Abstract
Increasing evidence suggests that CUL4A, a ubiquitin ligase, is involved in the promotion of cancer malignancy and correlated with worse clinical prognosis in several kinds of human cancers. Although its effect and mechanism on the progression of colorectal cancer (CRC) remain unknown. Our clinical data show that CUL4A protein is overexpressed, positively associated with lymph nodes status, differentiation degree, tumor size, and poor prognosis in 80 CRC patients. CUL4A overexpression promotes cell proliferation and colony formation of CRC cells. Knockdown of CUL4A inhibits cell proliferation and migration. CUL4A can significantly promote the in vitro migration of CRC cells via induction of the epithelial–mesenchymal transition process. And the modulation of CUL4A expression altered the level of H3K4 trimethylation at the E-cadherin, N-cadherin, and vimentin gene promoters, which in turn transcriptionally regulated their expression. Moreover, knockdown of CUL4A also decreased the tumor volume and tumor weight in vivo. Together, our results reveal that CUL4A plays as an oncogene in CRC and may become a potential therapeutic target in the treatment of colorectal cancer.
Introduction
In 2013, colorectal cancer (CRC) was the third most common cancer and the fourth leading cause of death worldwide.Citation1 An increasing number of oncogenes have been reported to be responsible for the development of CRC, such as S100P, K-Ras, BRAF, visfatin, and pinin.Citation2–Citation6 Primary CRC derives from the epithelial cells of the gastrointestinal tract.Citation7 During epithelial–mesenchymal transition (EMT) progression, cancer cells are thought to acquire a phenotype of the mesenchymal cell, which allows them to lose their junctions, invade surrounding tissues, and migrate to distant organs.Citation7 EMT is initiated by a set of transcription factors, such as Snail, Twist1, and ZEB, which execute EMT by repressing epithelial genes and activating mesenchymal genes.Citation8 H3K4 trimethylation (H3K4me3) is a prevalent mark that is exclusively associated with actively transcribed genes, whereas trimethylation of H3K27 (H3K27me3) is associated with gene repression.Citation9
CUL4A is a member of the evolutionally conserved cullin family, which is evolutionally conserved and includes seven-related cullins (Cul1, Cul2, Cul3, Cul4A, Cul4B, Cul5, and Cul7).Citation10 CUL4A constitutes the ubiquitin ligase E3 complex, and plays crucial roles in DNA replication, cell cycle regulation, and genomic instability.Citation11–Citation15 Previous studies have shown that CUL4A has been reported to have an essential role in the ubiquitination of several well-defined tumor suppressor genes, for instance p21, p27, p53, and DDB2, which provides evidence that CUL4A might be a potential oncogene.Citation15–Citation18 CUL4A overexpression has been discovered in several kinds of human cancers, including breast cancer, prostate cancer, non-small cell lung cancer (NSCLC), and malignant pleural mesothelioma (MPM).Citation19–Citation23
In this study, we provide evidence that CUL4A was overexpressed in primary CRC and cell line HCT-116 and predicted poor overall patient survival. Moreover, CUL4A promoted cell proliferation, colony formation, and migration of HCT-116. We also found that CUL4A promoted CRC development through EMT pathway and CUL4A triggered the EMT process via regulation of H3K4me3. Finally, knockdown of CUL4A inhibited tumor size and weight in vivo. In conclusion, these data suggest that CUL4A is a potential novel target for CRC diagnosis and therapy.
Materials and methods
Patients and specimens
Primary CRC tissues and their corresponding adjacent normal tissues were obtained from the CRC patients treated in the Affiliated Huai’an Hospital of Xuzhou Medical University. The study was approved by the Medical Ethics Committee of the Affiliated Huai’an Hospital of Xuzhou Medical University and written informed consent was obtained from all the patients involved in this study. Detailed clinical histopathologic factors were presented in . For the measurement of prognosis, we analyzed the clinical data overall survival (OS), defined as the time from surgery to death. All recruited patients had been followed-up periodically until the patients death.
Table 1 Association between CUL4A expression and clinicopathologic features of colorectal cancer patients
Antibodies and Western blot
H3K4me3 rabbit mAb, E-cadherin mouse mAb, N-cadherin rabbit mAb, and β-actin mouse mAb were purchased from Cell Signaling Technology (Danvers, MA, USA). The cells were lysed in a buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, and 1% Nonidet P 40 with a mixture of protease inhibitors before Western blot assay.
Immunohistochemistry (IHC) analysis
The CRC patients specimens were fixed in 4% paraformaldehyde solution overnight and embedded in paraffin wax the next day. For histologic analysis, sections were cut at a thickness of 4 μm and followed by hematoxylin and eosin staining. All sections were processed for IHC using an ABC kit (Vector Laboratories, Burlingam, CA, USA) according to the manufacturer’s instruction and observed under a microscope (Olympus Corporation, Tokyo, Japan). Representative photographs were taken.
Cell culture, lentivirus packaging, and infection
HEK293T and human CRC cell line HCT-116 were purchased from Bank/Stem Cell Bank, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences (Shanghai, People’s Republic of China). HEK293T cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific). Then HCT-116 cells were cultured in Roswell Park Memorial Institute 1640 (Thermo Fisher Scientific) supplemented with 10% FBS.
For lentivirus packaging, HEK293T cells (7×106) were plated in a 15 cm dish, incubated for 24 hours (h), and then transfected with 15 μg of lentivirus plasmids. After 48 h, the virus containing medium was filtered through a 0.45 μm filter (EMD Millipore, Billerica, MA, USA) and collected as the first supernatant. Additional DMEM medium with FBS was added into the dish and the virus-containing medium was filtered and collected as the second supernatant after another 24 h. Both of the first and second supernatant was then centrifuged at 20,000× g for 2 h. The supernatant was abandoned and the precipitate was resuspended in 100 μL DMEM without FBS.
For infection, gradient virus-contained DMEM was added with polybrene (Sigma-Aldrich Co., St Louis, MO, USA, 4 μg/mL), and then the culture dishes were incubated at 37°C for 6 h and replaced by fresh medium. After incubation for 36–48 h, the infected cell populations were confirmed by fluorescence microscope for green fluorescent protein expression to evaluate the virus titer. Target cells were plated in a 6-well plate for infection by appropriate volume of virus-contained DMEM.
RNA isolation and quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells by Trizol reagent (Thermo Fisher Scientific) then reverse transcribed and synthesized to cDNA using avian myeloblastosis virus reverse transcriptase (Takara, Dalian, People’s Republic of China) according to these manufacturers’ instructions. The quantification of gene transcripts was determined by real-time PCR using SYBR Green Real time PCR Master Mix (Toyobo) and Mx3000P quantitative PCR (qPCR) system (Stratagene, La Jolla, LA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control. The qPCR primers are 5′-CTCCAAGAAGCTGGTCATCA-3′ and 5′-GAGCTCCTCGAGGTTGTACC-3′ for CUL4A; 5′-TACACTGCCCAGGAGCCAGA-3′ and 5′-TGGCACCAGTGTCCGGATTA-3′ for E-cadherin; 5′-GACGGTTCGCCATCCAGAC-3′ and 5′-TCGATTGGTTTGACCACGG-3′ for N-cadherin; 5′-TGAGTACCGGAGACAGGTGCAG-3′ and 5′-TAGCAGCTTCAACGGCAAAGTTC-3′ for vimentin; 5′-GCACCGTCAAGGCTGAGAAC-3′ and 5′-TGGTGAAGACGCCAGTGGA-3′ for GAPDH.
Colony formation and wound healing assays
For colony formation assay, the HCT-116 cells were seeded in to a 6-well plate (400 cells/well). After 5 days, the colonies were washed with 1× phosphate-buffered saline (PBS) and stained with crystal violet for 20 min, then imaged and quantified.
For wound-healing assay, HCT-116 cells (5×105 cells/well) were seeded into a 6-well plate. After incubation for 24 h, a wound was created by scratching the confluent monolayer layer with a yellow tip. After washing with 1× PBS three times, cells were incubated with serum-free medium. Images were taken immediately and 24 h later under a microscope (Olympus Corporation).
Chromatin immunoprecipitation (ChIP) and PCR detection
ChIPs were performed using the ChIP Assay Kit (Upstate Biotechnology, Lake Placid, NY, USA) and the H3K4me3 antibody (9751) from Cell Signaling Technology. Primers used for PCR detection are listed as follows: 5′-CCCGCCCCAGCTGTGTCATTTT-3′ and 5′-AATGGTGCCCATCCACGTGG-3′ for E-cadherin (−80 to +88); 5′-CCAAAGTGCTGGTATTCCGCTGTAAG-3′ and 5′-GTGTGCTCCCAGAGTCGGGTTTGC-3′ for N-cadherin (−5,112 to −4,961), 5′-GGTGTGGTTTCATGGGGGGAGG-3′ and 5′-CCCTAAGTTTTTAATAACTCGCTAAAG-3′ for vimentin (−116 to +91).
Nude mice xenograft model
The animal study was performed in strict accordance with the National Institutes of Health, Guide for the Care and Use of Laboratory Animals. All the animal experiments were approved by the institutional review board of The Affiliated Huai’an Hospital of Xuzhou Medical University. The nude mice (4 weeks old, male) were randomly divided into two groups (n=6/group). Two HCT-116 cell lines were established, including CUL4A knockdown cell line and its control cell line. The cells in the logarithmic phase of growth were trypsinized and rinsed with 1× PBS three times. Each nude mouse was injected with HCT-116 cells (5×106 cells) in 100 μL of 1× PBS in the upper right shoulder subcutaneously. Tumor sizes were measured by measuring two perpendicular diameters with digital calibers every 5 days, and tumor volume was calculated using the equation: (Length × WidthCitation2)/2.
Statistical analysis
All data were expressed as the mean ± standard deviation from three independent experiments. Student’s t-test was used to evaluate the data. χ2 test was used to analyze the differences between groups of CUL4A expression in CRC tissues. The log-rank test was used to explore the association between CUL4A expression and the OS of CRC patients. SPSS version 19.0 was adopted for clinical data analysis. Values were considered to be statistically significant at P<0.05 and P<0.01.
Results
CUL4A is upregulated in primary CRC tissues
To explore the potential impact of CUL4A in CRC, we examined CUL4A expression in 80 CRC tissues and 30 normal tissues by IHC. CUL4A expression was significantly increased in CRC tissues compared with normal tissues (). Next, we investigated the mRNA level of CUL4A in primary CRC by quantitative RT-PCR (qRT-PCR). The mRNA level of CUL4A was determined in tumors and their corresponding paracancerous histologic normal tissue from 10 clinically and pathologically annotated cases of CRC. The results demonstrated that CUL4A was robustly increased in 70% (7 of 10) of CRC ().
Figure 1 CUL4A is overexpressed and associated with prognosis in colorectal cancer.
Abbreviation: qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
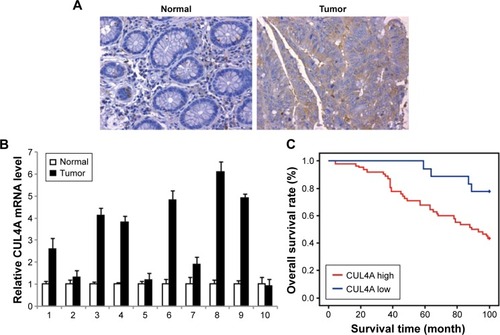
Next, Kaplan–Meier survival analysis was conducted to investigate the correlation between CUL4A expression and CRC patient prognosis. The patients were divided into two groups with high expression or low expression level of CUL4A. The Kaplan–Meier survival test revealed that CRC patients with high CUL4A expression had shorter OS than those patients with low CUL4A expression (P=0.001; ). The expression level of CUL4A was correlated significantly with the OS of CRC patients.
With clinicopathologic correlation analysis, we found that CUL4A protein level was positively correlated with lymph nodes status, differentiation degree, tumor size band poor prognosis in 80 CRC patients ().
CUL4Apromotes cell proliferation and colony formation
To detect the role of CUL4A in CRC, we created a CUL4A gain of function in CRC cells. Overexpression of CUL4A in HCT-116 cell was performed by a lentivirus-based method. The mRNA level of CUL4A in these cells was determined by qRT-PCR (). The cell proliferation rate of these cells was investigated by 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay, and CUL4A overexpression significantly increased the cell growth rate (). The knockdown of CUL4A was performed by a lentivirus-based method. A control short hairpin RNA (shRNA) or CUL4A-specific shRNA was introduced into HCT-116 cells. As confirmed by qRT-PCR, the mRNA level of CUL4A was successfully reduced by a CUL4A-specific shRNA (). Knockdown of CUL4A decreased cell proliferation rate of HCT-116 cells as shown by MTT assay ().
Figure 2 CUL4A promotes colorectal cancer cell line HCT-116 growth and migration.
Abbreviations: MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
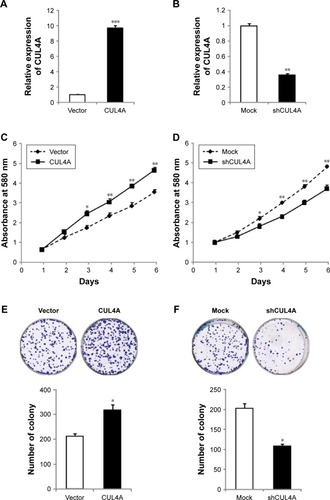
Next, colony formation was used to investigate the potential of tumorigenesis of HCT-116 cells. We found that CUL4A overexpression significantly promoted colony formation in these cells (), whereas the capacity of colony formation was reduced by CUL4A knockdown ().
CUL4A promotes migration of CRC cell line
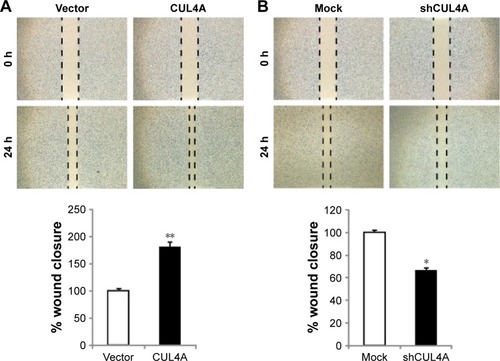
Clinical data revealed that CUL4A is positively correlated with lymph node metastasis of CRC patients, we then investigated the effects of CUL4A on the in vitro motility of CRC cells. Overexpression of CUL4A significantly promoted wound closure of HCT-116 cells (). Whereas silencing of CUL4A by its specific shRNA inhibited the wound closure as compared to the control group (). Therefore, these results indicate that in addition to the regulatory impact on cell proliferation, CUL4A has an effect on the migration of CRC cells.
Figure 3 CUL4A promotes migration of colorectal cancer cell line HCT-116.
Abbreviations: SD, standard deviation; h, hours.
CUL4A regulates EMT of CRC cells
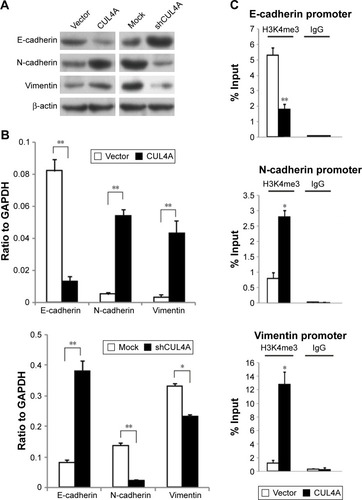
To further understand the mechanisms by which CUL4A engaged in CRC development and progression, Western blot was conducted to analyze CUL4A overexpression, knockdown, and vector and mock control cells in HCT-116. Overexpression of CUL4A obviously downregulated the expression of epithelial marker E-cadherin, whereas increased the expression of mesenchymal markers N-cadherin and Vimentin. Repression of CUL4A caused the shift in expression of mesenchymal markers Vimentin, N-cadherin to epithelial marker E-cadherin compared with the control cells (). Similarly, results of mRNA expression profiles of EMT markers in HCT-116 cells also confirmed that overexpression of CUL4A triggered EMT. Furthermore, the silencing of CUL4A by shRNA exhibited the opposite effect (). Collectively, these observations showed a critical role of CUL4A in the EMT and metastatic phenotypes of CRC cells.
Figure 4 CUL4A promotes epithelial–mesenchymal transition by changing the H3K4me3 level in promoter regions of E-cadherin, N-cadherin and vimentin.
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IgG, immunoglobulin G; H3K4me3, H3K4 trimethylation; PCR, polymerase chain reaction; qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
CUL4A triggers the EMT via regulation of H3K4me3
We then detected how CUL4A regulates EMT at the transcriptional level. Cullin-RING E3 ubiquitin ligase are frequently involved in histone modification.Citation19,Citation24,Citation25 To explore whether CUL4A regulates specific histone methylation in CRC cells, H3K4me3, and H3K27me3 were measured after overexpression of CUL4A. For the reason that H3K4me3 is associated with transcriptional activation, we tested whether CUL4A overexpression was associated with the H3K4me3 modification at the promoters of E-cadherin, N-cadherin, and vimentin in CRC cells. Quantitative ChIP (qChIP) assay was performed in HCT-116 cells. We found that CUL4A expression was associated with decreased H3K4me3 levels at region −80 to +88 bp of the E-cadherin promoter. In contrast, the H3K4me3 levels of −5,112 to −4,961 bp of the N-cadherin promoter and −116 to +91 bp of the vimentin promoter were increased in HCT-116 cells (). Therefore, these data suggest that CUL4A induces transcriptional activation of N-cadherin and vimentin through regulating H3K4me3 and enriching H3K4me3 to their promoters.
Knockdown of CUL4A inhibits CRC cell growth in vivo
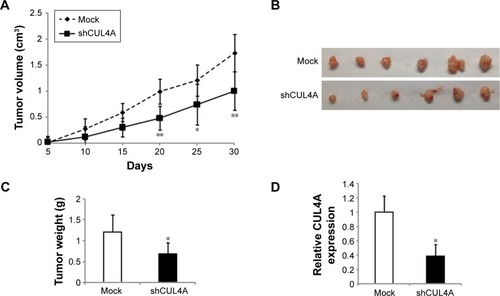
To further explore the influence of CUL4A knockdown on CRC cell growth, we investigated the effects of CUL4A on the xenograft model. As evaluated by tumor volumes, knockdown of CUL4A substantially decreased HCT-116 cells growth (). In addition, CUL4A knockdown led to a significant decrease in both tumor size and weight compared with the control cells (). Then, we did qRT-PCR to confirm CUL4A expression was knocked down by shCUL4A in these xenograft tumors. The results indicated that CUL4A protein was significantly reduced by shRNA (). In conclusion, these data demonstrate that CUL4A promotes CRC tumorigenesis and knockdown of CUL4A has a negative effect on tumor growth in vivo.
Figure 5 Knockdown of CUL4A suppresses tumor growth in vivo.
Abbreviations: qPCR, quantitative polymerase chain reaction; SD, standard deviation.
Discussion
To the best of our knowledge, it is the first systematic study to evaluate the role CUL4A plays in CRC patients. Our study revealed that increased expression of CUL4A resulted in a more aggressive phenotype in CRC patients. Furthermore, CUL4A overexpression in CRC cell line HCT-116 induced proliferation and migration in vitro. Consistently, knockdown of CUL4A reversed these events. We also found the relation between CUL4A and EMT through CUL4A-mediated regulation of H3K4me3, which results in transcriptional downregulation of epithelial marker E-cadherin and upregulation of mesenchymal markers N-cadherin and vimentin expression. Generally, the present study revealed that CUL4A can be considered as a therapy target for CRC.
Abnormal gene expression changes are involved in tumorigenesis, which will induce a series of target gene alterations and subsequent biological changes and this cascade of events is crucial to tumorigenesis.Citation26 Previous studies have provided evidence that CUL4A might serve as an oncogene in several kinds of cancers for the reason that CUL4A is highly expressed in malignant tumors, such as breast cancer, prostate cancer, NSCLC, and MPM relative to normal tissues.Citation19–Citation23 Deletion of CUL4A in mouse resulted in dramatically increased resistance to ultraviolet-induced skin carcinogenesis.Citation27 Furthermore, as CUL4A ubiquitinates and degrades several well-known tumor suppressor genes p21, p27, p53, and DDB2, CUL4A can be considered an oncogene in certain contexts.Citation15–Citation18 Consistent with these previous studies, CUL4A overexpression promoted CRC cell line HCT-116 proliferation, migration, and invasion both in vitro and in vivo. Our study found a novel function of CUL4A in CRC metastasis through regulating EMT by H3K4me3 modulation.
Conclusion
In this study, we found that the levels of H3K4me3 at the E-cadherin, N-cadherin, and vimentin gene promoters could be altered by CUL4A expression, which subsequently controlled their expression. However, we did not detect the influence of CUL4A expression on the methylation status of H3K9, H3K27, and other histone modifications. We conclude that CUL4A transcriptionally regulates E-cadherin, N-cadherin, and vimentin expression through regulation of H3K4me3 and recruitment of H3K4me3 to these gene promoters, and consequently promotes EMT. Although how CUL4A modulates H3K4me3 in CRC requires further clarification. In summary, our experiments provides evidence that CUL4A is both a potential therapeutic target and biomarker for CRC.
Disclosure
The authors report no conflicts of interest in this work.
References
- FitzmauriceCDickerDPainAGlobal Burden of Disease Cancer CollaborationThe global burden of cancer 2013JAMA Oncol20151450552726181261
- ShenZDengHFangYIdentification of the interplay between SOX9 and S100P in the metastasis and invasion of colon carcinomaOncotarget2015624206722068426009899
- VincenziBCremoliniCSartore-BianchiAPrognostic significance of K-Ras mutation rate in metastatic colorectal cancer patientsOncotarget2015631316043161226384309
- YiCHuangYYuXClinicopathologic distribution of KRAS and BRAF mutations in a Chinese population with colorectal cancer precursor lesionsOncotarget2016713172651727426910894
- YangJZhangKSongHVisfatin is involved in promotion of colorectal carcinoma malignancy through an inducing EMT mechanismOncotarget2016722323063231727058759
- WeiZMaWQiXPinin facilitated proliferation and metastasis of colorectal cancer through activating EGFR/ERK signaling pathwayOncotarget2016720294292943927107420
- BrabletzTHlubekFSpadernaSInvasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-cateninCells Tissues Organs20051791–2566515942193
- LamouilleSXuJDerynckRMolecular mechanisms of epithelial-mesenchymal transitionNat Rev Mol Cell Biol201415317819624556840
- BernsteinBEMikkelsenTSXieXA bivalent chromatin structure marks key developmental genes in embryonic stem cellsCell2006125231532616630819
- ZhongWFengHSantiagoFEKipreosETCUL-4 ubiquitin ligase maintains genome stability by restraining DNA-replication licensingNature2003423694288588912815436
- LeeJZhouPPathogenic role of the CRL4 ubiquitin ligase in human diseaseFront Oncol201222122649780
- SugasawaKThe CUL4 enigma: culling DNA repair factorsMol Cell200934440340419481520
- HanJZhangHZhangHWangZZhouHZhangZA Cul4 E3 ubiquitin ligase regulates histone hand-off during nucleosome assemblyCell2013155481782924209620
- HuJXiongYAn evolutionarily conserved function of proliferating cell nuclear antigen for Cdt1 degradation by the Cul4-Ddb1 ubiquitin ligase in response to DNA damageJ Biol Chem200628173753375616407242
- LiBJiaNKapurRChunKTCul4A targets p27 for degradation and regulates proliferation, cell cycle exit, and differentiation during erythropoiesisBlood2006107114291429916467204
- NishitaniHShiomiYIidaHMichishitaMTakamiTTsurimotoTCDK inhibitor p21 is degraded by a proliferating cell nuclear antigen-coupled Cul4-DDB1Cdt2 pathway during S phase and after UV irradiationJ Biol Chem200828343290452905218703516
- NagABagchiSRaychaudhuriPCul4A physically associates with MDM2 and participates in the proteolysis of p53Cancer Res200464228152815515548678
- LiJWangQEZhuQDNA damage binding protein component DDB1 participates in nucleotide excision repair through DDB2 DNA-binding and cullin 4A ubiquitin ligase activityCancer Res200666178590859716951172
- WangYWenMKwonYCUL4A induces epithelial–mesenchymal transition and promotes cancer metastasis by regulating ZEB1 expressionCancer Res201474252053124305877
- MelchorLSaucedo-CuevasLPMuñoz-RepetoIComprehensive characterization of the DNA amplification at 13q34 in human breast cancer reveals TFDP1 and CUL4A as likely candidate target genesBreast Cancer Res2009116R8619995430
- RenSXuCCuiZOncogenic CUL4A determines the response to thalidomide treatment in prostate cancerJ Mol Med (Berl)201290101121113222422151
- WangYZhangPLiuZCUL4A overexpression enhances lung tumor growth and sensitizes lung cancer cells to erlotinib via transcriptional regulation of EGFRMol Cancer20141325225413624
- HungMSMaoJHXuZCul4A is an oncogene in malignant pleural mesotheliomaJ Cell Mol Med201115235035819929949
- JacksonSXiongYCRL4s: the CUL4-RING E3 ubiquitin ligasesTrends Biochem Sci2009341156257019818632
- HigaLAWuMYeTKobayashiRSunHZhangHCUL4–DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylationNat Cell Biol20068111277128317041588
- OxnardGRBinderAJännePANew targetable oncogenes in non–small-cell lung cancerJ Clin Oncol20133181097110423401445
- LiuLLeeSZhangJCUL4A abrogation augments DNA damage response and protection against skin carcinogenesisMol Cell200934445146019481525
