizumab but with the advantage that it does not alter systemic VEGF levels.Citation37 This is a very important point to be considered for the use of IVR in preterm babies under organogenesis period, but despite of these apparent advantages on pharmacokinetics related to ranibizumab, when compared with bevacizumab for the use in premature infants, this drug has only recently been used for the treatment of severe presentations of ROP.Citation22,Citation23,Citation38
In this study, IVR injections were used in 57 eyes and a regression of plus disease and retinal neovascularization was registered in 43 of the treated eyes. This represents 75.4% of favorable outcomes. Fourteen eyes had unfavorable evolution after treatment and progressed to stage 4B or 5 of ROP. Sixteen eyes were included in group 1 and 14 eyes achieved favorable outcomes (87.5%). It was remarkable that all eyes that achieved favorable outcomes in the IVR group remained with avascular peripheral retina up to 6 months after treatment. These findings are according to previous reports on the use of bevacizumab.Citation34 Group 2 comprised 41 eyes. Twenty-nine eyes achieved favorable outcomes (70.7%) in the entire group. Thirteen eyes (group 2A) received IVR as initial treatment and, after failure, received rescue laser photocoagulation in zones II and III, but not in zone I. ROP reactivation after treatment with IVR was reported by Wong et al.Citation39 The shorted life of ranibizumab justified it. Krohne et al determined an aqueous half-life of 7.19 days in humans.Citation40 Zhou et al reported that IVR reduce plasma VEGF levels only for 1 week.Citation41 Twenty-eight eyes (group 2B) received laser photocoagulation by initial treatment and IVR was used after laser failure. It is important to mention that in group 2, better favorable outcomes were observed when patients received initial IVR injection and complementary laser treatment (group 2A – 100% of favorable outcomes) than in patients who were initially treated by laser photocoagulation followed by IVR (group 2B – 57.1% of favorable outcomes).
This study showed no complications related to the procedure itself, that is, neither systemic side effects nor adverse events. Delayed retinal vascularization after IVR injection was observed, as described in earlier studies, and thus demanding longer ophthalmological follow-up of those patients.Citation39–Citation44
Further studies are necessary to evaluate safety and efficacy of new anti-VEGF drugs to minimize potential systemic long-term consequences and to evaluate retinal functions in preterm babies.
Conclusion
We reported favorable outcomes after the use of IVR, as monotherapy or combined with laser photocoagulation, to treat severe cases of AP-ROP in zone I and posterior zone II, as well as in severe ROP 3 plus in posterior zone II. Final favorable outcomes with regression of retinal neovascularization and control of the ROP were obtained in 43 eyes of the 57 treated eyes (75.4%). During the follow-up period, no ocular or systemic complications were registered in our patients. The shorter half-life of ranibizumab can be associated with the higher chance of reactivations or persistent avascular peripheral retina.
Acknowledgments
The affiliated institutions provided all funding sources supporting this study.
Disclosure
The authors declare no conflicts of interest in this work.
References
- International Committee for the Classification of Retinopathy of PrematurityThe international classification of retinopathy of prematurity revisitedArch Ophthalmol2005123799199916009843
- KychenthalADortaPKatzXZone I retinopathy of prematurity: clinical characteristics and treatment outcomesRetina2006267 supplS11S1516946670
- YokoiTHiraokaMMiyamotoMVascular abnormalities in aggressive posterior retinopathy of prematurity detected by fluorescein angiographyOphthalmology200911671377138219481811
- SanghiGDograMRDasPVinekarAGuptaADuttaSAggressive posterior retinopathy of prematurity in Asian Indian babies: spectrum of disease and outcome after laser treatmentRetina20092991335133919574949
- Multicenter trial of cryotherapy for retinopathy of prematurityPreliminary results. Cryotherapy for retinopathy of prematurity cooperative groupArch Ophthalmol198810644714792895630
- FielderARPreliminary results of treatment of eyes with high-risk prethreshold retinopathy of prematurity in the early treatment for retinopathy of prematurity randomized trialArch Ophthalmol2003121121769177114662598
- FlynnJTChan-LingTRetinopathy of prematurity: two distinct mechanisms that underlie zone 1 and zone 2 diseaseAm J Ophthalmol20061421465916815250
- O’KeefeMLaniganBLongVWOutcome of zone 1 retinopathy of prematurityActa Ophthalmol Scand200381661461614641264
- KatzXKychenthalADortaPZone I retinopathy of prematurityJ AAPOS20004637337611124674
- SanghiGDograMRKatochDGuptaAAggressive posterior retinopathy of prematurity: risk factors for retinal detachment despite confluent laser photocoagulationAm J Ophthalmol2013155115916423022161
- DrenserKATreseMTCaponeAJrAggressive posterior retinopathy of prematurityRetina2010304 supplS37S4020224476
- ChenJSmithLERetinopathy of prematurityAngiogenesis200710213314017332988
- HellstromAPerruzziCJuMLow IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurityProc Natl Acad Sci U S A200198105804580811331770
- SmithLEPathogenesis of retinopathy of prematurityGrowth Horm IGF Res200414suppl AS140S14415135797
- SmithLEPathogenesis of retinopathy of prematurityActa Paediatr Suppl200291437262812200894
- TravassosATeixeiraSFerreiraPIntravitreal bevacizumab in aggressive posterior retinopathy of prematurityOphthalmic Surg Lasers Imaging200738323323717552391
- SangtamTVinekarAMaheshwarBDograMREongKGIntravitreal bevacizumab (Avastin) for post-laser photocoagulation anterior segment ischemia in aggressive posterior retinopathy of prematurityIndian J Ophthalmol200755431731817595489
- ChungEJKimJHAhnHSKohHJCombination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurityGraefes Arch Clin Exp Ophthalmol2007245111727173017690897
- HondaSHirabayashiHTsukaharaYNegiAAcute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurityGraefes Arch Clin Exp Ophthalmol200824671061106318320201
- HosseiniHKhaliliMRNowroozizadehSIntravitreal injection of bevacizumab (Avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone IIRetina200929456256419262431
- Mintz-HittnerHAKennedyKAChuangAZEfficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurityN Engl J Med2011364760361521323540
- ChenSNLianIHwangYCIntravitreal anti-vascular endothelial growth factor treatment for retinopathy of prematurity: comparison between ranibizumab and bevacizumabRetina201535466767425462435
- Orozco-GómezLPHernández-SalazarLMoguel-AncheitaSRamírez-MorenoMAMorales-CruzMVLaser-ranibizumab treatment for retinopathy of prematurity in umbral-preumbral disease. Three years of experienceCir Cir201179320721422380989
- ZouLLaiHZhouQXiaoFLasting controversy on ranibizumab and bevacizumabTheranostics2011139540222211145
- SchlachetzkiFZhuCPardridgeWMExpression of the neonatal Fc receptor (FcRn) at the blood-brain barrierJ Neurochem200281120320612067234
- KimHRobinsonSBCsakyKGFcRn receptor-mediated pharmacokinetics of therapeutic IgG in the eyeMol Vis2009152803281220019892
- SatoTWadaKArahoriHSerum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurityAm J Ophthalmol2012153232733321930258
- BakriSJSnyderMRReidJMPulidoJSSinghRJPharmacokinetics of intravitreal bevacizumab (Avastin)Ophthalmology2007114585585917467524
- BakriSJSnyderMRReidJMPulidoJSEzzatMKSinghRJPharmacokinetics of intravitreal ranibizumab (Lucentis)Ophthalmology2007114122179218218054637
- CarrionJZFortes FilhoJBTartarellaMBZinAJornada JrIDPrevalence of retinopathy of prematurity in Latin AmericaClin Ophthalmol201151687169522174577
- Fortes FilhoJBEckertGUValiattiFBDos SantosPGda CostaMCProcianoyRSThe influence of gestational age on the dynamic behavior of other risk factors associated with retinopathy of prematurity (ROP)Graefes Arch Clin Exp Ophthalmol2010248689390020016911
- Fortes FilhoJBEckertGUValiattiFBSantosPGCostaMCProcianoyRSPostconceptional age at the treatment of retinopathy of prematurity in inborn and referred preterm infants from the same institutionArq Bras Oftalmol201174425125422068850
- Early Treatment For Retinopathy Of Prematurity Cooperative GroupRevised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trialArch Ophthalmol2003121121684169414662586
- DaniCFrosiniSFortunatoPIntravitreal bevacizumab for retinopathy of prematurity as first line or rescue therapy with focal laser treatment. A case seriesJ Matern Fetal Neonatal Med201225112194219722506618
- HardALHellstromAOn the use of antiangiogenetic medications for retinopathy of prematurityActa Paediatr201110081063106521517962
- SpandauUTomicZEwaldULarssonEAkerblomHHolmströmGTime to consider a new treatment protocol for aggressive posterior retinopathy of prematurity?Acta Ophthalmol201391217017522268644
- CarneiroAMCostaRFalcãoMSVascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumabActa Ophthalmol2012901e25e3021958440
- HoersterRMuetherPDahlkeCSerum concentrations of vascular endothelial growth factor in an infant treated with ranibizumab for retinopathy of prematurityActa Ophthalmol2013911e74e7522672259
- WongRHubschmanSTsuiIReactivation of retinopathy of prematurity after ranibizumab treatmentRetina20153567568025768252
- KrohneTZengpingLHolzFMeyerCIntrocular pharmakinetics of ranibizumab following a single intravitreal injection in humansAm J Ophthalmol201215468268622818800
- ZhouYJiangYBaiYWenJChenLVascular endothelial growth factor plasma levels before an after treatment of retinopathy of prematurity with ranibizumabGraefes Arch Clin Exp Ophthalmol Epub201549
- CastellanosMASchwartzSGarcía-AguirreGQuiroz-MercadoHShort-term outcome after intravitreal ranibizumab injections for the treatment of retinopathy of prematurityBr J Ophthalmol201397781681923221964
- LinCChenSChangYFengJEffects of ranibizumab on very low birth weight infants with stage 3 retinopathy of prematuityTaiwan J Ophthalmol20122136139
- TahijaSHersetyatiRLamGKusakaSMcMenaminPGFluorescein angiographic observations of peripheral retinal vessel growth in infants after intravitreal injection of bevacizumab as sole therapy for zone I and posterior zone II retinopathy of prematurityBr J Ophthalmol20149850751224403566
