Abstract
The epidemic of insulin resistance, obesity, and metabolic syndrome has led to the emergence of nonalcoholic steatohepatitis (NASH) as the most common cause of liver disease in the US. Patients with NASH are at an increased risk for hepatic disease-related morbidity and death, and chronic inflammation in NASH patients can lead to hepatocellular carcinoma (HCC). The prevalence of HCC is higher in males than in females, and genetic studies have identified androgen and androgen receptors (ARs) as partially responsible for the gender disparity in the development of liver disease and HCC. Although many factors are known to play important roles in the progression of inflammation in NASH patients, the role of androgen and AR in the progression of NASH to HCC has been understudied. This review summarizes the evidence for a potential role of androgen and the AR pathway in the development of NASH-related HCC and in the treatment of HCC. It has been proposed that AR plays a role in the progression of HCC: inhibitory roles in early stages of hepatocarcinogenesis and tumor-promoting roles in advanced stages. AR can be activated by several pathways, even in the absence of androgen. While AR has been explored as a potential therapeutic target in HCC, several clinical trials have failed to demonstrate a clinical benefit of antiandrogen drugs in HCC. This review discusses the potential reason for these observations and discuss the potential future trials design in this important setting.
Introduction
Hepatocellular carcinoma (HCC) is an aggressive neoplasm with a poor prognosis, resulting in a 5-year relative survival rate of only 17.2% and an estimated 24,550 deaths in the US in 2015.Citation1 Over the last three decades, the incidence of HCC in the US has increased from 1.4 per 100,000 to 8.2 per 100,000 per year.Citation2 The incidence of HCC is higher in developing countries, particularly those in the Asian Pacific regions, owing to the high prevalence of chronic hepatitis B and C viral infections. However, the incidence in developed countries has also been increasing owing to the emergence of nonalcoholic steatohepatitis (NASH) as an important risk factor.Citation3,Citation4 In fact, recently, a large retrospective study demonstrated that the proportion of non-virus–related HCC increased from 10% in 1991 to 24.1% in 2010, with most cases related to nonalcoholic fatty liver disease (NAFLD) and diabetes.Citation5–Citation7 In a Japanese study, the 5-year HCC development rates in cirrhosis patients were 11.3% in NAFLD cirrhosis, 12.5% in alcoholic cirrhosis, and 30.5% in hepatitis C virus cirrhosis, showing similar rates of HCC development in both alcoholic and NAFLD-related cirrhosis.Citation8 Although metabolic syndrome had the lowest relative risk among other factors for HCC (1.5%−2.5%), its high prevalence in the general population (30%−40%) led to the highest population-attributable fraction.Citation9
Regardless of the underlying etiology, the incidence of HCC is higher in males, with male-to-female ratios varying between 3:1 and 4:1 depending on geographic location.Citation10,Citation11 Moreover, males have poorer survival despite there being many treatment options.Citation12–Citation14 The increased incidence and disease aggressiveness in males suggest that androgen and androgen receptors (ARs) might promote HCC development and progression and/or that estrogen and estrogen receptors might suppress HCC development.Citation15 This review summarizes the evidence for the potential role of androgen and ARs in NAFLD/NASH-related HCC.
NAFLD pathogenesis
NAFLD is the most common cause of liver dysfunction, with a prevalence of 20%−30% in the general population and up to 57%−74% among obese patients.Citation16 NAFLD was usually considered to be one of the components of the metabolic syndrome and to be strongly linked to central obesity, insulin resistance, dyslipidemia, and hypertension ().Citation17–Citation22 Recent studies support the association of NAFLD with type 2 diabetes mellitus or metabolic syndrome, suggesting that NAFLD actually precedes the development of both conditions and is considered as a risk factor for development of type 2 diabetes mellitus.Citation23 NAFLD includes disorders ranging from isolated liver steatosis (in which triglycerides accumulate in the hepatocytes), characterized by macrovesicular fatty change with or without nonspecific inflammation in the absence of cellular injury (ballooning), to NASH (characterized by the presence of additional cellular ballooning) and, subsequently, to cirrhosis and even HCC.Citation24–Citation26 Fibrosis is the most important determinant of the outcome.
Table 1 Five parameters of the metabolic syndrome according to the WHO and AACE
The molecular pathogenesis of liver steatosis and its progression to cirrhosis and eventually HCC is not clearly understood. Previously, a two-hit theory had been proposed to explain the molecular changes through which fatty liver leads to lipid peroxidation, cytokine production, and Fas ligand induction.Citation27 However, more recently, a multiple-hit theory which better explains NAFLD development and progression to HCC has gained ground. The first hit is caused by insulin resistance and leads to fat accumulation in the hepatocytes, induced by both lipolysis and hyperinsulinemia. This is followed by multiple hits with many factors playing different roles, including genetic predisposition, obesity, oxidative stress and mitochondrial dysfunction, inflammation, adipokines, small intestinal microbacteria, and others. Hyperinsulinemia could also lead to increased levels of insulin growth factor-1, which causes stimulation of insulin growth factor receptors and promotion of proliferative and antiapoptotic effects, and vascular endothelial growth factor–mediated promotion of angiogenesis.Citation28
Thus, in this setting, the liver is more susceptible to oxidative stress and inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which promote progression to NASH and fibrosis. IL-6 activates signal transducer and activator of transcription 3, which induces cell proliferation and antiapoptotic mechanisms. TNF-α activates pro-oncogenic pathways, including c-Jun N-terminal kinase, nuclear factor kappa-light-chain-enhancer of activated B cells, mammalian target of rapamycin, and the extracellular signal-regulated kinases.Citation29–Citation32 interestingly, several studies indicated that both dietary and genetic obesity could promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF-α expression.Citation33–Citation36
Finally, the high circulating levels of leptin in NAFLD have been recently shown to exert proinflammatory and profibrogenic effects.Citation37,Citation38 In addition, there is evidence that lipid peroxides and free radicals are elevated in metabolic syndrome; these may cause oxidative injury, endoplasmic reticulum stress, mitochondrial dysfunction, and apoptosis.Citation15
Role of gender differences in NAFLD
Several studies have shown that gender differences play a role in various liver disorders. Earlier beliefs that NAFLD/NASH was a female-predominant condition have been dispelled; recent studies have shown higher prevalence in males.Citation39–Citation46 Interestingly, in a preclinical study, a high-fat, high-cholesterol diet induced NASH and hepatic ballooning in ovariectomized mice, which showed that estrogen deficiency promoted NASH progression, while estrogen treatment reversed it.Citation47 Furthermore, many studies have shown that NAFLD patients have significantly lower levels of sex hormone binding globulin, a glycoprotein that binds to sex hormones (both estrogen and androgen).Citation48,Citation49 Increased prevalence of NAFLD has also been reported in patients with polycystic ovary syndrome (PCOS).Citation50,Citation51 Diethylnitrosamine administration caused greater increase in serum IL-6 in male than in female mice. Also, ablation of IL-6 abolished the gender differences in hepatocarcinogenesis in mice.Citation52,Citation53
However, the roles of androgen in the development of NAFLD remain unclear, as the results of experimental and clinical studies have been inconsistent.Citation54,Citation55 For example, Jones et al found a significantly higher degree of hepatic steatosis in high-androgen-expressed PCOS patients as compared to low-androgen-expressed PCOS patients or controls.Citation56 Another study observed that androgenic steroid usage by bodybuilders could be a possible risk factor for NAFLD.Citation57 In contrast, Haider et al showed that normalizing serum testosterone levels in obese hypogonadal males could improve their weight loss and metabolic state and suppress the development of NASH.Citation58
The role of ARs in the pathogenesis of NAFLD is also unclear. An experimental study showed that whole-body AR-knockout mice fed a high-fat diet developed liver steatosis and insulin resistance, possibly through either 1) suppression of fatty acid synthesis by decreased sterol regulatory element-binding protein 1 (SREBP1) expression or 2) increased insulin sensitivity by suppression of phosphoenolpyruvate carboxykinase and protein tyrosine phosphatase 1B.Citation59 Notably, other studies showed that activation of AR in orchidectomized mice led to obesity and altered lipid metabolism, and progression of NASH to HCC.Citation60 This may have been mediated either through downregulation of liver X receptor (which complements SREBP2 activation and increases cellular cholesterol levels) or through upregulation of AR messenger RNA levels and increased activity of CYP27A1 (an enzyme that plays a significant role in cholesterol homeostasis and vitamin D3 metabolism through activation of the c-Jun N-terminal kinase pathway).Citation61,Citation62
Prevention of NASH/metabolic syndrome-related HCC using existing drugs
Statins (3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors) are commonly prescribed for prevention of cardiovascular disease and act by decreasing the biosynthesis of cholesterol. Statins may play a role in the prevention of cancer development by inducing apoptosis and inhibiting cellular proliferation, angiogenesis, inflammation, and immu-nomodulation.Citation63 In multiple observational studies,Citation64–Citation67 the use of statins has been associated with a decreased risk of HCC in patients with viral hepatitis and those with diabetes, and statins may also decrease the risk of HCC recurrence after surgical treatment.Citation68 However, a randomized controlled trial did not demonstrate a significant difference in HCC incidence between the statin and placebo groups.Citation69
In diabetic patients, the use of both metformin and thiazolidinediones (peroxisome proliferator-activated receptor gamma agonists) was associated with a decreased cancer risk, whereas sulfonylurea was correlated with an increased overall risk of cancer.Citation70 Also, the use of combined statin and metformin in diabetic patients showed no benefit to reduce the risk of HCC in Asian population.Citation71 Metformin has been shown to inhibit hepatocellular proliferation and leads to arrest of the cell cycle at the G0/G1 phase by downregulation of cyclin D1,Citation72 while thiazolidinediones were reported to decrease the risk of HCC by accumulation of p27, inhibition of ubiquitin-proteasome, MEK-ERK signaling, and induction of apoptosis.Citation70,Citation73,Citation74 Also, drugs of proven antifibrogenic efficacy may potentially decrease the risk of developing HCC.Citation75
Mechanistic pathway of androgen and ARs in hepatocarcinogenesis
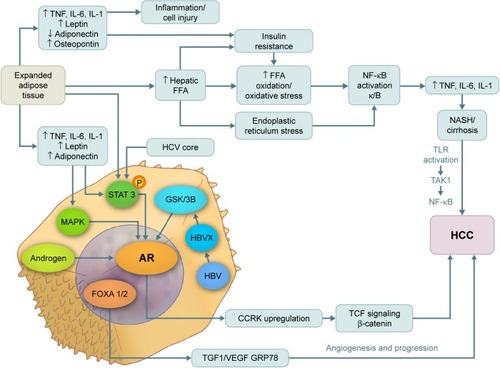
Both estrogen and androgen are steroid hormones that mediate their action by binding to nuclear receptors and acting as transcription factors to regulate the expression of multiple genes. Progression from hyperplasia to HCC is associated with suppression of estrogen receptors and elevated AR expression.Citation76–Citation80 In addition, several studiesCitation81 have shown that AR messenger RNA protein is expressed at higher levels in hepatic tumor tissue than in normal hepatic tissue. The AR gene is located on the X chromosome with a single copy in males. The AR molecule is a ligand-activated transcriptional factor with three domains – the N-terminal domain, the DNA-binding domain, and the ligand-binding domain.Citation82 ARs can be activated directly by androgen, inducing cell cycle-relatd kinase (CCRK) transcription through promoter binding; CCRK then upregulates β-catenin/T-cell factor signaling, leading to promotion of hepatocarcinogenesis.Citation83 ARs can also be activated directly in the absence of androgen by many alternative pathways such as the mitogen-activated protein kinase, AKt, and signal transducer and activator of transcription pathways, which are also involved in hepatocarcinogenesis ().Citation84,Citation85 In an experimental study, adding functional ARs to HCC cells promoted cell growth and increased cellular oxidative stress, DNA damage, and suppression of the p53-mediated sensing/repairing system and of cell apoptosis.Citation86 Furthermore, several studies have shown that AR is expressed in both normal liver and malignant tissues, but at higher levels in HCC tumor tissue.Citation56,Citation61,Citation86
Figure 1 Pathways of androgen and AR in the pathogenesis of NASH, cirrhosis, and hepatocellular carcinoma.
Abbreviations: AR, androgen receptor; CCRK, cycle cycle-related kinase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IL, interleukin; MAPK, mitogen-activated protein kinase; NASH, nonalcoholic steatohepatitis; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; STAT 3, signal transducer and activator of transcription 3; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; GSK3b, glycogen synthase kinase 3 beta; FFA, free fatty acid; HBVX, hepatitis B virus protein X; TGF1, tumor growth factor 1.
Clinical evidence of androgen’s role in promoting HCC recurrence and metastasis
Several clinical studies have reported a strong correlation between AR expression and the rate of HCC recurrence (summarized in ).Citation87–Citation89 However, there is controversy regarding the relationship between AR and tumor characteristics such as size, another factor that affects tumor recurrence.Citation57,Citation70
Table 2 Clinical studies that evaluated the role of androgen and its receptors in the recurrence of surgically treated HCC
Moreover, the role of AR in promoting metastasis in HCC has been understudied. A few studies have reported a role for AR in metastatic HCC lesions, which may include the following: 1) AR activation by its ligand leads to increased expression of ID1 (a metastasis-promoting gene), which causes HCC cell migration and invasionCitation90 or 2) tighter and faster adhesion of the cancer cells to collagen IV and various extracellular membranes occurs through β1 integrin expression induced by activated ARs.Citation91 The latter effect of AR on cell adhesion was shown to be mediated by the β1 integrin-phosphoinositide-3-kinase (PI3K)/Akt pathway. In contrast, another study found that AR expression was significantly reduced in advanced metastatic lesions compared with early primary HCC lesions, with the AR upregulated in tumors <3 cm.Citation92
Effect of antiandrogen treatment on HCC outcome
Despite the mounting evidence suggesting the potential role of the AR pathway as a therapeutic target in HCC, data on the use of antiandrogens are limited to a few clinical trials and no definite activity has been reported using this strategy ().Citation87,Citation93–Citation99 One reason for the failure of these clinical trials could be the fact that these studies included mostly advanced and metastatic HCC patients, and the role of AR in late-stage HCC – whether it promotes or suppresses invasion and metastasis – remains unclear. Also, AR expression might be more critical than androgen concentration in HCCCitation92 and no correlative studies have tested the predictive value of expression of AR in the tissue of HCC patients. Finally, most trials were performed on viral hepatitis–induced HCC, which may not have the same histologic characteristics or pathogenesis as non-hepatitis–induced HCC. The biological heterogeneity of HCC makes the prognosis of tumor growth, survival of patients, and treatment outcomes difficult.Citation100–Citation102
Table 3 Clinical studies that evaluated the role of antiandrogen drugs in the treatment of HCC
Current systemic therapy in HCC: potential role of AR
Currently, single-agent sorafenib, a putative multitargeted kinase inhibitor, is the only systemic therapy approved by the US Food and Drug Administration to treat patients with advanced HCC. Sorafenib prolongs the overall survival by approximately 3 months in this populationCitation13 Phase II studies of other targeted drugs such as sunitinib, linifanib, erlotinib, ramucirumab, and everolimus demonstrated promising results in the management of late-stage HCC, but Phase III studies of these agents did not show overall survival benefit in unselected patient populations.Citation103–Citation105 Notably, subgroup analyses of the Sorafenib HCC Assessment Randomized Protocol (SHARP) trial suggested that survival outcomes varied with patient demographics, geographic location, and risk factors. Interestingly, recent Phase II studies of other agents, including two studies in the first-line treatment setting conducted by the group using the combination of bevacizumab and erlotinib, showed activity in HCC,Citation103,Citation106–Citation111 which also suggested differential outcomes based on patients’ demographics, geographic locations, and risk factors.
Another important factor that affects the outcome in HCC patient population is their tolerance to therapies, given the coexistence of HCC tumors and underlying liver disease in most of the cases. Although the adverse effects of targeted therapy are usually tolerable, serious complications can develop, especially with higher doses or in combination with another angiogenic agent or with chemotherapy. Fatigue, diarrhea, and hand/foot skin reaction are the most common dose-limiting side effects, with bleeding, arterial thromboembolism, and other fatal complications also possible.Citation13 Therefore, increasing the efficiency of sorafenib and other targeted therapies by combination with other drugs that increase their efficacy while maintaining a lower dose is essential.
AR was found to suppress HCC metastasis by modulating p38. Also, the addition of functional AR in SKhep1 and HepG2 HCC cells was found to decrease p38, leading to enhanced effectiveness of sorafenib against HCC cells.Citation92 In preclinical studies, sorafenib treatment had greater anti-metastatic effects against AR-positive than AR-negative HCC (66.7% vs 0%, respectively; P=0.0109). Another study showed that sorafenib with or without AR inhibitors induced significant apoptosis in HCC cells with knocked out AR when compared with unmanipulated HCC cells; the authors concluded that inhibition of AR in combination with sorafenib may be beneficial for the treatment of HCC.Citation90,Citation111 These data suggest that AR may be a target of combination therapy, and that AR expression may be a potential biomarker of response to sorafenib in HCC.
Conclusion
Androgen and ARs may play a critical role in hepatocarcinogenesis and could mediate, in part, the mechanisms responsible for the gender disparity in HCC incidence. ARs can be activated both by androgen and, in the absence of androgen, by alternative pathways. Liver steatosis can progress into NASH, which can lead to cirrhosis and HCC. The high prevalence of NASH in the general population led to recent significant increase in incidence of NASH-related HCC cases. Gender disparity appears to play a significant role in the development of NASH, as NASH is more common in males than in females and also contributes to higher risk of HCC development. Nevertheless, the clinical trials conducted so far have failed to demonstrate a significant benefit of antiandrogen drugs in the treatment of HCC patients. This failure could be explained, at least partially, by 1) the marked heterogeneity of HCC, with most trials performed on virus-induced HCC; 2) the dual, yet opposite effects of AR during early and late HCC, with most trials performed on advanced HCC only; and 3) the direct stimulation of AR by pathways other than androgen. Therefore, it is critical to understand the molecular mechanisms associated with HCC in males with NASH to design successful targeted therapy studies focusing on AR pathway.
Acknowledgments
Editorial assistance was provided by Michael Worley and the Department of Scientific Publications at MD Anderson Cancer Center. This work was supported by MD Anderson Cancer Center (to AOK).
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- El-SeragHBLauMEschbachKDavilaJGoodwinJEpidemiology of hepatocellular carcinoma in hispanics in the United StatesArch Intern Med2007167181983198917923599
- WelzelTMGraubardBIZeuzemSEl-SeragHBDavilaJAMcGlynnKAMetabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare databaseHepatology (Baltimore, Md)2011542463471
- BaffyGBruntEMCaldwellSHHepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menaceJ Hepatol20125661384139122326465
- TateishiROkanoueTFujiwaraNClinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort studyJ Gastroenterol201550335036024929638
- WhiteDLKanwalFEl-SeragHBAssociation between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic reviewClin Gastroenterol Hepatol2012101213421359.e223041539
- FacciorussoAThe influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: recent findings and new perspectivesCurr Diabetes Rev20139538238623845075
- TokushigeKHashimotoEKodamaKHepatocarcinogenesis in nonalcoholic fatty liver disease in JapanJ Gastroenterol Hepatol201328Suppl 4889224251711
- WelzelTMGraubardBIQuraishiSPopulation-attributable fractions of risk factors for hepatocellular carcinoma in the United StatesAm J Gastroenterol201310881314132123752878
- HefaiedhREnnaiferRRomdhaneHGender difference in patients with hepatocellular carcinomaTunis Med2013918–950550824227507
- El-SeragHBHepatocellular carcinomaN Engl J Med2011365121118112721992124
- YamashitaYIYoshidaYKuriharaTSurgical results for recurrent hepatocellular carcinoma after curative hepatectomy: repeat hepatectomy vs. salvage living donor liver transplantationLiver Transpl201521796196825772591
- LlovetJMRicciSMazzaferroVSorafenib in advanced hepatocellular carcinomaN Engl J Med2008359437839018650514
- LeeDHLeeJMLeeJYKimSHHanJKChoiBIRadiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma: long-term results and prognostic factors in 168 patients with cirrhosisCardiovascu Intervent Radiol2014373705715
- CazanaveSCMottJLElmiNAJNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosisJ Biol Chem200928439265912660219638343
- AnguloPNonalcoholic fatty liver diseaseN Engl J Med2002346161221123111961152
- AlbertiKGZimmetPZDefinition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultationDiabet Med19981575395539686693
- GrundySMCleemanJIDanielsSRAmerican Heart AssociationNational Heart, Lung, and Blood InstituteDiagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific StatementCirculation2005112172735275216157765
- AlbertiKGZimmetPShawJMetabolic syndrome – a new worldwide definition. A consensus statement from the international diabetes federationDiabet Med200623546948016681555
- IsomaaBAlmgrenPTuomiTCardiovascular morbidity and mortality associated with the metabolic syndromeDiabetes Care200124468368911315831
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in AdultsExecutive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III)JAMA2001285192486249711368702
- EssahPANestlerJEThe metabolic syndrome in polycystic ovary syndromeJ Endocrinol Invest200629327028016682845
- LonardoABallestriSMarchesiniGAnguloPLoriaPNonalcoholic fatty liver disease: a precursor of the metabolic syndromeDig Liver Dis201547318119025739820
- ByrneCDOlufadiRBruceKDCagampangFRAhmedMHMetabolic disturbances in non-alcoholic fatty liver diseaseClin Sci (Lond)2009116753956419243311
- RinellaMENonalcoholic fatty liver disease: a systematic reviewJAMA2015313222263227326057287
- KubesPMehalWZSterile inflammation in the liverGastroenterology201214351158117222982943
- WiegandJMossnerJTillmannHLNon-alcoholic fatty liver disease and non-alcoholic steatohepatitisDer Internist2007482154163 German17226007
- TomimaruYKogaHYanoHde la MonteSWandsJRKimMUpregulation of T-cell factor-4 isoform-responsive target genes in hepatocellular carcinomaLiver Int20133371100111223651211
- BianZMaXLiver fibrogenesis in non-alcoholic steatohepatitisFront Physiol2012324822934006
- WuJZhangJShenBLong noncoding RNA lncTCF7, induced by IL-6/STAT3 transactivation, promotes hepatocellular carcinoma aggressiveness through epithelial-mesenchymal transitionJ Exp Clin Cancer Res20153411626452542
- JungIHChoiJHChungYYLimGLParkYNParkSWPredominant activation of JAK/STAT3 pathway by Interleukin-6 is implicated in hepatocarcinogenesisNeoplasia201517758659726297436
- WanSZhaoEKryczekITumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cellsGastroenterology201414761393140425181692
- BugianesiEPagottoUManiniRPlasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severityJ Clin Endocrinol Metab20059063498350415797948
- MussoGGambinoRDurazzoMAdipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver diseaseHepatology20054251175118316231364
- DingXSaxenaNKLinSXuASrinivasanSAnaniaFAThe roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biologyAm J Pathol200516661655166915920151
- ParkEJLeeJHYuGYDietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expressionCell2010140219720820141834
- AwazawaMUekiKInabeKAdiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathwayBiochem Biophys Res Commun20093821515619254698
- IkejimaKOkumuraKKonKTakeiYSatoNRole of adipocytokines in hepatic fibrogenesisJ Gastroenterol Hepatol200722Suppl 1S87S9217567476
- WestonSRLeydenWMurphyRRacial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver diseaseHepatology200541237237915723436
- BaigSGender disparity in infections of hepatitis B virusJ Coll Physicians Surg Pak200919959860019728952
- BrowningJDSzczepaniakLSDobbinsRPrevalence of hepatic steatosis in an urban population in the United States: impact of ethnicityHepatology20044061387139515565570
- BaconBRFarahvashMJJanneyCGNeuschwander-TetriBANonalcoholic steatohepatitis: an expanded clinical entityGastroenterology19941074110311097523217
- MarchesiniGBugianesiEForlaniGNonalcoholic fatty liver, steatohepatitis, and the metabolic syndromeHepatology200337491792312668987
- AngelicoFDel BenMContiRNon-alcoholic fatty liver syndrome: a hepatic consequence of common metabolic diseasesJ Gastroenterol Hepatol200318558859412702052
- KralJGSchaffnerFPiersonRNJrWangJBody fat topography as an independent predictor of fatty liverMetabolism19934255485518492707
- OmagariKKadokawaYMasudaJFatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristicsJ Gastroenterol Hepatol200217101098110512201871
- KamadaYKisoSYoshidaYEstrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol dietAm J Physiol Gastrointest Liver Physiol20113016G1031G104321885686
- PolyzosSAKountourasJTsatsoulisASex steroids and sex hormone-binding globulin in postmenopausal women with nonalcoholic fatty liver diseaseHormones201312340541624121382
- ShinJYKimSKLeeMYSerum sex hormone-binding globulin levels are independently associated with nonalcoholic fatty liver disease in people with type 2 diabetesDiabetes Res Clin Pract201194115616221862168
- VassilatouENonalcoholic fatty liver disease and polycystic ovary syndromeWorld J Gastroenterol201420268351836325024594
- CiottaLPaganoIStracquadanioMFormusoCPolycystic ovarian syndrome incidence in young women with non-alcoholic fatty liver diseaseMinerva Ginecol2011635429437 Italian21926952
- NauglerWESakuraiTKimSGender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 productionScience2007317583412112417615358
- PrietoJInflammation, HCC and sex: IL-6 in the centre of the triangleJ Hepatol200848238038118093689
- ZhangHLiuYWangLDifferential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male ratJ Lipid Res201354234535723175777
- ChowJDJonesMEPrelleKSimpsonERBoonWCA selective estrogen receptor alpha agonist ameliorates hepatic steatosis in the male aromatase knockout mouseJ Endocrinol2011210332333421705395
- JonesHSprungVSPughCJPolycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistanceJ Clin Endocrinol Metab201297103709371622837189
- SchwingelPACotrimHPSallesBRAnabolic-androgenic steroids: a possible new risk factor of toxicant-associated fatty liver diseaseLiver Int201131334835321040407
- HaiderASaadFDorosGGoorenLHypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational studyObes Res Clin Pract201484e339e34925091355
- LinHYYuICWangRSIncreased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptorHepatology (Baltimore, Md)200847619241935
- Moverare-SkrticSVenkenKAnderssonNDihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized miceObesity (Silver Spring, Md)2006144662672
- NorlinMPetterssonHTangWWikvallKAndrogen receptor-mediated regulation of the anti-atherogenic enzyme CYP27A1 involves the JNK/c-jun pathwayArch Biochem Biophys2011506223664121134350
- KrycerJRBrownAJCross-talk between the androgen receptor and the liver X receptor: implications for cholesterol homeostasisJ Biol Chem201128623206372064721489984
- LonardoALoriaPPotential for statins in the chemoprevention and management of hepatocellular carcinomaJ Gastroenterol Hepatol201227111654166422849701
- LaiSWLiaoKFLaiHCMuoCHSungFCChenPCStatin use and risk of hepatocellular carcinomaEur J Epidemiol201328648549223681775
- TsanYTLeeCHWangJDChenPCStatins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infectionJ Clin Oncol201230662363022271485
- El-SeragHBJohnsonMLHachemCMorganaROStatins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetesGastroenterology200913651601160819208359
- SinghSSinghPPSinghAGMuradMHSanchezWStatins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysisGastroenterology2013144232333223063971
- WuCYChenYJHoHJHsuYCKuoKNWuMSAssociation between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resectionJAMA2012308181906191423162861
- EmbersonJRKearneyPMBlackwellLCholesterol Treatment Trialists’ (CTT) CollaborationLack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapyPLoS One201271e2984922276132
- ChangCHLinJWWuLCLaiMSChuangLMOral insulin secretagogues, insulin, and cancer risk in type 2 diabetes mellitusJ Clin Endocrinol Metab2012977E1170E117522563104
- ChenHHLinMCMuoCHYehSYSungFCKaoCHCombination therapy of mtetformin and statin may decrease hepatocellular carcinoma among diabetic patients in AsiaMedicine20159424e101326091447
- ChenHPShiehJJChangCCMetformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studiesGut201362460661522773548
- OkumuraTMechanisms by which thiazolidinediones induce anticancer effects in cancers in digestive organsJ Gastroenterol201045111097110220824291
- RuiterRVisserLEvan Herk-SukelMPLower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up studyDiabetes Care201235111912422100960
- SinghSKheraRMuradMHLoombaRReplyHepatology (Baltimore, Md)2016642694
- EagonPKElmMSEpleyMJShinozukaHRaoKNSex steroid metabolism and receptor status in hepatic hyperplasia and cancer in ratsGastroenterology19961104119912078613010
- OstrowskiJLIngletonPMUnderwoodJCParsonsMAIncreased hepatic androgen receptor expression in female rats during diethylnitrosamine liver carcinogenesis. A possible correlation with liver tumor developmentGastroenterology1988945 Pt 1119312003350289
- TejuraSRodgersGRDunionMHParsonsMAUnderwoodJCIngletonPMSex-steroid receptors in the diethylnitrosamine model of hepatocarcinogenesis: modifications by gonadal ablation and steroid replacement therapyJ Mol Endocrinol1989332292372590384
- NakataniTRoyGFujimotoNAsaharaTItoASex hormone dependency of diethylnitrosamine-induced liver tumors in mice and chemo-prevention by leuprorelinJpn J Cancer Res200192324925611267934
- FengHChengASTsangDPCell cycle-related kinase is a direct androgen receptor-regulated gene that drives beta-catenin/T cell factor- dependent hepatocarcinogenesisJ Clin Invest201112183159317521747169
- TianYWongVWChanHLChengASEpigenetic regulation of hepatocellular carcinoma in non-alcoholic fatty liver diseaseSemin Cancer Biol2013236 Pt B47148224018165
- GelmannEPMolecular biology of the androgen receptorJ Clin Oncol200220133001301512089231
- BoltonECSoAYChaivorapolCHaqqCMLiHYamamotoKRCell- and gene-specific regulation of primary target genes by the androgen receptorGenes Dev200721162005201717699749
- CuligZAndrogen receptor coactivators in regulation of growth and differentiation in prostate cancerJ Cell Physiol2016231227027426201947
- AgoulnikIUWeigelNLCoactivator selective regulation of androgen receptor activitySteroids200974866967419463689
- KalraMMayesJAssefaSKaulAKKaulRRole of sex steroid receptors in pathobiology of hepatocellular carcinomaWorld J Gastroenterol200814395945596118932272
- NagasueNKohnoHChangYCAndrogen and estrogen receptors in hepatocellular carcinoma and the surrounding liver in womenCancer19896311121162535950
- BoixLCastellsABruixJAndrogen receptors in hepatocellular carcinoma and surrounding liver: relationship with tumor size and recurrence rate after surgical resectionJ Hepatol19952266166227560855
- ZhangXHeLLuYLiuMHuangXAndrogen receptor in primary hepatocellular carcinoma and its clinical significanceChin Med J1998111121083108611263369
- AoJMengJZhuLActivation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasionMol Oncol20126550751522819717
- MaWLJengLBLaiHCLiaoPYChangCAndrogen receptor enhances cell adhesion and decreases cell migration via modulating beta1-integrin-AKT signaling in hepatocellular carcinoma cellsCancer Lett20143511647124944078
- MaWLHsuCLYehCCHepatic androgen receptor suppresses hepatocellular carcinoma metastasis through modulation of cell migration and anoikisHepatology (Baltimore, Md)2012561176185
- ForbesAWilkinsonMLIqbalMJJohnsonPJWilliamsRResponse to cyproterone acetate treatment in primary hepatocellular carcinoma is related to fall in free 5 alpha-dihydrotestosteroneEur J Cancer Clin Oncol19872311165916642828073
- GuptaSKorulaJFailure of ketoconazole as anti-androgen therapy in nonresectable primary hepatocellular carcinomaJ Clin Gastroenterol19881066516542466073
- ChaoYChanWKHuangYSPhase II study of flutamide in the treatment of hepatocellular carcinomaCancer19967746356398616754
- GrimaldiCBleibergHGayFEvaluation of antiandrogen therapy in unresectable hepatocellular carcinoma: results of a European Organization for Research and Treatment of Cancer multicentric double-blind trialJ Clin Oncol19981624114179469323
- ManesisEKGiannoulisGZoumboulisPVafiadouIHadziyannisSJTreatment of hepatocellular carcinoma with combined suppression and inhibition of sex hormones: a randomized, controlled trialHepatology (Baltimore, Md)199521615351542
- Groupe d’Etude et de Traitement du Carcinome HépatocellulaireRandomized trial of leuprorelin and flutamide in male patients with hepatocellular carcinoma treated with tamoxifenHepatology (Baltimore, Md)200440613611369
- Di MaioMDe MaioEMorabitoAHormonal treatment of human hepatocellular carcinomaAnn N Y Acad Sci2006108925226117261772
- VillaECritelliRLeiBNeoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective studyGut201665586186925666192
- WeiskirchenRIntratumor heterogeneity, variability and plasticity: questioning the current concepts in classification and treatment of hepatocellular carcinomaHepatobiliary Surg Nutr20165218318727115013
- LiLWangHHeterogeneity of liver cancer and personalized therapyCancer Lett2015379219119726213370
- HsuCHKangYKYangTSBevacizumab with erlotinib as first-line therapy in Asian patients with advanced hepatocellular carcinoma: a multicenter phase II studyOncology2013851445223838576
- CainapCQinSHuangWTLinifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trialJ Clin Oncol201533217217925488963
- WelkerMWTrojanJAntiangiogenic treatment in hepatocellular carcinoma: the balance of efficacy and safetyCancer Manag Res2013533734724204170
- GovindarajanRSiegelEMakhoulIWilliamsonSBevacizumab and erlotinib in previously untreated inoperable and metastatic hepatocellular carcinomaAm J Clin Oncol201336325425722643560
- KasebAOGarrett-MayerEMorrisJSEfficacy of bevacizumab plus erlotinib for advanced hepatocellular carcinoma and predictors of outcome: final results of a phase II trialOncology2012822677422327795
- PhilipPAMahoneyMRHolenKDPhase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancerCancer201211892424243021953248
- YauTWongHChanPPhase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory diseaseInvest New Drugs20123062384239022402942
- ThomasMBMorrisJSChadhaRPhase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinomaJ Clin Oncol200927684385019139433
- JiangXKandaTNakamotoSMiyamuraTWuSYokosukaOInvolvement of androgen receptor and glucose-regulated protein 78 kDa in human hepatocarcinogenesisExp Cell Res2014323232633624583399
