Abstract
Previously, several polymorphisms in TGFB1 have been identified in non-small-cell lung cancer (NSCLC), and the variants, C-509T, T869C, and G915C, have been demonstrated to associate with higher circulating levels of TGF-β1. However, little is known about the prognostic value of TGF-β1 polymorphisms in cancers. In this study, by genotyping the TGF-β1 T869C polymorphism in a total of 261 patients with NSCLC using DNA from blood lymphocytes, we first found that NSCLC patients, especially those with allele C carriers, had significantly higher serum TGF-β1 levels than healthy individuals. By using chi-square (χ2) test and Fisher’s exact test, we noticed that TC/CC genotypes were positively correlated with smoking, clinical TNM stage, lymph node, and distant metastasis in NSCLC patients. Kaplan–Meier analysis showed that patients with TT genotype had a better overall survival than the allele C carriers (TC + CC). Finally, multivariate analysis confirmed histology, lymph node, and distant metastasis but not T869C polymorphism as independent prognostic factors for NSCLC. Taken together, our data, as a proof of principle, suggest that T869C polymorphism in TGFB1 may act as a genetic modifier in NSCLC progression and a promising prognostic marker of survival in NSCLC patients.
Introduction
Lung cancer is a leading cause of cancer-related deaths worldwide.Citation1 Nearly 75%–85% of patients with primary lung cancer are pathologically diagnosed with non-small-cell lung cancer (NSCLC). Despite intensive progress in preclinical and clinical studies, the prognosis of NSCLC is still poor, with a 5-year survival rate of only 15%.Citation2,Citation3 Currently, accumulating studies are underway to reveal the specific prognostic biomarkers that can predict the prognosis in patients with NSCLC.Citation4 This may largely help to guide medical care of NSCLC patients, with either conventional chemotherapy or targeted therapies.
Genetic factors are critically involved in modulating cellular microenvironment and affecting carcinogenesis in a complicated manner.Citation5,Citation6 Single-nucleotide polymorphisms (SNPs) are the most common form of human genetic variation. Emerging evidence has demonstrated that SNPs affect gene expression and further increase the potential risk of cancer.Citation7,Citation8 Meanwhile, the prognostic value of SNPs has been revealed in multiple human cancers, including NSCLC.Citation9–Citation11
TGF-β1 is a multifunctional cytokine that regulates many aspects of cellular function, including cellular proliferation, differentiation, migration, apoptosis, and immune surveillance.Citation12 In cancers, it has been reported that TGF-β1 acts as a tumor suppressor at early stages by inhibiting cell proliferation and as a tumor promoter at enhanced stages by facilitating tumor progression and metastasis.Citation13 In lung cancer, several polymorphisms in the TGFB1 gene have been reported previously, including rs1800469, rs1800471, rs1982073, rs4803455, rs11466345, rs12983047, rs10417924, and rs10980942.Citation14 Importantly, the association between TGFB1 polymorphisms and the risk of lung cancer has been well studied.Citation15–Citation18 However, limited knowledge is known about the prognostic value of these polymorphisms in lung cancer. In this study, we evaluated the prognostic value of an SNP of TGFB1 gene, rs1982073, which is also known as T869C, in a large-scale cohort.
Materials and methods
Study population
A total of 261 NSCLC patients were recruited from The First Affiliated Hospital of Harbin Medical University between January 2006 and December 2012. All patients were diagnosed and histopathologically confirmed with NSCLC and without prior history of other cancers. None of the patients included in this study received radiotherapy, chemotherapy, hormone therapy, or other related antitumor therapies prior to surgery. Blood samples were collected from these patients for genotyping and detection of serum TGF-β1 levels. Follow-ups were done every 3 months from the enrolled time until death or the last time of follow-up. The maximum follow-up time was 72 months, and the median follow-up time was 22.7 months. As a result, 213 patients with complete follow-ups were subjected to survival analysis. For certification of smoking, nonsmokers were defined as those who smoked <1 cigarette per day for <1 year; otherwise, they were considered as smokers. The control subjects were matched to the cancer cases on the basis of gender and age (±5 years). All the cases and the controls were ethnic Chinese, and they resided in Harbin City or in the surrounding regions.
This study was approved by the ethics committees of The First Affiliated Hospital of Harbin Medical University, and written informed consent was obtained from each individual for this study.
TGFB1 T869C genotyping
Genomic DNA was extracted from blood samples using commercially available QIAamp DNA purification kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The SNP T869C of the TGFB1 gene was determined by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) using primers 5′-TATGAGGATGTGGTGCGTGT-3′ (forward) and 5′-TGGGGTGGTGTTTACGTGATG-3′ (reverse). The PCR was performed in a GeneAmp PCR system 9700 (Applied Biosystems, San Francisco, CA, USA) thermal cycler. Each PCR amplification reaction was conducted in a total volume of 25 μL. The reaction mixture included 50 ng genomic DNA, 2.5 μM MgCl2, 200 μM dNTPs, 1 unit of Taq polymerase (Qiagen), and 200 μM primers. The PCR amplification consisted of a preliminary denaturation at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 15 s and annealing at 61°C for 1 min. Genotyping results and statistical analyses were determined independently by two authors in a blinded manner.
Enzyme-linked immunosorbent assay (ELISA) analysis of serum TGF-β1 levels
After centrifuging blood samples, serum samples were obtained and stored frozen at −70°C until used. Quantitative determination of the serum levels of TGF-β1 was measured by a commercial ELISA kit (R&D Systems, Minneapolis, MN, USA). Briefly, each serum sample or recombinant human TGF-β1 was diluted and added to the microtiter plates precoated with TGF-β-soluble receptor type II and allowed to incubate overnight at 4°C. After the incubation, the plate was washed three times with 1× Phosphate Buffered Saline add Tween-20 (PBS-T) and incubated with biotinylated anti-human TGF-β1 for 2 hours at room temperature. Subsequently, horseradish peroxidase (HRP)-conjugated streptavidin was added to the plate and allowed to incubate for 30 minutes at room temperature. Color development was performed using a tetramethyl benzidine–H2O2 mixture and was terminated by 0.5 mol/L sulfuric acid. Finally, the absorbance of each well at 490 nm was determined using a spectrophotometer.
Statistical analysis
All statistical analyses were performed using SPSS19.0 software (SPSS Company, Chicago, IL, USA). Hardy–Weinberg equilibrium (HWE) of the T869C polymorphism was tested by standard χ2 test. Survival time was calculated from the date of diagnosis to the date of death or to the last time of follow-up. Correlation of TGFB1 SNP with clinicopathological parameters in patients with NSCLC was evaluated by chi-square (χ2) test or Fisher’s exact test. The different survival times based on TGFB1 SNP were estimated by using the Kaplan–Meier method and compared by the log-rank test. Univariate or multivariate Cox regression analysis was done to determine the prognostic factors of NSCLC prognosis by estimating the crude hazard ratios (HRs). P-values <0.05 were considered statistically significant.
Results
TGFB1 T869C polymorphism contributes to elevated serum TGF-β1 levels in NSCLC
The demographics of the cases and controls enrolled in the current study are shown in . No significant differences were found between the cases and controls regarding the age or gender distribution and smoking history. A total of 261 cases with NSCLC were enrolled in our study. The general clinical characteristics of the enrolled patients are summarized in . The genotype distributions of TGFB1 T869C polymorphism among the cases were in HWE. TGFB1 T869C polymorphism results in a Leu10Pro substitution in the hydrophobic core of signal peptide (). Notably, this transition has been associated with higher circulating TGF-β1 levels, and this variant has been found to increase the risk of breast cancer.Citation19 By ELISA analysis of the serum TGF-β1 levels in 261 NSCLC patients and 95 healthy controls, we noticed that the mean serum TGF-β1 value (17.1±4.9 ng/mL) was significantly higher than that of healthy controls (10.6±2.6 ng/mL; ). Moreover, in both cases and controls, TGF-β1 level was certainly increased in individuals with smoking history (). Specially, patients with TC or CC genotype showed increased serum TGF-β1 levels compared with TT genotype carriers, indicating that T869C polymorphism also certainly contributes to increased serum TGF-β1 levels in NSCLC ().
Figure 1 TGFB1 T869C polymorphism contributes to elevated serum TGF-β1 level in NSCLC.
Abbreviations: NSCLC, non-small-cell lung cancer; 3D, three-dimensional; ELISA, enzyme-linked immunosorbent assay; aa, amino acid.
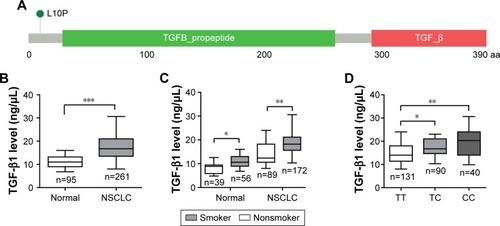
Table 1 Characteristics of the study population
Table 2 Correlations between clinicopathological features and TGFB1 T869C genotype frequencies in 261 patients with NSCLC
TGFB1 T869C polymorphism predicts a poor prognosis in NSCLC patients
To further determine the prognostic value of TGFB1 T869C polymorphism in NSCLC, the χ2 test was used to evaluate the correlation between T869C genotypes and corresponding patients’ clinicopathological features, including age, gender, smoking, TNM stage, histology, lymph node metastasis, and distant metastasis. As shown in , allele C was positively associated with smoking history (P=0.046), clinical stage (P=0.000), lymph node metastasis (P=0.000), and distant metastasis (P=0.000), whereas no significant correlations were found in age, gender, and histology.
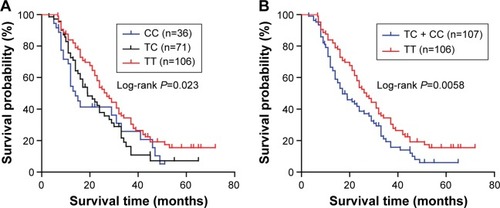
Then, by Kaplan–Meier analysis and log-rank test, we observed a different survival rate between different genotypes of TGFB1 T869C. The survival rate for NSCLC patients with TT or TC genotype was significantly lower than that for patients with CC genotype (, P=0.023). However, no remarkable difference in the survival rate was found between TT and TC genotype ().
Figure 2 Kaplan–Meier curve of overall survival according to TGFB1 T869C polymorphism in NSCLC.
Abbreviation: NSCLC, non-small-cell lung cancer.
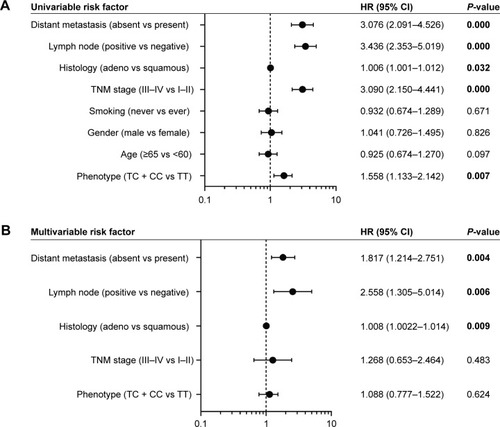
Moreover, to identify the risk factors that correlated with the patients’ outcome, we carried out univariate and multivariate analyses. The univariate Cox regression analyses showed that the genotype of TGFB1 T869C, histology, TNM stage, lymph node metastasis, and distant metastasis might be associated with the prognosis of NSCLC (). The multivariate Cox regression analysis revealed histology (risk ratio [RR] =1.008, 95% confidence interval [CI]: 1.002–1.014, P=0.009), lymph node metastasis (RR =2.558, 95% CI: 1.305–5.014, P=0.006), and distant metastasis (RR =1.817, 95% CI: 1.817–2.751, P=0.004) as independent prognostic factors of the overall survival in patients with NSCLC ().
Figure 3 Prognostic factors in Cox proportional hazards model.
Abbreviations: NSCLC, non-small-cell lung cancer; CI, confidence interval; HR, hazard ratio.
Discussion
In the present study, we demonstrated the prognostic value of TGFB1 T869C polymorphisms in patients with NSCLCs. The results suggest that testing for the existence of these three genotypes in TGFB1 T869C may help to identify patient subgroups at high risk of poor prognosis, thereby helping to make therapeutic decisions in the management of NSCLC. To the best of our knowledge, this is the first study to determine the prognostic effect of TGFB1 polymorphism on the clinical outcomes of patients with NSCLC.
Previously, a large body of evidence has demonstrated that TGFB1 polymorphisms contribute to lung cancer susceptibility.Citation18,Citation20 Several studies have also been conducted to determine the function of TGFB1 polymorphisms in radiation pneumonia susceptibility in lung cancer patients.Citation14,Citation16 These findings suggest that several polymorphisms of TGFB1 gene could be used as genetic biomarkers for predicting the susceptibility of lung cancer. Notably, several studies have shown that C-509T, T869C, and G915C polymorphisms of TGFB1 gene are associated with increased serum TGF-β1 levels.Citation18,Citation21 Consistent with this notion, in this study, compared to healthy controls, patients with NSCLC had a higher serum TGF-β1 level. Moreover, patients with a TC or CC genotype in the T869C polymorphism of TGFB1 gene had a higher TGF-β1 level than those with a TT genotype.
Mutations in TGFB1 and its receptors, or associated downstream kinases of TGF-β1 signaling, play key roles in the development of cancer.Citation22 Most NSCLC patients exhibit resistance to the tumor-suppressive role of TGF-β1 at advanced stage as a result of several alterations, such as mutations in SMAD4 and aberrant serum TGF-β1 levels.Citation12,Citation23,Citation24 TGF-β1 acts as a tumor promoter through interactions with tumor microenvironment. It is widely reported that TGF-β1 can suppress a repertoire of immune response, including cytotoxic T lymphocytes, macrophages, and natural killer cells.Citation25 Therefore, we hypothesized that dysregulation of TGF-β1 signaling induced by T869C polymorphism of TGFB1 gene might contribute to a poor prognosis in patients with NSCLC. In this study, we noticed that the genotypic distribution of T869C polymorphism in TGFB1 gene showed significant correlation with smoking history, clinical stage, lymph node metastasis, and distant metastasis. In addition, allele C of TGFB1 (T869C) was associated with increased risk of tumor-related death, suggesting that SNP T869C of TGFB1 gene might be a potential prognostic marker for patients with NSCLC. However, the clear mechanisms underlying the unconfirmed roles of TGF-β1 in NSCLC warrant further investigation.
Besides, several limitations in the current study must be specified. First, the healthy controls enrolled in our study were selected on the basis of gender and age, which might contribute to a bias. Second, replication in a validation cohort was not carried out. Finally, other SNPs (C-509T and G915C) of TGFB1 gene that contribute to increased TGF-β1 levels should also be evaluated to test the common roles of those SNPs in NSCLC.
Conclusion
Our findings suggest that the T869C polymorphism of TGFB1 gene was correlated with clinical progression of NSCLC patients and might be a genetic predictor for NSCLC prognosis. Furthermore, validation in a larger cohort and related functional characterizations are needed to confirm these observations.
Acknowledgments
This work was supported by Education Department of Heilongjiang Province (12541550).
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- GoldbergSBWillersHHeistRSMultidisciplinary management of small cell lung cancerSurg Oncol Clin N Am201322232934323453338
- SpiraAEttingerDSMultidisciplinary management of lung cancerN Engl J Medi20043504379392
- LudwigJAWeinsteinJNBiomarkers in cancer staging, prognosis and treatment selectionNat Rev Cancer200551184585616239904
- HillMJInteractions between genetic and environmental factors in colorectal carcinogenesisEur J Cancer Prev199761129161805
- LangevinSMKratzkeRAKelseyKTEpigenetics of lung cancerTransl Res20151651749024686037
- SigurdsonAJBrennerAVRoachJASelected single-nucleotide polymorphisms in FOXE1, SERPINA5, FTO, EVPL, TICAM1 and SCARB1 are associated with papillary and follicular thyroid cancer risk: replication study in a German populationCarcinogenesis201637767768427207655
- MacPhersonGHealeyCSTeareMDAssociation of a common variant of the CASP8 gene with reduced risk of breast cancerJ Natl Cancer Inst200496241866186915601643
- LiuYQingHSuXWangCLiZLiuSAssociation of CD44 gene polymorphism with survival of NSCLC and risk of bone metastasisMed Sci Monit2015212694270026356590
- DongSGuoALChenZHRRM1 single nucleotide polymorphism-37C–>A correlates with progression-free survival in NSCLC patients after gemcitabine-based chemotherapyJ Hematol Oncol201031020226083
- KimMJKangHGLeeSYAKT1 polymorphisms and survival of early stage non-small cell lung cancerJ Surg Oncol2012105216717421842521
- BlobeGCSchiemannWPLodishHFRole of transforming growth factor beta in human diseaseN Engl J Med2000342181350135810793168
- ElliottRLBlobeGCRole of transforming growth factor Beta in human cancerJ Clin Oncol20052392078209315774796
- NiuXLiHChenZA study of ethnic differences in TGF-beta1 gene polymorphisms and effects on the risk of radiation pneumonitis in non-small-cell lung cancerJ Thorac Oncol20127111668167523059779
- Gonzalez-Zuloeta LaddAMArias-VasquezASiemesCTransforming-growth factor beta1 Leu10Pro polymorphism and breast cancer morbidityEur J Cancer200743237137417035001
- HeJDengLNaFXueJGaoHLuYThe association between TGF-beta1 polymorphisms and radiation pneumonia in lung cancer patients treated with definitive radiotherapy: a meta-analysisPLoS One201493e9110024642488
- WangHBSongWGLiuHQFangFXiaoYRole of TGFB1 polymorphism in the development of metastatic brain tumors in non-small cell lung cancer patientsGenet Mol Res20151423545355025966122
- KangHGChaeMHParkJMPolymorphisms in TGF-beta1 gene and the risk of lung cancerLung Cancer20065211716499994
- DunningAMEllisPDMcBrideSA transforming growth factorbeta1 signal peptide variant increases secretion in vitro and is associated with increased incidence of invasive breast cancerCancer Res200363102610261512750287
- TeixeiraALAraujoACoelhoAInfluence of TGFB1+869T>C functional polymorphism in non-small cell lung cancer (NSCLC) riskJ Cancer Res Clin Oncol2011137343543920449615
- GraingerDJHeathcoteKChianoMGenetic control of the circulating concentration of transforming growth factor type beta1Hum Mol Genet19998193979887336
- BierieBMosesHLTumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancerNat Rev Cancer20066750652016794634
- GlickABTGFbeta1, back to the future: revisiting its role as a transforming growth factorCancer Biol Ther20043327628315034302
- NagatakeMTakagiYOsadaHSomatic in vivo alterations of the DPC4 gene at 18q21 in human lung cancersCancer Res19965612271827208665501
- Prud’hommeGJPathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerationsLab Invest200787111077109117724448
