Abstract
Caveolin-1 (Cav-1), a major structural protein of caveolae, is an integral membrane protein which plays an important role in the progression of carcinoma. However, whether Cav-1 acts as a tumor promoter or a tumor suppressor still remains controversial. For example, the tumor-promoting function of Cav-1 has been found in renal cancer, prostate cancer, tongue squamous cell carcinoma (SCC), lung SCC and bladder SCC. In contrast, Cav-1 also plays an inhibitory role in esophagus adenocarcinoma, lung adenocarcinoma and cutaneous SCC. The role of Cav-1 is still controversial in thyroid cancer, hepatocellular carcinoma, gastric adenocarcinoma, colon adenocarcinoma, breast cancer, pancreas cancer, oral SCC, laryngeal SCC, head and neck SCC, esophageal SCC and cervical SCC. Besides, it has been reported that the loss of stromal Cav-1 might predict poor prognosis in breast cancer, gastric cancer, pancreas cancer, prostate cancer, oral SCC and esophageal SCC. However, the accumulation of stromal Cav-1 has been found to be promoted by the progression of tongue SCC. Taken together, Cav-1 seems playing a different role in different cancer subtypes even of the same organ, as well as acting differently in the same cancer subtype of different organs. Thus, we hereby explore the functions of Cav-1 in human adenocarcinoma and SCC from the perspective of clinical significances and pathogenesis. We envision that novel targets may come with the further investigation of Cav-1 in carcinogenesis.
Introduction
Caveolins, the major structural proteins of caveolae, consist of caveolin-1 (Cav-1), Cav-2 and Cav-3. Interestingly, the expression patterns of Cav-1 and Cav-2 are largely distinct from that of Cav-3. Cav-1 and Cav-2 have similar distribution; they are highly expressed in adipocytes, endothelial cells, pneumocytes, fibroblasts and these terminal differentiation cells, whereas Cav-3 expressed limited to muscle cell types.Citation1 Besides, it has been found that Cav-1 and Cav-2 collectively promote the malignant progress of carcinoma. Recently, the oncogenic role of Cav-1 and Cav-2 has been identified in breast cancer,Citation2 prostate cancerCitation3 and esophageal squamous cell carcinoma (SCC).Citation4 Furthermore, Cav-1 and Cav-2 may be act as novel therapeutic targets in prostate cancerCitation3 and breast cancerCitation2 as well as potential marker in esophageal SCC.Citation4 However, the function of Cav-3 in tumor is under exploration, whereas Cav-1 is involved in multiple cancer-associated processes, including cellular transformation, tumor growth, cell migration and metastasis, cell death and survival, multidrug resistance (MDR) and angiogenesis.Citation5
At present, whether Cav-1 functions as an oncogene or a tumor suppressor in cancer progression is still controversial. Certain studies reported that Cav-1 is downregulated in pancreatic cancer,Citation6 ovarian cancer,Citation7 breast cancer,Citation8 laryngeal SCC,Citation9 lung adenocarcinomaCitation10 and esophageal adenocarcinoma (EAC).Citation11 Consistent with these observations, the human Cav-1 gene locates at chromosome 7q31.1, which has a high incidence of tumor suppressor gene loss in a broad range of tumor types.Citation12 These evidences indicate that Cav-1 may regard as a tumor suppressor. In contrast, the expression of Cav-1 was reported to increase in prostate cancer,Citation13 bladder cancer,Citation14 renal cancerCitation15 and esophageal SCC,Citation4,Citation16 head and neck SCC (HNSCC),Citation17 cervical SCCCitation18 and tongue SCC.Citation19 Interestingly, these upregulations have also been associated with advanced tumor stage, lymph node metastasis and poor prognosis of cancer patients, which may imply that Cav-1 can function as a tumor promoter. In the initial stages of tumor progression, tissue cells can undergo oncogenic transformation through various mechanisms. The decreased expression of Cav-1 can promote the rapid expansion of these abnormal cells. In the later stages, with the larger tumor and malignant progression, cancer cells have to adapt a complex microenvironment. Cav-1 overexpression can suppress apoptosis and acquire MDR. As a consequence, the elevated expression of Cav-1 can enhance the survival ability of cancer cells.Citation20
There is a growing recognition that cancer cells are not independent existence, but surrounded by stromal components in the tumor microenvironment, which are composed of the extracellular matrix (ECM), fibroblasts and myofibroblasts/cancer-associated fibroblasts (CAFs), immune cells, blood and lymphoid vessels.Citation21,Citation22 Recent studies have shown that the expression of stromal Cav-1 has an influence on the progression of carcinoma.Citation23,Citation24 The absence of stromal Cav-1 in CAFs and the loss of Cav-1 may affect the survival of cancer, autophagic tumor stroma model of cancer metabolism. It is well known that cancer cells or hypoxia induces oxidative stress and activate two proautophagic drivers, namely, HIF-1α and nuclear factor kappa-B (NF-κB), in adjacent fibroblasts. Thus, oxidative stress can lead to autophagic/lysosomal degradation of Cav-1. Simultaneously, mitophagy in CAFs would secrete the high-energy nutrients (such as lactate and pyruvate) by aerobic glycolysis that can directly transfer to epithelial cancer cells via a monocarboxylate transporter (MCT). The epithelial cancer cells would apply nutrients to the mitochondrial tricarboxylic acid cycle, thereby promoting ATP generation via oxidative phosphorylation. Moreover, CAFs can upregulate the expression of antiapoptotic protein TP53-induced glycolysis regulatory phosphatase in adjacent epithelial cancer cells, so protecting cancer cells from apoptosis and autophagy.Citation21,Citation25–Citation27 Antioxidants (such as N-acetyl cysteine, metformin or quercetin) or lysosomal inhibitors (eg, chloroquine) can effectively inhibit the degradation of Cav-1, directly implicating autophagy in this process.Citation28 In conclusion, a loss of stromal Cav-1 can promote cancer cell survival and resist to apoptosis. Thus, the absence of stromal Cav-1 is one of the powerful predictor about oxidative stress, autophagic CAFs and reverse Warburg effect.
Downregulation of stromal Cav-1 can promote cancer survival and predict a poor prognosis. Recently, some reporters suggested that the absence of the stromal Cav-1 can cause a “lethal” breast cancer microenvironment and associated with cancer recurrence, metastasis and poor clinical outcome.Citation22,Citation24,Citation29,Citation30 Similar results were obtained from prostate cancer patients; specifically, a loss of stromal Cav-1 is also correlated with reduced relapse-free survival and is functionally relevant to cancer progression.Citation31 Furthermore, the loss of stromal Cav-1 expression in colorectal cancer (CRC) is associated with poor prognosis and could be a prognostic factor for CRC patients.Citation32 The above studies have shown that stromal Cav-1 may play an inhibited role. In contrast, the accumulation of stromal Cav-1 in tongue SCC is significantly associated with poor prognosis.Citation33
Taken together, there is a huge difference about Cav-1 expression in different cancers or different stages of the same cancer. Whether Cav-1 plays a tumor-inhibiting role or a tumor-promoting role may quite depend on the subtypes and stages of cancers. Meanwhile, the stromal Cav-1 may play a complex role in the progression of cancer; therefore, further studies are needed to clarify these contradictory phenomena. In this review, we try to explore the function of Cav-1 in the human adenocarcinomas and SCCs.
The structure of Cav-1
Cav-1 is a 21–24 kDa integral membrane protein, consisting of two isoforms, α-isoform with a slower migration (containing residues 1–178) and β-isoform with a faster migration (containing residues 32–178).Citation34,Citation35 Previous study also demonstrated that both isoforms contain the oligomerization residues 61–101.Citation36 It was reported that Cav-1 has a central hydrophobic domain (residues 102–134), which are considered to form an unusual hairpin loop structure in the membrane, thus leading to both the amino-terminal domain (residues 1–101) and the carboxyl-terminal domain (residues 135–178) of Cav-1 face with the cytoplasm.Citation37
The residues between 80 and 101 have termed the caveolin scaffolding domain (CSD).Citation38 Two caveolin-binding motifs (φχφχχφ and φχχχχφχχ, where φ represents aromatic residues, such as Trp, Phe or Tyr and χ represents non-aromatic amino residues) exist in most caveolae-associated proteins.Citation39 Numerous signal molecules can interact with Cav-1 via the CSD, including Src family tyrosine kinases, Rho-GTPases, growth factor receptors, endothelial nitric oxide synthase (eNOS), G-proteins and G-protein-coupled receptors. However, Cav-1 can negatively or positively regulate these signaling molecules, thus playing a vital role in cancer progression.
The role of Cav-1 in invasion, migration and metastasis
Cav-1 and Rho-GTPases
There is increasing evidence that Rho-GTPases are likely to play a role in tumor metastasis and invasion.Citation40,Citation41 Previous studies also have demonstrated the pivotal role of Cav-1 in regulating the activity of Rho-GTPases in various cancers. The cooperation between Cav-1 and Rho-GTPases promotes tumor metastasis, which mainly depend on the elevated expression of α5-integrin and the enhanced activation of Src, Ras and Erk.Citation42 Moreover, an increased expression of Cav-1 can promote the activation of AKT1, leading to the increased phosphorylation of RhoC GTPase (). As a consequence, the invasion capacity of inflammatory breast cancer cells is significantly elevated.Citation43 These phenomena are not in accordance with the study reported by Lin et al who indicated that Cav-1 expression inhibits RhoC GTPase activation and subsequently activates the p38 mitogen-activated protein kinase (MAPK) pathway, thus restricting migration and invasion of primary pancreatic cancer cells.Citation44
Figure 1 Cav-1 plays an important role in tumor migration, invasion and metastasis by regulating the activity of Rho-GTPases, EMT and MMPs.
Abbreviations: Cav-1, caveolin-1; EGFR, epidermal growth factor receptor; EMT, epithelial-to-mesenchymal transition; GSK, glycogen synthase kinase; LEF, lymphoid enhancer factor; MMPs, matrix metalloproteinase; MT, membrane type; PI3K, phosphatidylinositol 3-kinase; STAT3, signal transducer and activator of transcription 3; TCF, T-cell factor.
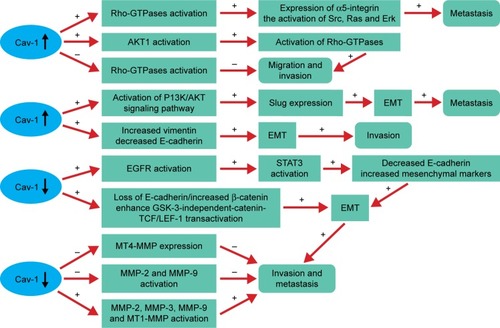
Cav-1 and epithelial-to-mesenchymal transition (EMT)
Evidences suggested that Cav-1 can mediate the invasion and metastasis of cancer and often accompanied by the EMT. Some studies from different angles have been referred to clarify the effect of Cav-1 in regulating EMT in cancers. Cav-1 can promote bladder cancer metastasis via inducing EMT by activating the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway, thus upregulating Slug expression.Citation14 In addition, overexpression of Cav-1 significantly increases vimentin expression, but decreases E-cadherin expression, caused the change of EMT, which may explain the motility and invasion ability in hepatocellular carcinoma (HCC).Citation45 Whereas the reduced levels of Cav-1 function by hypoxia increases epidermal growth factor receptor (EGFR) activation, leading to the activation of signal transducer and activator of transcription 3 (STAT3), resulting in the downregulation of E-cadherin and upregulation of mesenchymal markers (Slug, a-SMA, N-cadherin and vimentin), suggesting that Cav-1 can mediate the EMT and promote the invasive potency in gastric cancer (GC).Citation46 Furthermore, the decreased expression of Cav-1 by EGF leads to the loss of E-cadherin, disruption of cell–cell contacts and enhances glycogen synthase kinase 3 (GSK-3)-independent-catenin-T-cell factor (TCF)/lymphoid enhancer factor 1 (LEF-1) transactivation and increased transcriptional activity of β-catenin, resulting in enhancing cancer cells invasion and metastasis.Citation47
Cav-1 and matrix metalloproteinase (MMP)
MMPs are a family of zinc-containing proteolytic enzymes that degrade various components of ECM.Citation48 Numerous studies indicated that high expression levels of certain MMPs are related to the cancer invasion and metastasis capacity.Citation45,Citation49–Citation52 At present, many researches have investigated the relationship between Cav-1 and MMP, but came out with different results. It has been demonstrated that membrane type 1 (MT1)-MMP colocalizes with caveolae and Cav-1.Citation53 Cav-1 may function as a negative regulator by inhibiting MT4-MMP expression, which is associated with the metastasis in colon cancer.Citation54 Furthermore, Cav-1 overexpression can reduce the metastasis and invasion capacity of metastatic mammary tumor cells by inhibiting the activity of MMP-2 and MMP-9.Citation55 In contrast, the motility and invasion-promoting effect of Cav-1 overexpression in HCC may be partly through increasing secreted MMP-2 and expression levels of MMP-9 and MT1-MMP, as well as inducing an EMT-like phenotype.Citation45 Consistent with the above results, Cav-1 might promote the invasion and metastasis potential via decreasing of E-cadherin protein expression and activate the enzyme activity of MMP3 in human small cell lung cancer NCI-H446 cell.Citation50
The role of Cav-1 in cell cycle
Cav-1 mediates the development of tumor by inversely regulating the cell cycle progression. The control of the cell cycle involved in two major “checkpoints/transitions”, more specifically, the G1→S transition and the G2→M transition. Besides, cyclins/cyclin-dependent kinases (CDKs) and CDK inhibitors are the key regulatory factors of the two transitions.Citation56 Cav-1 may negatively regulate the transcriptional activity of two major components (cyclinD1 and CDC25A) of the cell cycle regulatory apparatus that governs DNA synthesis and cell transformation.Citation57 Moreover, the decreased expression of Cav-1 by small interfering RNA significantly reduces the expression of phospho-AKT, cyclinD1 and CDK4, downstream transducers phosphorylated ERK and STAT3, thus leading to the inhibition of the metastatic lung cancer cells proliferation.Citation58 In addition, Cav-1 expression negatively regulates cell cycle progression by inducing G0/G1 arrest via a p53/p21WAF1/Cip1-dependent pathway.Citation59
The role of Cav-1 in apoptosis
Apoptosis is an active and physiological process of cell death, and the imbalance is one of the important factors for the formation of malignant tumor. Yet, the role of Cav-1 on apoptosis regulation fails to reach a consensus ().
Figure 2 The role of Cav-1 in apoptosis fails to reach a consensus.
Abbreviations: BCL, B-cell lymphoma; Cav-1, caveolin-1; EGFR, epidermal growth factor receptor; EMT, epithelial-to-mesenchymal transition; GSK, glycogen synthase kinase; IGF, insulin-like growth factor; IR, insulin receptor; LEF, lymphoid enhancer factor; MAPKs, mitogen-activated protein kinases; PI3K, phosphatidylinositol 3-kinase; PP, protein phosphatase; TCF, T-cell factor.
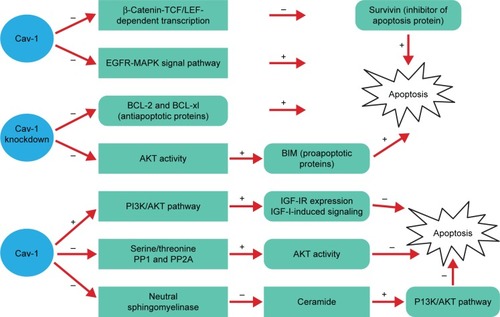
Many previous studies have demonstrated that Cav-1 is capable of promoting cell apoptosis. The study of HEK293T and ZR75 cells indicated that antiproliferative and proapoptotic properties of Cav-1 may be attributed to reducing survivin (inhibitor of apoptosis protein) expression via a mechanism involving diminished β-catenin-TCF/LEF-dependent transcription.Citation60 What is more, overexpression of Cav-1 exhibits slower growth and promotes cell apoptosis in human cancer by inhibiting the activities of EGFR-MAPK signaling pathway.Citation6,Citation9
However, Cav-1 can inhibit apoptosis through various signal pathways. It has been shown that the absence of Cav-1 significantly reduces the activation of the AKT pathway mediated by TGF-β, thus contributing to the increased expression of proapoptotic proteins BIM. Besides, a significant decrease of antiapoptotic protein B-cell lymphoma (BCL)-2 and BCL-xl expression is observed, suggesting the elevation of the hepatocyte apoptosis.Citation61 Furthermore, the expression of Cav-1 in human breast cancer cells is able to inhibit anoikis and enhances matrix-independent survival by a mechanism, which involves upregulation of insulin-like growth factor (IGF)-insulin receptor (IR) expression and IGF-I-induced PI3K/AKT signaling pathway.Citation62 From another point of view, Cav-1 could maintain phosphorylated AKT through scaffolding binding site interaction and inhibition of serine/threonine protein phosphatases (PP1 and PP2A), and that elevated AKT activities are largely responsible for Cav-1-mediated survival in prostate carcinoma cells.Citation63 Based on the research of the breast cancer cell line Hs578T, the results suggested that Cav-1 overexpression significantly reduces staurosporine-induced apoptosis through inhibition of neutral sphingomyelinase, decrease of ceramide, which can inhibit PI3K/AKT pathway mediating cell apoptosis.Citation64 Taken together, the effect of Cav-1 on cell apoptosis is an extremely complex process.
The different functions of Cav-1 in adenocarcinoma
Cav-1 and thyroid carcinoma
Cav-1 plays a different role in different subunits of thyroid carcinoma. A study has demonstrated that Cav-1 is frequently positive in papillary thyroid carcinoma (PTC), significantly decreases in undifferentiated carcinomas (anaplastic carcinomas), but not the follicular-type carcinomas (FTC). In addition, Cav-1 overexpression plays an important role in the early phase and its decreased expression is associated with the aggressive characteristics including dedifferentiation in PTC, whereas it has little influence on follicular tumors.Citation65 Consistent with the results, Cav-1 expression in the cancer cells is more intense in classical PTC than the other histologic subtypes. In contrast, stromal Cav-1 expression is stronger in the follicular, solid and trabecular PTC variants than in classical PTC. Moreover, clinicopathologic parameters showed that upregulation of Cav-1 in thyroid epithelia correlates with lymph node metastasis, whereas downregulation of Cav-1 in the stromal compartment correlates with the degree of neoplastic infiltration. The study first reported that downregulation of Cav-1 in epithelia and stroma is coincided with BRAF mutation of PTC.Citation66 Inconsistent with the findings, Cav-1 is expressed in the cancer cells with similar frequencies in PTC, diffuse sclerosing variant of papillary carcinoma (DSVPC) and AC, whereas the stromal Cav-1 expression is more frequent in AC compared with PTC and DSVPC.Citation67 In addition to those results, one study showed that galectin-3 can override the tumor suppressor activity of Cav-1, the coordinated expression of Cav-1 and galectin-3; represent a highly precise marker for differentiated thyroid cancer (DTC) diagnosis; and synergistically promote focal adhesion signaling, tumor cell migration, progression and aggressivity of DTC.Citation68 In summary, the different expression of Cav-1 in PTC, AC, FTC and DTC reflects that Cav-1 may play a complicate role in the different histologic subtypes of adenocarcinoma from the same organ.
Cav-1 and EAC
Positive staining of Cav-1 was lost in 95% of EAC specimens (n=100) by immunohistochemistry (IHC) on microar-rays, predominantly concentrating on the mechanism that bile acids could mediate downregulation of Cav-1 expression in EAC cells through the sterol-responsive element-binding protein-1 (SREBP1)/SREBP cleavage-activating protein and nuclear receptors farnesoid X receptor (FXR)/FXR target genes small heterodimer partner-mediated posttranscriptional events. Thus, loss of Cav-1 may activate EGFR signaling and destabilize cell–cell and cell–matrix contacts, contributing to the onset of Barrett esophagus metaplasia and progression to Barrett adenocarcinoma of the esophagus.Citation11 Furthermore, Cav-1 normalized methylation value in EAC is significantly higher than that in corresponding normal esophagus. Whereas OE33 cells are subjected to demethylation by 5-AzadC treatment and could increase Cav-1 mRNA expression, which interpreted that DNA hypermethylation could make Cav-1 gene silencing, and hypermethylation of Cav-1 promoter is a common event in human EAC and occurs early during Barrett’s-associated EAC.Citation69 These two studies suggested that Cav-1 may inhibit the progression of EAC.
Cav-1 and lung adenocarcinoma
It was reported that the positive staining of Cav-1 is downregulated in lung adenocarcinomas and loss of Cav-1 may be a critical step for tumor extension and dedifferentiation.Citation10 Whereas our previous study verified that no correlation is detected between Cav-1 expression and clinicopathologic parameters of lung adenocarcinomas.Citation70 Even upregulation of Cav-1 mRNA expression is found in lung adenocarcinomas (29.7%), which is higher than lung SCC (15.8%) but it is significantly lower when compared with matched tumor-free tissues or noncancerous lung tissue. Furthermore, its overexpression is correlated with the advanced pathologic stage and shorter survival rates in lung adenocarcinoma patients.Citation71 Moreover, Cav-1 overexpression is necessary for mediating filopodia formation in human lung adenocarcinoma cell lines, thus promoting the ability of cell invasion and migration.Citation72 The effect and mechanism of Cav-1 in cancer MDR is still controversial. Cav-1 is upregulated in several MDR cancer cells, including taxol-resistant lung adenocarcinoma A549 cell line,Citation73 bleomycin treatment of A549 cell lineCitation74 and taxol-resistant A2780 ovarian carcinoma cell line.Citation75 More importantly, A549 cell lines transduced with the Cav-1 K176R mutant would enhance drug resistance of doxorubicin and cisplatin through the influence of interaction between Cav-1 and P-glycoprotein.Citation76 However, low expression of Cav-1 in A549/Taxol cells by transfecting with a Cav-1 shRNA lentiviral vector, inhibited cell proliferation, cell invasion ability and induced G0/G1 arrest and cell apoptosis.Citation77 Taken together, these results may suggest that the Cav-1 serves as a suppressor in the early stage, while shifting its function in the later stage of lung adenocarcinoma patients.
Cav-1 expression in CAFs is closely related with pathologic T stage, lymphatic permeation, vascular invasion and pleural invasion and predicts a poor outcome of lung adenocarcinoma patients, but it cannot serve as an independent prognostic factor partly due to its strong associations with pathologic vascular and/or pleural invasion. Taken together, Cav-1-positive CAFs play a tumor-promoting role in lung adenocarcinoma.Citation78
Cav-1 and breast cancer
There is considerable controversy about the role of Cav-1 in breast cancer. Some people put forward evidence to prove that Cav-1 serves as a tumor suppressor in breast cancer. The decreased Cav-1 expression level is observed in non-metastatic primary tumors, but much lower level is found in highly metastatic tumor.Citation8 Besides, Cav-1 overexpression can suppress the primary breast cancer growth and brain metastases via STAT3 inhibition.Citation79 In addition, Cav-1 can limit the activation of large conductance Ca2+-activated potassium (BKCa) channel, leading to the suppression of the proliferation and invasiveness in breast cancer cells.Citation80 Cav-1 also can regulate breast cancer cell metabolism, the elevated Cav-1 expression is inversely correlated with NF-E2-related factor 2 (Nrf2) and Mn superoxide dismutase (MnSOD) expression, thus preventing MnSOD-driven glycolysis in breast cancer MCF7 cell line.Citation81 These may be critical for understanding the tumor inhibitory mechanisms of Cav-1.
In contrast, Cav-1 overexpression is associated with tumor malignant progression. The increased expression of Cav-1 can elevate the invasion capacity of inflammatory breast cancer cell by activating AKT1.Citation43 Moreover, the coexpression of Cav-1 and MT1-MMP is observed in human breast cancer cell lines, both are required for invadopodia formation, which regulates the invadopodia-mediated invasion by degrading ECM.Citation82 Furthermore, one study also indicated that Cav-1 can inhibit apoptosis and enhance matrix-independent survival in human breast cancer cells.Citation62
Cav-1 can mediate MDR by positively regulating the activity of BCRP in breast cancer.Citation83 Cav-1 knockdown could significantly reduce the tumorigenicity and chemoresistance of breast cancer stem cells by downregulating the β-catenin/ABCG2 pathway.Citation84 Moreover, breast cancer aggressiveness is associated with Cav-1 CGI shore methylation levels.Citation85 An interesting study revealed that 19% estrogen receptor-positive breast cancers involve Cav-1 P132L mutation.Citation86 Further study demonstrated that Cav-1 (P132L) mutation dramatically accentuates cell migration, invasion and metastasis capacity via activating EGF, HGF and TGF signaling pathways.Citation87 However, another study reported that Cav-1 P132L mutation has not found in breast cancer.Citation88 Therefore, further studies are needed to explore the possible mechanism of the Cav-1 P132L mutation on tumorigenicity of breast cancer.
According to the previous reports, epithelial Cav-1 expression could not be an independent prognostic factor for clinical outcome of breast cancer patients.Citation22,Citation89–Citation91 However, Cav-1 expression with tumor (++)/stromal (−) is closely associated with unfavorable prognosis.Citation92 Whereas an absence of stromal Cav-1 has been regarded as an independent poor prognostic indicator of breast cancer and specifically associated with early disease recurrence, advanced tumor stage, lymph node metastasis, a shorter disease-free survival and an overall survival.Citation22,Citation24,Citation29,Citation30
Cav-1 and GC
Cav-1 is frequently lost or reduced in primary GC tissue by comparison with nonneoplastic mucosa, but it is increased in distant metastases cell lines by comparison with primary tumor cell lines.Citation93 In addition, via the activation of the EGFR, TGF-β, Wnt signaling and their downstream signal pathways, the low expression of Cav-1 plays a vital role in enhancing cell proliferation, cell survival and upregulating EMT, which results in promoting GC progression under hypoxic condition.Citation46 Conversely, one study revealed that positive staining of Cav-1 is shown in 22 (5.4%) of 405 cases in GC, significantly correlated with advanced pTNM stage, lymph node metastasis and poor prognosis, while Cav-1 is not expressed in nonneoplastic gastric mucosa.Citation94 However, another study reported that Cav-1 immunoexpression is found in 46 (94%) of 49 cases from surgically resected GC tissues, but it appear that Cav-1 is neither stage-specific nor related to prognosis.Citation95 Furthermore, a meta-analysis showed that Cav-1 expression is only correlated with Lauren classification and its overexpression could predict a better overall survival. Therefore, Cav-1 is a novel prognostic biomarker of GCs.Citation96
In the stromal of gastric adenocarcinoma, our group previously showed that low stromal Cav-1 levels and high autophagy levels may cooperatively promote GC development, and downregulation of stromal Cav-1 is a novel predictor of poor GC prognosis.Citation97 Consistent with our results, loss of stromal Cav-1 can significantly activate fibroblasts in GC microenvironment, and it may function as a potential biomarker for GC progression.Citation98
The role of Cav-1 in GC is so complicated that the above studies have yet to reach a consensus. There are many possible reasons that account for the differences, such as the small sample size, difference in pathological subtypes, using the different antibodies or the different proportion of tumor histologic stage and histologic grade.
Cav-1 and pancreatic carcinoma
In pancreatic carcinoma, the negative regulation of the cell growth and cell invasion capacity, as well as the promotion of apoptosis may be critical for understanding the mechanism of Cav-1 in tumor suppression through downregulating EGFR–MAPK signaling pathway.Citation6 Some reports showed that Cav-1 plays an important role in regulating the migration and invasion in pancreatic carcinoma. Cav-1 expression can restrict migration and invasion of primary pancreatic carcinoma, via inhibiting RhoC GTPase activation.Citation44 This is in contrast to which RhoC-mediated migration and invasion in inflammatory breast cancer.Citation43 Together with Cav-1, expression in pancreatic carcinoma induces an epithelial phenotype and promotes cell–cell contact, with increased expression of plasma membrane bound E-cadherin and β-catenin, resulting in reducing cell migration and invasion. Consistent with Cav-1 as a negative regulator of some signaling pathway, one report indicated that Cav-1 gene could inhibit pancreatic carcinoma cell invasion, at least in part, probably through downregulating ERK-MMP signaling pathways.Citation99 Then, Cav-1 also attenuates doxorubicin-chemoresistance of pancreatic cancer cells.Citation100 Disagreeing with the above results, a previous study suggested that Cav-1 overexpression is positively associated with tumor progression, indicating a poor prognosis for certain patients undergoing surgical resection for pancreatic carcinoma.Citation101 What is more, Cav-1 can make an independent poor prognostic factor and its elevated level is correlated with histologic stage and tumor aggressiveness.Citation102–Citation104 Furthermore, the elevated Cav-1 expression is resistant to therapies.Citation103 Beyond that, Cav-1 is indispensable for the tumor-promoting effect of Cav-1 and promoting its prognostic potency.Citation104 The above results demonstrated that Cav-1 function is very complicated in the pancreatic carcinoma cells.
Stromal Cav-1 expression in CAFs is lower than that in paracancerous associated and normal fibroblasts, and its absence is closely related to TNM stage, lymph node metastasis, distant metastasis and HER-2/neu amplification, they also indicated that the loss of stromal Cav-1 is an independent prognostic indicator in pancreatic carcinoma.Citation105
Cav-1 and HCC
Comparing with normal liver cell line and nontumorous liver tissues, increased expression of Cav-1 is found in metastatic HCC cell lines and tumor tissues.Citation45,Citation106,Citation107 In addition, Cav-1 overexpression is correlated with invasion and poor prognosis of HCC.Citation49,Citation108 Some reports suggested that Cav-1 contributes to HCC progression and metastasis through the inhibition of autophagyCitation107 or inducing EMT via Wnt/β-catenin pathway.Citation109 Furthermore, Cav-1 overexpression induced by GLI1 also plays a vital role in GLI1-driven EMT.Citation110 In addition, Cav-1 may function as an initiator of the HCC via triggering c-Met signal transduction and the interaction between them will be beneficial to the invasive phenotype.Citation111 Even Cav-1 also can negatively regulate TRAIL-induced apoptosis in hepatoma HepG2 cells.Citation112 These evidences suggest the oncogenic role of Cav-1 in HCC. However, Cav-1 has been reported to function as a tumor suppressor role through its modulation of eNOS in HCC and Cav-1 overexpression means a better overall survival.Citation113
Cav-1 and colon adenocarcinoma
Compared to normal colonic epithelium and adenomas, the expression of Cav-1 is elevated in colon adenocarcinoma.Citation114 In addition, its overexpression is directly associated with the growth rate, contributing to tumorigenesis.Citation115 Moreover, Cav-1 can stimulate the activation of a small GTPase Rab5, thus enhancing the activity of ras-related C3 botulinum toxin substrate 1 (Rac1) and cell migration, invasion in metastatic human colon adenocarcinoma HT-29 (US) cells.Citation116 Whereas Cav-1 may play an inhibitory role during early stages of colon adenocarcinoma, and the decreased expression of Cav-1 is correlated with the loss of wild-type adenomatous polyposis coli via downregulation of Forkhead box protein O1a and upregulation of c-myc transcription factors.Citation117
Cav-1 and renal cancer
Previous studies have reached an agreement about the role of Cav-1 in renal cell carcinoma (RCC). Using IHC, increased Cav-1 expression correlates with tumor progression and predicts a poor prognosis in RCC.Citation118 Furthermore, Cav-1 mRNA expression is remarkably increased in RCC by comparison with normal renal tissue, and the elevated Cav-1 mRNA levels are associated with tumor stage and predicted malignant progression.Citation119 In addition, the coexpression of Cav-1 and activated AKT/mTOR pathway can predict a poor disease-free survival, contributing to disease progression and metastasis in RCC.Citation120 Moreover, Cav-1 in coordination with pERK can predict metastasis risk in RCC.Citation15 Furthermore, Cav-1 may boost the angiogenesis, associating with microvessel density (MVD), and the coexpression of Cav-1 and MVD is significantly correlated with metastasis and a worse prognosis in clear cell RCC. Taken together, Cav-1 plays an important role in the progression of RCC.Citation121
Cav-1 and prostate cancer
It is widely accepted that Cav-1 is elevated in prostate cancer cells and its overexpression is associated with disease progression. High expression of Cav-1 has a significant positive association with higher stage and grade tumor.Citation31 Cav-1 also might help to identify patients at high risk of developing aggressive prostate cancer recurrence.Citation122 Recently, the role of Cav-1 in prostate cancer is explored in some researches from different angles. Cav-1 can cause phosphorylated AKT, and elevated AKT activities are benefit to Cav-1-mediated survival activities.Citation63 Moreover, the positive combination of c-myc and Cav-1 likely reflects the aggressiveness of prostate carcinoma and may offer prognostic value for this malignancy.Citation123 Furthermore, Cav-1 mediates angiogenesis via cooperating with VEGFR2 during prostate cancer progression.Citation124 Besides, the physical interaction between Id-1 and Cav-1 plays a key role in the EMT and increases cell migration rate as well as resistance to taxol-induced apoptosis in prostate cancer cells.Citation125 This phenomenon is in accordance with other researches that have shown a link between Cav-1 expression and EMT in bladderCitation14 and pancreatic cancers.Citation126
Compared to stromal Cav-1 expression in patients with benign prostatic hypertrophy, primary prostate cancers and prostate cancer metastases, the results indicated that the loss of stromal Cav-1 is associated with advanced prostate cancer and metastatic disease, and it could be a powerful prognostic marker for patients with prostate cancer.Citation127 In concordance with their findings, the low expression of stromal Cav-1 is correlated with clinical stage, increased Gleason score and reduced relapse-free survival. What is more, an absence of stromal Cav-1 confers the metastatic capacity of tumor cells by upregulation of TGF-β1 and γ-synuclein via AKT activation.Citation128
The different functions of Cav-1 in SCCs
Cav-1 and cutaneous SCC
Cav-1 can be observed in the cytoplasm of cutaneous SCC, and its expression level is significantly lower than that of normal skin cells.Citation129 Furthermore, Cav-1 overexpression has the ability to suppress cutaneous SCC progression by decreasing cell proliferation, migration and invasion capabilities. Whereas Cav-1 knockdown occurs the opposite results, simultaneously, increases the invasive ability and incidence of spontaneous lymph node metastasis. The possible molecular mechanism is that Cav-1 can negatively regulate the MAPK/activator protein-1 pathway activation.Citation130
Cav-1 and oral SCC (OSCC)
The role of Cav-1 in OSCC still remains complex and controversial. Cav-1 overexpression is observed in the cytoplasm of OSCC by comparison with normal oral mucosa and serves as a prognostic marker for poor prognosis in OSCC.Citation131,Citation132 Moreover, Cav-1 upregulation plays an important role in the tumor progression and correlates with cisplatin sensitivity in OSCC.Citation133 The opposite results are found in other studies, and genetic evidence showed the inactivation of Cav-1 by a mutation or reduced expression may take effect in the pathogenesis of OSCC.Citation134 Whereas an increased Cav-1 expression is seen in the stepwise carcinogenesis from normal oral mucosa (8%), noncancerous-matched tissues (18%), oral precancerous lesions (53%) to primary OSCC (79%), a drastic decrease in expression from primary OSCC to metastatic OSCC (37%), which indicates the value to explore its biphasic functions in oral carcinogenesis.Citation135
In the tumor stroma, a reverse Warburg metabolism in OSCC is not dependent upon myofibroblasts,Citation136 and no negative correlation is detected between the loss of stromal Cav-1 expression and the elevated expression of MCT4, which has been shown in breast cancer.Citation137 Furthermore, the reduced Cav-1 can lead to an increase in oxidative stress in OSCC microenvironment.Citation138
Cav-1 and tongue SCC
Regarding the correlation between the expression of Cav-1 and tongue SCC, little research has been reported. Our previous reports showed that an increased expression of Cav-1 in the carcinogenesis from normal tongue mucosa, hyperplastic tongue mucosa, tongue precancerous lesions to tongue SCC by quantum dots immunofluorescence histochemistry, moreover, Cav-1 expression level is correlated positively with clinical stage and histologic grade in tongue SCC, which suggested that Cav-1 might be an oncogene in the development of tongue SCC.Citation19 Whereas Cav-1 expression in the tumor microenvironment components of human tongue SCC is higher than those in the tumor cells, and it had a negative prognostic value. One of the ways can enable the accumulation of Cav-1 in the tumor microenvironment, which is secreted by exosomes. As such, exosomal Cav-1 can be present in the ECM or it may be ingested by cells that undergo EMT, by CAFs or by other types of cells. The secretion of exosomes is a way to enhance the accumulation of Cav-1 in the tumor microenvironment.Citation33 In agreement with the idea, increased stromal Cav-1 is found in the human renal carcinoma, colon carcinoma and melanoma and it would promote local invasiveness and metastasis via remodeling of ECM, and also linked to poor survival.Citation139 However, these clinical findings appear to conflict with those reports from prostate cancer,Citation127,Citation128 breast cancer,Citation24 pancreatic cancerCitation105 and esophageal SCCCitation23 patients, where the loss of stromal Cav-1 expression is associated with recurrent disease, advanced stage, metastatic spread and poor survival.
Cav-1 and laryngeal SCC
Cav-1 is capable of inhibiting the growth of human laryngeal SCC HEp-2 cell line both in vitro and in vivo, reducing the capacity of anchorage-independent growth, inducing G0/G1 arrest in a cell cycle and increasing the apoptotic cell fraction. The mechanism of its tumor suppressing action may be interpretation via the negative regulation of the EGFR–MAPK signaling pathway.Citation9 Other study demonstrated that Cav-1 overexpression induced by low-intensity ultrasound will be involved in low-intensity ultrasound-induced apoptosis via downregulation of STAT3 in HEp-2 cells.Citation140 In contrast, the univariate analysis indicates that Cav-1 overexpression in membranous and cytoplasmic means a worse disease-specific survival (DSS). However, Cav-1 cannot act as an independent prognostic factor for DSS due to its strong associations with other recognized clinical prognostic factors.Citation141
Cav-1 and HNSCC
Cav-1 overexpression in HNSCC is associated with the simultaneous abnormal expression of at least one member of the E-cadherin/α-β catenin complex and multiple ErbB receptors as well as with lymph node metastases.Citation142 The elevated expression of Cav-1 may promote tumor cell migration and invasion in HNSCC due to the loss of miR-133a.Citation17 Furthermore, increased expression of Cav-1 may play a pivotal role in the metastasis of HNSCC, possibly through the induction of EMT and the formation of cancer stem cells.Citation143 However, Cav-1 may have the potential to suppress carcinogenesis and lung metastasis via disrupting integrin β1/Src-mediated tumor cell growth, invasion and survival.Citation144 Similarly, low levels of Cav-1 can enable HNSCC cells to undergo EMT and enhanced prometastatic properties by inducing the expression of α5β1 integrins.Citation145
Cav-1 and esophageal SCC
In esophageal SCC, overexpression of Cav-1 is associated with lymph node metastasis and poor prognosis for long-term survival.Citation16 In another research, positive Cav-1 immunostaining is correlated with T factor, lymphatic invasion, vein invasion, differentiation and overall survival of esophageal SCC patients.Citation4 In vitro study demonstrated that Cav-1 is upregulated in esophageal SCC cell lines using human cancer cDNA arrays.Citation146 Furthermore, Cav-1 can mediate MDR by positively regulating the expressions of P-glycoprotein and MDR1 in esophageal SCC cell line Ec9706.Citation147 Downregulation of miR-138 promotes lipid raft formation via upregulating multiple components of lipid rafts, including flotillin-1 (FLOT1) and FLOT2, and then Cav-1 facilitates the recruitment of the tumor necrosis factor receptor and inhibitor of NF-κB kinase signalosome into lipid rafts and activates the NF-κB signaling pathway, consequently leading to the progression of aggressiveness and poorer clinical outcomes in human esophageal SCCs.Citation148 Moreover, there is evidence that Cav-1 downregulation induced by β-carotene enhances apoptosis via inhibiting the AKT/NF-κB pathway in esophageal SCC cells.Citation149 Inconsistent with the previous reports, hypermethylation of the Cav-1 promoter in esophageal SCC is significantly higher than that in corresponding normal esophagus, which can lead to gene silencing. These inconsistent results may be due to the different analytic methods used, ethnic groups studied and smaller sample sizes in the previous studies.Citation69 However, downregulation of stromal Cav-1 expression in esophageal SCC may promote malignant progression and heralds worse outcome, which is significantly associated with lymph node metastases, early tumor recurrence and poor prognosis.Citation23
Cav-1 and lung SCC
Many researchers have reported that increased expression of Cav-1 has been observed in lung SCC.Citation10,Citation70,Citation150 Overexpression of Cav-1 may be correlated with tumor extension,Citation10 advanced pathologic stage, pT and poor prognosis in lung SCC.Citation151 Whereas our previous study reported that its overexpression is only correlated with lymph node metastasis in lung SCC, suggesting that Cav-1 plays a critical role in the metastasis of lung SCC.Citation70 Even there is no significant correlation between Cav-1 expression and clinicopathologic parameters of lung SCC.Citation71 Taken together, Cav-1 plays a tumor-promoting role in the progression of lung SCC, whereas the relationship between Cav-1 and clinicopathologic parameters is still controversial and further studies are needed.
Cav-1 and bladder SCC
Hypermethylation of the Cav-1 promoter is found in bladder SCC (25.9%) by comparison with adenocarcinomas (0%), nonneoplastic urothelium (0%) via methylation-specific PCR. However, a strong diffuse immunostaining of Cav-1 protein is detected in all the specimens of bladder SCC, suggesting that aberrant methylation and protein expression of the Cav-1 are related to bladder SCC. Whereas any expression of Cav-1 is not detected in the normal transitional cell epithelium and adenocarcinomas, supporting epigenetic control of Cav-1 gene is not involved in the histogenesis of adenocarcinomas.Citation152,Citation153 These results may be due to the small sample size, 5 normal sample and 10 adenocarcinomas sample are involved in this study.Citation153
Cav-1 and cervical SCC
Cav-1 expression is dramatically reduced in a human cervical SCC SiHa cell. The possible molecular mechanisms is that the human papillomavirus (HPV) oncoprotein E6 can downregulate Cav-1 via the inactivation of p53, and restore Cav-1 expression that can partially revert HPV-mediated cell transformation, which supports an emerging role for Cav-1 as a tumor suppressor in cervical SCC.Citation154 This is not in accordance with our previous study, which demonstrated that the positive rates of Cav-1 protein by quantum dot-based immunofluorescence staining from normal cervical mucosa, CIN, cervical adenocarcinoma and SCC were 0%, 33%, 19% and 55%, respectively. Furthermore, Cav-1 has a positive correlation with the PCNA protein and high-risk of HPV infection; however, there is no significant association between Cav-1 and any other clinicopathologic characteristics in cervical SCC. Furthermore, a study revealed that Cav-1 is critical for cervical SCC invasion and metastasis.Citation155 These results suggested that Cav-1 might be an oncogene in the progress of cervical SCC.Citation18
The absence of stromal Cav-1 is detected in 39 patients (67%) of cervical SCC. However, stromal Cav-1 expression is not correlated with cell differentiation degree and invasive range. At present, the role of Cav-1 in the stromal microenvironment remains unknown.Citation18 Therefore, further studies are needed to discover the functions of stromal Cav-1 in cervical SCC.
Conclusion
Expression of Cav-1 may affect the malignant progression of carcinoma. From and , we make a conclusion that Cav-1 plays a different role in different histologic types, such as adenocarcinoma and SCC. Even in the same histologic type, its expression is significantly different. In fact, the expression level of Cav-1 has differences even in the different histologic types of the same organ as shown in . Cav-1 plays a complex role in different subunits of the same histologic type in the same organ. Stromal Cav-1 may also play a vital role in influencing the progression of carcinoma. Taken together, the multifunction of Cav-1 can regulate cell proliferation, migration, apoptosis, autophagy and cell cycle in cancer by various pathways. Therefore, we need to further explore the mechanism of Cav-1, which may shed light on the discovery of new targets for cancer treatment.
Table 1 The different functions of Cav-1 in human adenocarcinoma
Table 2 The different functions of Cav-1 in human SCC
Table 3 The role of Cav-1 in different histological types of the same organ
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of Hubei Province (No ZRY2015001716) and Public Welfare Technology Application Research of Zhejiang Province (No 2016C33236).
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenDCheGValue of caveolin-1 in cancer progression and prognosis: emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression (Review)Oncol Lett2014841409142125202343
- Van den EyndenGGVan LaereSJVan der AuweraIOverexpres-sion of caveolin-1 and -2 in cell lines and in human samples of inflammatory breast cancerBreast Cancer Res Treat200695321922816244790
- SugieSMukaiSYamasakiKKamibeppuTTsukinoHKamotoTSignificant association of caveolin-1 and caveolin-2 with prostate cancer progressionCancer Genomics Proteomics201512639139626543085
- AndoTIshiguroHKimuraMThe overexpression of caveolin-1 and caveolin-2 correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinomaOncol Rep200718360160917671707
- GoetzJGLajoiePWisemanSMNabiIRCaveolin-1 in tumor progression: the good, the bad and the uglyCancer Metastasis Rev200827471573518506396
- HanFGuDChenQZhuHCaveolin-1 acts as a tumor suppressor by down-regulating epidermal growth factor receptor-mitogen-activated protein kinase signaling pathway in pancreatic carcinoma cell linesPancreas200938776677419893453
- WiechenKDiatchenkoLAgoulnikACaveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor geneAm J Pathol200115951635164311696424
- SloanEKStanleyKLAndersonRLCaveolin-1 inhibits breast cancer growth and metastasisOncogene200423477893789715334058
- GuDLiHWangZChenQJiangJZhuHCaveolin-1 inhibits the growth of human laryngeal squamous cell carcinoma and down regulates EGFR-MAPKs signaling pathwayLaryngoscope2007117101782178917906498
- KatoTMiyamotoMKatoKDifference of caveolin-1 expression pattern in human lung neoplastic tissue. Atypical adenomatous hyper-plasia, adenocarcinoma and squamous cell carcinomaCancer Lett2004214112112815331180
- PradeETobiaschMHitkovaIBile acids down-regulate caveolin-1 in esophageal epithelial cells through sterol responsive element-binding proteinMol Endocrinol201226581983222474125
- HurlstoneAFReidGReevesJRAnalysis of the CAVEOLIN-1 gene at human chromosome 7q31.1 in primary tumours and tumour-derived cell linesOncogene199918101881189010086342
- YangGGoltsovAARenCCaveolin-1 upregulation contributes to c-Myc-induced high-grade prostatic intraepithelial neoplasia and prostate cancerMol Cancer Res201210221822922144662
- LiangWHaoZHanJLZhuDJJinZFXieWLCAV-1 contributes to bladder cancer progression by inducing epithelial-to-mesenchymal transitionUrol Oncol201432685586324968949
- CampbellLAl-JayyoussiGGutteridgeRCaveolin-1 in renal cell carcinoma promotes tumour cell invasion, and in co-operation with pERK predicts metastases in patients with clinically confined diseaseJ Transl Med20131125524119769
- KatoKHidaYMiyamotoMOverexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stageCancer200294492993311920460
- NohataNHanazawaTKikkawaNCaveolin-1 mediates tumor cell migration and invasion and its regulation by miR-133a in head and neck squamous cell carcinomaInt J Oncol201138120921721109942
- SunJGaoJHuJBExpression of Cav-1 in tumour cells, rather than in stromal tissue, may promote cervical squamous cell carcinoma proliferation, and correlates with high-risk HPV infectionOncol Rep20122761733174022378247
- XueJChenHDiaoLChenXXiaDExpression of caveolin-1 in tongue squamous cell carcinoma by quantum dotsEur J Histochem2010542e2020558341
- CarverLASchnitzerJECaveolae: mining little caves for new cancer targetsNat Rev Cancer20033857158112894245
- ZhaoXHeYChenHAutophagic tumor stroma: mechanisms and roles in tumor growth and progressionInt J Cancer201313211822684793
- WitkiewiczAKDasguptaASotgiaFAn absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancersAm J Pathol200917462023203419411448
- JiaYWangNWangJDown-regulation of stromal caveolin-1 expression in esophageal squamous cell carcinoma: a potent predictor of lymph node metastases, early tumor recurrence, and poor prognosisAnn Surg Oncol201421132933623982252
- KooJSParkSKimSILeeSParkBWThe impact of caveolin protein expression in tumor stroma on prognosis of breast cancerTumour Biol201132478779921584795
- Martinez-OutschoornUETrimmerCLinZAutophagy in cancer associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microen-vironmentCell Cycle20109173515353320855962
- PavlidesSWhitaker-MenezesDCastello-CrosRThe reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stromaCell Cycle20098233984400119923890
- PavlidesSTsirigosAMignecoGThe autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolismCell Cycle20109173485350520861672
- Martinez-OutschoornUEPavlidesSHowellAStromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironmentInt J Biochem Cell Biol20114371045105121300172
- WitkiewiczAKKlineJQueenanMMolecular profiling of a lethal tumor microenvironment, as defined by stromal caveolin-1 status in breast cancersCell Cycle201110111794180921521946
- El-GendiSMMostafaMFEl-GendiAMStromal caveolin-1 expression in breast carcinoma. Correlation with early tumor recurrence and clinical outcomePathol Oncol Res201218245946922057638
- GumulecJSochorJHlavnaMCaveolin-1 as a potential high-risk prostate cancer biomarkerOncol Rep201227383184122159333
- ZhaoZHanFHYangSBHuaLXWuJHZhanWHLoss of stromal caveolin-1 expression in colorectal cancer predicts poor survivalWorld J Gastroenterol20152141140114725632186
- VeredMLehtonenMHotakainenLCaveolin-1 accumulation in the tongue cancer tumor microenvironment is significantly associated with poor prognosis: an in-vivo and in-vitro studyBMC Cancer2015152525633184
- SchererPETangZChunMSargiacomoMLodishHFLisantiMPCaveolin isoforms differ in their N-terminal protein sequence and sub-cellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probeJ Biol Chem19952702716395164017608210
- PodarKTaiYTColeCEEssential role of caveolae in interleukin- 6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cellsJ Biol Chem200327885794580112482878
- SargiacomoMSchererPETangZOligomeric structure of caveolin: implications for caveolae membrane organizationProc Natl Acad Sci U S A19959220940794117568142
- OkamotoTSchlegelASchererPELisantiMPCaveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membraneJ Biol Chem199827310541954229488658
- LiSCouetJLisantiMPSrc tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinasesJ Biol Chem19962714629182291908910575
- CouetJLiSOkamotoTIkezuTLisantiMPIdentification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteinsJ Biol Chem199727210652565339045678
- SahaiEMarshallCJRHO-GTPases and cancerNat Rev Cancer20022213314212635176
- NarumiyaSTanjiMIshizakiTRho signaling, ROCK and mDia1, in transformation, metastasis and invasionCancer Metastasis Rev2009281–2657619160018
- ArpaiaEBlaserHQuintela-FandinoMThe interaction between caveolin-1 and Rho-GTPases promotes metastasis by controlling the expression of alpha5-integrin and the activation of Src, Ras and ErkOncogene201231788489621765460
- JoglekarMElbazantiWOWeitzmanMDLehmanHLvan GolenKLCaveolin-1 mediates inflammatory breast cancer cell invasion via the Akt1 pathway and RhoC GTPaseJ Cell Biochem2015116692393325559359
- LinMDiVitoMMMerajverSDBoyanapalliMvan GolenKLRegulation of pancreatic cancer cell migration and invasion by RhoC GTPase and caveolin-1Mol Cancer2005412115969750
- CokakliMErdalENartDDifferential expression of Caveolin-1 in hepatocellular carcinoma: correlation with differentiation state, motility and invasionBMC Cancer200996519239691
- KannanAKrishnanAAliMSubramaniamSHalagowderDSivasithamparamNDCaveolin-1 promotes gastric cancer progression by up-regulating epithelial to mesenchymal transition by crosstalk of signalling mechanisms under hypoxic conditionEur J Cancer201450120421524070739
- LuZGhoshSWangZHunterTDownregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasionCancer Cell20034649951514706341
- AnnabiBLachambreMBousquet-GagnonNPageMGingrasDBeliveauRLocalization of membrane-type 1 matrix metalloproteinase in caveolae membrane domainsBiochem J2001353Pt 354755311171051
- TangYZengXHeFLiaoYQianNToiMCaveolin-1 is related to invasion, survival, and poor prognosis in hepatocellular cancerMed Oncol201229297798421416157
- YehDChenCSunMZCaveolin-1 is an important factor for the metastasis and proliferation of human small cell lung cancer NCI-H446 cellAnat Rec (Hoboken)2009292101584159219718715
- WengYCaiMZhuJMatrix metalloproteinase activity in early-stage lung cancerOnkologie201336525625923689219
- MakinenLKHayryVHagstromJMatrix metalloproteinase-7 and matrix metalloproteinase-25 in oral tongue squamous cell carcinomaHead Neck201436121783178824488688
- PuyraimondAFridmanRLemesleMArbeilleBMenashiSMMP-2 colocalizes with caveolae on the surface of endothelial cellsExp Cell Res20012621283611120602
- NimriLBarakHGraeveLSchwartzBRestoration of caveolin-1 expression suppresses growth, membrane-type-4 metalloproteinase expression and metastasis-associated activities in colon cancer cellsMol Carcinog2013521185987022674854
- WilliamsTMMedinaFBadanoICaveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretionJ Biol Chem200427949516305164615355971
- QinLYangYBTuoQHEffects and underlying mechanisms of curcumin on the proliferation of vascular smooth muscle cells induced by Chol:MbetaCDBiochem Biophys Res Commun2009379227728219101502
- HulitJBashTFuMThe cyclin D1 gene is transcriptionally repressed by caveolin-1J Biol Chem200027528212032120910747899
- PancottiFRoncuzziLMaggioliniMGasperi-CampaniACaveolin-1 silencing arrests the proliferation of metastatic lung cancer cells through the inhibition of STAT3 signalingCell Signal20122471390139722406084
- GalbiatiFVolonteDLiuJCaveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanismMol Biol Cell20011282229224411514613
- TorresVATapiaJCRodriguezDACaveolin-1 controls cell proliferation and cell death by suppressing expression of the inhibitor of apoptosis protein survivinJ Cell Sci2006119Pt 91812182316608879
- MeyerCLiuYKaulAPeipeIDooleySCaveolin-1 abrogates TGF-beta mediated hepatocyte apoptosisCell Death Dis20134e46623328673
- RavidDMaorSWernerHLiscovitchMCaveolin-1 inhibits anoikis and promotes survival signaling in cancer cellsAdv Enzyme Regul20064616317516857240
- LiLRenCHTahirSARenCThompsonTCCaveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2AMol Cell Biol200323249389940414645548
- WuPQiBZhuHZhengYLiFChenJSuppression of staurosporine-mediated apoptosis in Hs578T breast cells through inhibition of neutral-sphingomyelinase by caveolin-1Cancer Lett20072561647217618736
- ItoYYoshidaHNakanoKCaveolin-1 overexpression is an early event in the progression of papillary carcinoma of the thyroidBr J Cancer200286691291611953823
- PaskasSJankovicJMareckoICaveolin-1 expression in papillary thyroid carcinoma: correlation with clinicopathological parameters and BRAF mutation statusOtolaryngol Head Neck Surg2014150220120924255086
- KimDKimHKooJSExpression of caveolin-1, caveolin-2 and caveolin-3 in thyroid cancer and stromaPathobiology201279111022236542
- ShankarJWisemanSMMengFCoordinated expression of galectin-3 and caveolin-1 in thyroid cancerJ Pathol20122281566622513979
- JinZWangLCaoZTemporal evolution in caveolin 1 methy-lation levels during human esophageal carcinogenesisBMC Cancer20141434524885118
- ChenHLFanLFGaoJOuyangJPZhangYXDifferential expression and function of the caveolin-1 gene in non-small cell lung carcinomaOncol Rep201125235936621165568
- ZhanPShenXKQianQExpression of caveolin-1 is correlated with disease stage and survival in lung adenocarcinomasOncol Rep20122741072107822200856
- HoCCHuangPHHuangHYChenYHYangPCHsuSMUp-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formationAm J Pathol200216151647165612414512
- YangCPGalbiatiFVolonteDHorwitzSBLisantiMPUpregulation of caveolin-1 and caveolae organelles in Taxol-resistant A549 cellsFEBS Lett199843933683729845355
- LingeAMeleadyPHenryMClynesMKasperMBarthKBleomycin treatment of A549 human lung cancer cells results in association of MGr1-Ag and caveolin-1 in lipid raftsInt J Biochem Cell Biol20114319810520937408
- WangNNZhaoLJWuLNMechanistic analysis of taxol-induced multidrug resistance in an ovarian cancer cell lineAsian Pac J Cancer Prev20131494983498824175763
- LeeCYLaiTYTsaiMKThe influence of a caveolin-1 mutant on the function of P-glycoproteinSci Rep201662048626843476
- HanFZhangLZhouYYiXCaveolin-1 regulates cell apoptosis and invasion ability in paclitaxel-induced multidrug-resistant A549 lung cancer cellsInt J Clin Exp Pathol2015888937894726464635
- ShimizuKKiritaKAokageKClinicopathological significance of caveolin-1 expression by cancer-associated fibroblasts in lung adenocarcinomaJ Cancer Res Clin Oncol Epub20161022
- ChiuWTLeeHTHuangFJCaveolin-1 upregulation mediates suppression of primary breast tumor growth and brain metastases by stat3 inhibitionCancer Res201171144932494321622714
- DuCChenLZhangHCaveolin-1 limits the contribution of BKCa channel to MCF-7 breast cancer cell proliferation and invasionInt J Mol Sci20141511207062072225397596
- HartPCRattiBAMaoMCaveolin-1 regulates cancer cell metabolism via scavenging Nrf2 and suppressing MnSOD-driven glycolysisOncotarget20167130832226543228
- YamaguchiHTakeoYYoshidaSKouchiZNakamuraYFukamiKLipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cellsCancer Res200969228594860219887621
- HerzogMStorchCHGutPKnockdown of caveolin-1 decreases activity of breast cancer resistance protein (BCRP/ABCG2) and increases chemotherapeutic sensitivityNaunyn Schmiedeberg’s Arch Pharmacol2011383111120936466
- WangZWangNLiWCaveolin-1 mediates chemoresistance in breast cancer stem cells via beta-catenin/ABCG2 signaling pathwayCarcinogenesis201435102346235625085904
- RaoXEvansJChaeHCpG island shore methylation regulates caveolin-1 expression in breast cancerOncogene201332384519452823128390
- LiTSotgiaFVuoloMACaveolin-1 mutations in human breast cancer: functional association with estrogen receptor alpha-positive statusAm J Pathol200616861998201316723714
- BonuccelliGCasimiroMCSotgiaFCaveolin-1 (P132L), a common breast cancer mutation, confers mammary cell invasiveness and defines a novel stem cell/metastasis-associated gene signatureAm J Pathol200917451650166219395651
- KoikeSKoderaYNakaoAIwataHYatabeYAbsence of the caveolin-1 P132L mutation in cancers of the breast and other organsJ Mol Diagn201012571271720581046
- LiedtkeCKerstingCBurgerHKieselLWulfingPCaveolin-1 expression in benign and malignant lesions of the breastWorld J Surg Oncol2007511017915016
- ElsheikhSEGreenARRakhaEACaveolin 1 and caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotypeBr J Cancer200899232733418612310
- MaXLiuLNieWPrognostic role of caveolin in breast cancer: a meta-analysisBreast201322446246923639584
- QianNUenoTKawaguchi-SakitaNPrognostic significance of tumor/stromal caveolin-1 expression in breast cancer patientsCancer Sci201110281590159621585620
- BurgermeisterEXingXRockenCDifferential expression and function of caveolin-1 in human gastric cancer progressionCancer Res200767188519852617875691
- NamKHLeeBLParkJHCaveolin 1 expression correlates with poor prognosis and focal adhesion kinase expression in gastric cancerPathobiology2013802879423038627
- BarresiVGiuffreGVitarelliETodaroPTuccariGCaveolin-1 immuno-expression in human gastric cancer: histopathogenetic hypothesesVirchows Arch2008453657157818936967
- YeYMiaoSHLuRZZhouJWPrognostic value of caveolin-1 expression in gastric cancer: a meta-analysisAsian Pac J Cancer Prev201415198367837025339030
- HeYZhaoXGaoJQuantum dots-based immunofluorescent imaging of stromal fibroblasts caveolin-1 and light chain 3B expression and identification of their clinical significance in human gastric cancerInt J Mol Sci20121311137641378023203033
- ShenXJZhangHTangGSCaveolin-1 is a modulator of fibroblast activation and a potential biomarker for gastric cancerInt J Biol Sci201511437037925798057
- HanFZhuHGCaveolin-1 regulating the invasion and expression of matrix metalloproteinase (MMPs) in pancreatic carcinoma cellsJ Surg Res2010159144345020031158
- SalemAFBonuccelliGBevilacquaGCaveolin-1 promotes pancreatic cancer cell differentiation and restores membranous E-cadherin via suppression of the epithelial-mesenchymal transitionCell Cycle201110213692370022041584
- SuzuokiMMiyamotoMKatoKImpact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinomaBr J Cancer200287101140114412402154
- TanaseCPCaveolin-1: a marker for pancreatic cancer diagnosisExpert Rev Mol Diagn20088439540418598222
- ChatterjeeMBen-JosefEThomasDGCaveolin-1 is associated with tumor progression and confers a multi-modality resistance phenotype in pancreatic cancerSci Rep201551086726065715
- LiuLXuHXWangWQCavin-1 is essential for the tumorpromoting effect of caveolin-1 and enhances its prognostic potency in pancreatic cancerOncogene201433212728273623770857
- ShanTLuHJiHLoss of stromal caveolin-1 expression: a novel tumor microenvironment biomarker that can predict poor clinical outcomes for pancreatic cancerPLoS One201496e9723924949874
- TseEYKoFCTungEKCaveolin-1 overexpression is associated with hepatocellular carcinoma tumourigenesis and metastasisJ Pathol2012226464565322072235
- LiuWRJinLTianMXCaveolin-1 promotes tumor growth and metastasis via autophagy inhibition in hepatocellular carcinomaClin Res Hepatol Gastroenterol201640216917826206578
- ZhangZBCaiLZhengSGXiongYDongJHOverexpression of caveolin-1 in hepatocellular carcinoma with metastasis and worse prognosis: correlation with vascular endothelial growth factor, microvessel density and unpaired arteryPathol Oncol Res200915349550219434519
- YuHShenHZhangYCAV1 promotes HCC cell progression and metastasis through Wnt/beta-catenin pathwayPLoS One201499e10645125180681
- GaiXLuZTuKLiangZZhengXCaveolin-1 is up-regulated by GLI1 and contributes to GLI1-driven EMT in hepatocellular carcinomaPLoS One201491e8455124454730
- KorhanPErdalEKandemisEReciprocal activating crosstalk between c-Met and caveolin 1 promotes invasive phenotype in hepa-tocellular carcinomaPLoS One201498e10527825148256
- ZhaoXLiuYMaQCaveolin-1 negatively regulates TRAIL-induced apoptosis in human hepatocarcinoma cellsBiochem Biophys Res Commun20093781212618992712
- YangSFYangJYHuangCHIncreased caveolin-1 expression associated with prolonged overall survival rate in hepatocellular carcinomaPathology201042543844520632820
- FineSWLisantiMPGalbiatiFLiMElevated expression of caveolin-1 in adenocarcinoma of the colonAm J Clin Pathol2001115571972411345836
- PatlollaJMSwamyMVRajuJRaoCVOverexpression of caveolin-1 in experimental colon adenocarcinomas and human colon cancer cell linesOncol Rep200411595796315069532
- DiazJMendozaPOrtizRRab5 is required in metastatic cancer cells for caveolin-1-enhanced Rac1 activation, migration and invasionJ Cell Sci2014127Pt 112401240624659799
- RoyUKHenkhausRSIgnatenkoNAMoraJFultzKEGernerEWWild-type APC regulates caveolin-1 expression in human colon adenocarcinoma cell lines via FOXO1a and C-mycMol Carcinog2008471294795518444242
- CampbellLGumbletonMGriffithsDFCaveolin-1 overexpression predicts poor disease-free survival of patients with clinically confined renal cell carcinomaBr J Cancer200389101909191314612902
- WaalkesSEggersHBlasigHCaveolin 1 mRNA is overexpressed in malignant renal tissue and might serve as a novel diagnostic marker for renal cancerBiomark Med20115221922521473727
- CampbellLJasaniBEdwardsKGumbletonMGriffithsDFCombined expression of caveolin-1 and an activated AKT/mTOR pathway predicts reduced disease-free survival in clinically confined renal cell carcinomaBr J Cancer200898593194018283322
- JooHJOhDKKimYSLeeKBKimSJIncreased expression of caveolin-1 and microvessel density correlates with metastasis and poor prognosis in clear cell renal cell carcinomaBJU Int200493329129614764125
- KaramJALotanYRoehrbornCGAshfaqRKarakiewiczPIShariatSFCaveolin-1 overexpression is associated with aggressive prostate cancer recurrenceProstate200767661462217299799
- YangGTimmeTLFrolovAWheelerTMThompsonTCCombined c-Myc and caveolin-1 expression in human prostate carcinoma predicts prostate carcinoma progressionCancer200510361186119415712208
- YangGAddaiJWheelerTMCorrelative evidence that prostate cancer cell-derived caveolin-1 mediates angiogenesisHum Pathol200738111688169517707459
- ZhangXLingMTWangQIdentification of a novel inhibitor of differentiation-1 (ID-1) binding partner, caveolin-1, and its role in epithelial-mesenchymal transition and resistance to apoptosis in prostate cancer cellsJ Biol Chem200728246332843329417855368
- HuangCQiuZWangLA novel FoxM1-caveolin signaling pathway promotes pancreatic cancer invasion and metastasisCancer Res201272365566522194465
- Di VizioDMorelloMSotgiaFPestellRGFreemanMRLisantiMPAn absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activationCell Cycle20098152420242419556867
- AyalaGMorelloMFrolovALoss of caveolin-1 in prostate cancer stroma correlates with reduced relapse-free survival and is functionally relevant to tumour progressionJ Pathol20132311778723729330
- LangloisSCowanKNShaoQCowanBJLairdDWThe tumor-suppressive function of Connexin43 in keratinocytes is mediated in part via interaction with caveolin-1Cancer Res201070104222423220406988
- TrimmerCBonuccelliGKatiyarSCav1 suppresses tumor growth and metastasis in a murine model of cutaneous SCC through modulation of MAPK/AP-1 activationAm J Pathol20131823992100423267770
- HuangCFYuGTWangWMLiuBSunZJPrognostic and predictive values of SPP1, PAI and caveolin-1 in patients with oral squamous cell carcinomaInt J Clin Exp Pathol2014796032603925337248
- AuzairLBVincent-ChongVKGhaniWMCaveolin 1 (Cav-1) and actin-related protein 2/3 complex, subunit 1B (ARPC1B) expressions as prognostic indicators for oral squamous cell carcinoma (OSCC)Eur Arch Otorhinolaryngol201627371885189326138391
- NakataniKWadaTNakamuraMUzawaKTanzawaHFujitaSExpression of caveolin-1 and its correlation with cisplatin sensitivity in oral squamous cell carcinomaJ Cancer Res Clin Oncol2005131744545215856296
- HanSEParkKHLeeGHuhYJMinBMMutation and aberrant expression of Caveolin-1 in human oral squamous cell carcinomas and oral cancer cell linesInt J Oncol200424243544014719121
- HungKFLinSCLiuCJChangCSChangKWKaoSYThe biphasic differential expression of the cellular membrane protein, caveolin-1, in oral carcinogenesisJ Oral Pathol Med200332846146712901727
- JensenDHTherkildsenMHDabelsteenEA reverse Warburg metabolism in oral squamous cell carcinoma is not dependent upon myofibroblastsJ Oral Pathol Med201544971472125420473
- MartinsDBecaFFSousaBBaltazarFParedesJSchmittFLoss of caveolin-1 and gain of MCT4 expression in the tumor stroma: key events in the progression from an in situ to an invasive breast carcinomaCell Cycle201312162684269023907124
- RoutraySCaveolin-1 in oral squamous cell carcinoma microenvironment: an overviewTumour Biol201435109487949525123270
- GoetzJGMinguetSNavarro-LeridaIBiomechanical remod-eling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasisCell2011146114816321729786
- YeQMengCShenYCaveolin-1 mediates low-intensity ultrasound-induced apoptosis via downregulation of signal transducer and activator of transcription 3 phosphorylation in laryngeal carcinoma cellsUltrasound Med Biol20164292253226027289429
- GrecoADe VirgilioARizzoMIPandolfiFRosatiDde VincentiisMThe prognostic role of E-cadherin and beta-catenin overexpression in laryngeal squamous cell carcinomaLaryngoscope20161264E148E15526511677
- MasuelliLBudillonAMarzocchellaLCaveolin-1 overexpression is associated with simultaneous abnormal expression of the E-cadherin/alpha-beta catenins complex and multiple ErbB receptors and with lymph nodes metastasis in head and neck squamous cell carcinomasJ Cell Physiol201222793344335322213373
- MasoodRHochstimCCervenkaBA novel orthotopic mouse model of head and neck cancer and lymph node metastasisOncogenesis20132e6824018643
- ZhangHSuLMullerSRestoration of caveolin-1 expression suppresses growth and metastasis of head and neck squamous cell carcinomaBr J Cancer200899101684169419002186
- JungACRayAMRamoluLCaveolin-1-negative head and neck squamous cell carcinoma primary tumors display increased epithelial to mesenchymal transition and prometastatic propertiesOncotarget2015639418844190126474461
- HuYCLamKYLawSWongJSrivastavaGProfiling of differentially expressed cancer-related genes in esophageal squamous cell carcinoma (ESCC) using human cancer cDNA arrays: overexpression of oncogene MET correlates with tumor differentiation in ESCCClin Cancer Res20017113519352511705871
- ZhangSCaoWYueMCaveolin-1 affects tumor drug resistance in esophageal squamous cell carcinoma by regulating expressions of P-gp and MRP1Tumour Biol20163779189919626768616
- GongHSongLLinCDownregulation of miR-138 sustains NF-kappaB activation and promotes lipid raft formation in esophageal squamous cell carcinomaClin Cancer Res20131951083109323319823
- ZhuXZhangYLiQBeta-carotene induces apoptosis in human esophageal squamous cell carcinoma cell lines via the Cav-1/AKT/NF-kappaB signaling pathwayJ Biochem Mol Toxicol201630314815726733226
- CassoniPDanieleLMaldiECaveolin-1 expression in lung carcinoma varies according to tumour histotype and is acquired de novo in brain metastasesHistopathology2009551202719614763
- YooSHParkYSKimHRExpression of caveolin-1 is associated with poor prognosis of patients with squamous cell carcinoma of the lungLung Cancer200342219520214568687
- KunzeEVon BoninFWernerCWendtMSchlottTTransitional cell carcinomas and nonurothelial carcinomas of the urinary bladder differ in the promoter methylation status of the caveolin-1, hDAB2IP and p53 genes, but not in the global methylation of Alu elementsInt J Mol Med200617131316328005
- KunzeESchlottTHigh frequency of promoter methylation of the 14-3-3 sigma and CAGE-1 genes, but lack of hypermethylation of the caveolin-1 gene, in primary adenocarcinomas and signet ring cell carcinomas of the urinary bladderInt J Mol Med200720455756317786288
- RazaniBAltschulerYZhuLPestellRGMostovKELisantiMPCaveolin-1 expression is down-regulated in cells transformed by the human papilloma virus in a p53-dependent manner. Replacement of caveolin-1 expression suppresses HPV-mediated cell transformationBiochemistry20003945139161392411076533
- ChengYMaDZhangYLiZGengLCervical squamous cancer mRNA profiles reveal the key genes of metastasis and invasionEur J Gynaecol Oncol201536330931726189259
- AgelakiSSpiliotakiMMarkomanolakiHCaveolin-1 regulates EGFR signaling in MCF-7 breast cancer cells and enhances gefitinib-induced tumor cell inhibitionCancer Biol Ther20098151470147719483462
- SavageKLambrosMBRobertsonDCaveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysisClin Cancer Res20071319010117200343
