Abstract
Bladder cancer (BC) is the second most common malignant tumor of the urinary tract in the world. In this study, we found that ubiquitin-specific protease (USP21) was upregulated in BC and the ectopic expression of USP21 was closely associated with tumor size and metastasis. Moreover, patients with higher levels of USP21 had poorer survival rate. Multiple function analysis such as CCK-8, colony formation, wound healing, and transwell analysis indicated that USP21 regulated cell proliferation and metastasis in bladder carcinoma cell lines. We also found that USP21 could facilitate epithelial–mesenchymal transition. As EZH2 has been reported to promote cell metastasis in BC, our work identified that USP21 deubiquitinated EZH2 and stabilized it. Our data demonstrated that USP21 might play a crucial role in regulating BC progression and could provide a potential therapeutic strategy for BC.
Keywords:
Introduction
Bladder cancer (BC) has become the second most common malignant tumor of the urinary tract in the world.Citation1,Citation2 The urothelial carcinoma of the bladder is the most common type and accounts for ~95% of bladder carcinoma.Citation3 Approximately 47% of transitional cell carcinoma recur as a nonlethal disease initially and ~9% will ultimately deteriorate to a muscle-invasive bladder carcinoma,Citation1,Citation2 which commonly occurs as metastasis and results in a high rate of death.Citation3 In patients with invasive and metastatic bladder carcinoma, the traditional therapy slightly improves the 5-year survival rate.Citation4 In the recent decade, although many targeted therapies have excelled in several cancers, such as gefitinib in lung carcinoma and sunitinib in kidney carcinoma, there is still no evidence of the efficiency of targeted therapeutic reagent for bladder carcinoma.Citation1,Citation2,Citation5 Hence, finding and developing a more efficient therapeutic target is urgently needed. Meanwhile, it is crucial to investigate the molecular mechanism of BC development in detail and it is beneficial for finding a potential molecular target for bladder carcinoma therapy.
Protein ubiquitination involves multiple cellular processes, such as protein degradation, transcriptional activation or inhibition, and immune signal transduction pathways.Citation6–Citation8 The deubiquitinase (DUB) family has been found in at least 79 members in humans. There are five subfamilies of DUB: ovarian tumor, ubiquitin C-terminal hydrolases, ubiquitin-specific proteases (USPs), Josephin domain, and JAB1/MPN/MOV34 proteases (JAMM) family.Citation9,Citation10 For example, USP7 and USP10 were found to regulate p53 localization and function.Citation11,Citation12 USP15, USP21, and USP31 have been found to play key roles in the regulation of the NF-κB pathway.Citation13–Citation18
USP21 belongs to the USPs family with a C-terminal catalytic DUB domain.Citation19 USP21 has been regarded as an USP, which catalyzes the hydrolysis of ubH2A and activates transcriptional initiation.Citation20 Moreover, USP21 regulates NF-κB signaling pathway or Th2-specific transcriptional factor GATA3 to modulate immune defense.Citation18,Citation21 Recently, one report indicated that USP21 promotes cell proliferation and invasion ability in human renal cell carcinoma.Citation22 However, the role of USP21 in bladder carcinoma is still unknown.
In our study, we suggested that USP21 was an oncogene in bladder carcinoma because it had an obviously high expression in BC tissue samples and cell lines; moreover, high expression of USP21 was closely associated with tumor size, metastasis, and poor prognosis. In addition, several functional experiments indicated that USP21 not only promoted cell proliferation but also facilitated metastasis through regulation epithelial–mesenchymal transition (EMT) process. Furthermore, we identified that USP21 directly regulated the protein level of EZH2 through its DUB activity. These findings demonstrated that USP21 could enhance the progression of bladder carcinoma and provide a novel potential targets for bladder carcinoma therapy.
Methods and materials
Cell culture and transfection
Human-immortalized bladder urothelial cell line (SV-HUC-1) and human BC cell lines (T24 and 5637) were purchased from Shanghai Cell Bank (People’s Republic of China) and cultured in Roswell Park Memorial Institute 1640 medium (HyClone, Logan, UT, USA) supplemented with 1% penicillin–streptomycin (HyClone) and 10% fetal bovine serum (HyClone) at 37°C with 5% CO2.
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used to transfect cells according to the manufacturer’s protocol. In brief, DNA plasmids or USP21 siRNA were mixed with Opti-MEM medium and lipofectamine 2000 reagents, gently vortexed and stranded for 10 min at room temperature. After adding it to the cells, the medium was replaced 6 h later. The transfection efficiency was determined after 48 h.
Bladder tissue samples
The 62 adjacent normal bladder tissues and BC tissues were obtained from The First Affiliated Hospital of Chongqing Medical University during 2015–2016. All patients were aware that their tissue sample would be used for research prior to the study and had provided written informed consent for the samples to be used. Our research was approved by the Institutional Research Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (FAHCM201503). All the tissue samples were collected and frozen in liquid nitrogen and stored at −80°C until processing.
Quantitative real time polymerase chain reaction (qRT-PCR)
RNeasy mini kit (Qiagen, Hilden, Germany) was used to extract total RNA from 40 mg tumor or normal tissue samples and cells and next cDNA was synthesized using PrimeScript RT reagent kit (Takara, Tokyo, Japan). For real-time qPCR, we used the 7500 Real-Time PCR System using SYBR® Green (Thermo Fisher Scientific, Waltham, MA, USA). The relative expression of mRNA was normalized against β-actin. All experiments were repeated at least three times.
Western blotting
After collecting and lysating cells in radio immunoprecipitation assay lysis buffer (BLKW, Fanke Biological Technology Co., Ltd, Shanghai, People’s Republic of China), we next measured protein concentrations with bicinchoninic acid assay kit (Pierce, Appleton, WI, USA). Equal amounts of protein were separated in 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred proteins to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). After blocking the membranes with 5% nonfat dry milk in Tris-buffered saline, Tween 20 (TBST) buffer, membranes were incubated with indicated primary antibodies at 4°C overnight. We washed the membranes with TBST buffer three times and incubated membranes with the horseradish peroxidase-labeled secondary antibodies at room temperature for 1 h. After washing the membranes with TBST buffer three times, the immunobands were visualized using the ECL reagents (ECL-Amersham, Piscataway, NJ, USA).
CCK-8 assays
The CCK-8 assay was used to determine the cell proliferation ability. After cells were transfected with vector, USP21, scramble siRNA (SCR), siUSP21 for 48 h, collected, and counted, 4×103 cells were placed in 96-well plates with 100 μL complete medium. Every 24 h, 10 μL CCK-8 were added in each well and then incubated at 37°C for 30 min. The absorbance was measured at 450 nm to assess the number of viable cells. All experiments were repeated at least three times.
Colony formation assay
Colony formation assay was used to determine the cell proliferation ability. After cells were transfected with vector, USP21, SCR, siUSP21 for 48 h, collected and counted, 3×103 cells were placed in six-well plates and incubated for 14 days with complete medium. The colonies were fixed with 10% formaldehyde and stained with 0.5% crystal violet. The colonies were counted under microscope. All experiments were repeated at least three times.
Transwell assay
Transwell assay was performed to detect the cell invasiveness ability. We used transwell chambers with 8 μm pores (Corning Costar, Corning, NY, USA) and Matrigel (BD, San Diego, CA, USA), and coated matrigel on chambers. After cells were transfected with vector, USP21, SCR, siUSP21 for 48 h, collected and counted, 2×103 cells were placed on top chambers with serum-free medium, whereas the lower chamber completes with the medium. After incubating cells at 37°C with 5% CO2 for 24 h, cotton swabs were used to remove the cells remained in the upper side, fixed and stained with 0.5% crystal violet. Cells were counted under microscope. All experiments were repeated at least three times.
Immunopurification and mass spectrometry (MS)
We overexpressed FLAG-USP21 in 5637 cells after 48 h of transfection, lysated whole cells, added 70 μL of 50% FLAG column, and incubated for 4 h at 4°C. After washing the column with phosphate-buffered saline (PBS) for three times, subsequently FLAG peptides (Sigma, New York, USA) were used to elute protein complex. The eluted solution was resolved on SDS-PAGE gel, stained gel with silver-stained kit (Pierce), and the special band were subjected to liquid chromatography–MS/MS sequencing.
Co-immunoprecipitation (co-IP)
For co-IP assays, we lysated whole cells by NETN lysis buffer (0.5 mM ethylenediaminetetraacetic acid [EDTA], 20 mM Tris-Cl [pH 8.0], 100 mM NaCl, 1× cocktail protease inhibitor, 0.5% Nonidet P-40 [NP-40]) and next incubated with 2 μg anti-USP21 or anti-EZH2 antibody and ~35 μL protein A-agarose beads at 4°C overnight. We washed the beads with NETN lysis buffer three times and eluted immunocomplexes via boiling in SDS sample buffer at least for 10 min. Finally, Western blotting was performed to identify protein.
GST pull-down analysis
We utilized 40 μL of 50% glutathione-Sepharose 4B beads (Solarbio, Beijing, People’s Republic of China) to immobilize GST fusion proteins in 500 μL binding buffer (5% glycerol, 100 mM KCl, 10 mM Hepes, 5 mM EDTA, 3 mM MgCl2, 0.5% CA630, and 3 mM MgCl2). We incubated GST fusion proteins and beads for 2 h at 4°C with rotation, washed beads with binding buffer for three times, and next resuspended and added 5 μL transcribed/translated EZH2, 4% bovine serum albumin and again incubated it for at least 90 min at 4°C with rotation. We washed beads with ice-cold PBS for three times and boiled the beads with 35 μL of 2× SDS loading buffer and resolved on 10% SDS-PAGE.
Statistical analysis
The GraphPad Prism 5 statistical software (GraphPad Software, La Jolla, CA, USA) was used to evaluate all the statistical analyses. The correlation between USP21 expression and patients’ clinical pathological characteristics was evaluated by chi-square test. The two-tailed Student’s t-test was used to assess the differences between two groups. The *P<0.05 was considered statistically significant. All experiments were repeated at least three times, and the values were shown as mean ± standard deviation.
Results
USP21 is highly expressed in BC tissues and cell lines and predicts poor prognosis of BC
To investigate the function of USP21 in BC, we first performed qRT-PCR to assess the expression level of USP21 in BC tissues and adjacent normal tissues. To our surprise, we found that USP21 was elevated in BC tissues compared with the adjacent normal tissues (). In addition, we further decipher the relationship of the expression level of USP21 with the pathological characteristics of BC. As shown in , high expression of USP21 was more closely correlated with high-grade BC than in low-grade tumors. Moreover, USP21 was found to be evaluated in high tumor stage, suggesting a potential relationship of tumor progression with the expression of USP21. Tumor size and lymphatic invasion also had association with high expression of USP21. This finding suggested that USP21 played an important role in tumor growth and metastasis in BC. According to the KM plot, we draw the survival curve. The survival curve revealed that the patient who had high expression of USP21 had a shorted survival time than the patient who had low expression of USP21 (). Together, our work revealed that the elevated expression of USP21 was closely correlated with the tumor aggressiveness, eventually resulted in poor prognoses. Subsequently, we determined the expression of USP21 in two BC cell lines (5637 and T24) and normal urothelial cell line (SV-HUC-1) as control. As shown in , both the mRNA level and the protein level of USP21 were highly expressed in BC cell lines, compared with SV-HUC-1.
Figure 1 USP21 is highly expressed in BC tissues and cell lines, and it predicted poor prognosis of BC.
Abbreviations: BC, bladder cancer; HUC, human urothelial cell; USP, ubiquitin-specific protease.
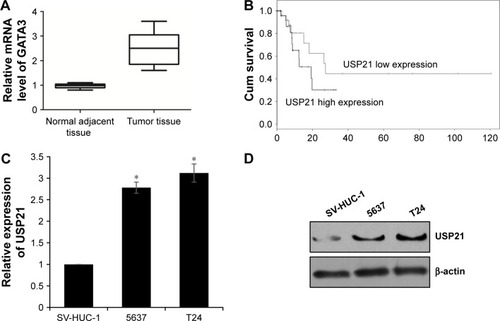
Table 1 Clinicopathologic variables in 66 bladder cancer patients
Overexpression of USP21 promotes proliferation of BC cells
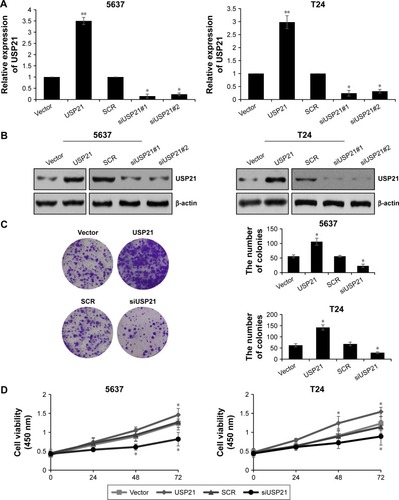
Our previous work indicated that high expression of USP21 was coordinated with tumor size, hence, we next investigated the involvement of USP21 in tumor progression. BC cell lines 5637 and T24 were transfected with vector, USP21 plasmid, SCR, or USP21 siRNA, and the expression of USP21 were detected. As shown in , the USP21 siRNA#1 was more efficient than USP21 siRNA#2, thus the subsequent experiments used USP21 siRNA#1. After transfection, we performed colony formation analysis and CCK-8 analysis to evaluate the functions of USP21 on cell proliferation. As shown in , ectopic expression of USP21 obviously increased the proliferation rates of 5637 and T24 cells, whereas the inhibition of USP21 expression significantly inhibited cell proliferation. Together, our findings demonstrated that USP21 promoted cell proliferation in BC.
Figure 2 Overexpression of USP21 promotes proliferation of bladder cancer cells.
Abbreviations: SCR, scramble siRNA; USP, ubiquitin-specific protease.
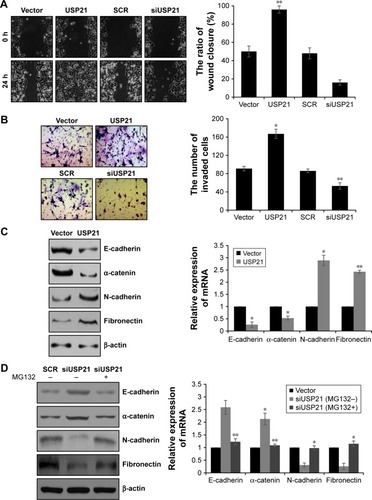
USP21 promotes migration and invasion ability of BC cells and facilitates EMT
Because lymphatic invasion also had association with high expression of USP21, we next performed wound healing analysis and transwell assay to determine the effect of USP21 in cell migration and invasion. As shown in , the cells with ectopic expression of USP21 migrated to ~100% of the wounded area, whereas the control groups migrated ~50% of the area. However, the cells with the inhibition of USP21 migrated to ~17% of the wounded area, whereas the control groups migrated ~50% of the area. The transwell assay revealed that the number of invaded cells was significantly increased in the USP21 overexpression group compared with the control group. The number of invaded cells was significantly increased in the USP21-depleted group compared with the control group (). These data revealed that USP21 not only promoted cell proliferation but also facilitated cell migration and invasion in BC. To further decipher the molecular mechanism of USP21 on BC cell migration and invasion, we aimed to identify whether USP21 regulated EMT. To verify our hypothesis, we assessed several EMT markers (E-cadherin, α-catenin, N-cadherin, and fibronectin) by qRT-PCR and Western blot. As expected, we found that E-cadherin and α-catenin were remarkably decreased and N-cadherin and fibronectin were obviously increased while overexpressing USP21 (). The contrary results were observed while USP21 was depleted (). To investigate whether USP21 regulated EMT through its DUB activity, we added MG132 in USP21-depleted cells. We found that the changes in epithelial and mesenchymal markers were obviously reverse (). These results suggested that USP21 facilitated BC cell metastasis through the promotion of the EMT process.
Figure 3 USP21 promotes migration and invasion ability of BC cells and facilitates EMT.
Abbreviations: BC, bladder cancer; EMT, epithelial–mesenchymal transition; SCR, scramble siRNA; USP, ubiquitin-specific protease.
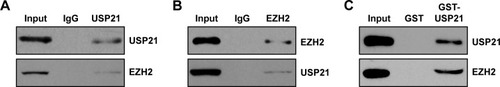
Identifying USP21 as a EZH2-interacting protein
To further decipher the cellular functions of USP21, we performed affinity purification to detect USP21-interacting protein through MS. We found that there were 11 matching peptides from EZH2, which suggested that USP21 was associated with EZH2 in vivo. EZH2 was reported to have a high expression in BC and correlated with EMT.Citation23–Citation26 Subsequently, we verified the interaction between USP21 and EZH2 through co-IP analysis. We performed co-IP assays in 5637 cell lysates with anti-USP21 antibody and then immunoblotting with antibodies against USP21 and EZH2. The results revealed that EZH2 was efficiently interacted with USP21 (). Reciprocal immunoprecipitation with anti-EZH2 and immunoblotting with EZH2 and USP21 also confirmed that USP21 interacts with EZH2 in vivo (). To further determine whether USP21 was directly interacted with EZH2, GST pull-down assay was performed. We expressed GST-USP21 in bacteria and then incubated with transcribed/translated FLAG-EZH2 (). The result showed that USP21 could interact with EZH2 directly in vitro. Together, our works suggested that USP21 was physically associated with EZH2 in vivo.
Figure 4 Identifying USP21 as a EZH2-interacting protein.
Abbreviations: co-IP, co-immunoprecipitation; USP, ubiquitin-specific protease.
USP21 stabilizes EZH2 through its ubiquitinase activity
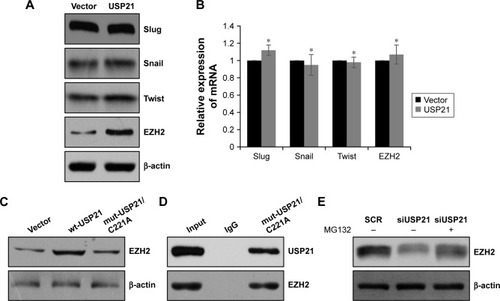
As a DUB, USP21 was reported to deubiquitinate Gli1 and RIG-I;Citation27,Citation28 therefore, we assumed that USP21 may regulate EZH2 and some protein, which was associated with EMT. We first detected several transcriptional factors, such as snail, slug, and twist, but there were no correlation between USP21 and those transcription factors, whereas the protein level of EZH2 was significantly upregulated while overexpressing USP21 (), but the mRNA level of EZH2 had no change (). The above data indicated that USP21 may regulate EZH2 through its DUB activity. To verify our hypothesis, we ectopically expressed wild-type USP21 (wt-USP21) and mutant USP21 (mut-USP21/C221A), which were catalytically inactive in 5637 cells. As expected, EZH2 only increased in cells with overexpressed wt-USP21; however, EZH2 had no change in cells with overexpressed mutant USP21 (USP21/C221A; ). Moreover, to determine whether mut-USP21/C221A had any effect on EZH2, because EZH2 had no interaction with mut-USP21/C221A. Co-IP assay was performed, as shown in , USP21/C221A could interact with EZH2. In addition, after the addition of MG132 in USP21-depleted 5637 cells, it was succeeded to rescue EZH2 protein from degradation (). Together, our works revealed that USP21 could stabilize EZH2 through its DUB activity.
Figure 5 USP21 stabilizes EZH2 through its ubiquitinase activity.
Abbreviations: Co-IP, co-immunoprecipitation; USP, ubiquitin-specific protease.
Discussion
Accumulating evidence has suggested that USP21 is closely correlated to the development of cancer.Citation18,Citation21,Citation22,Citation29 Many studies have reported that USP21 deubiquitinated H2A and several nonhistone, such as Gli1 and RIG-I.Citation27,Citation28
In our study, we found that USP21 was highly expressed in BC and the expression of USP21 was associated with tumor size, metastasis, and poor survival rate. Several functional experiments, including colony formation analysis and CCK-8 analysis, suggested that overexpression of USP21 promoted cell proliferation and inhibition of USP21 suppressed cell proliferation. However, the details of molecular mechanism remain unknown. Cell proliferation is a complex cell process. It is regulated by multiple proteins, such as cyclin-D1, cyclin-E1, p16, p21, etc.Citation30–Citation32 We assumed that USP21 might regulate some protein, which influenced those cell proliferations. Subsequently, transwell assay and wound healing assay confirmed that USP21 promoted BC cells metastasis. Furthermore, we found that USP21 also facilitated EMT. To further investigate the cellular function of USP21, we performed affinity purification to detect the USP21-interacting protein. To our surprise, EZH2, a component of PRC2 complex, was interacted with USP21. EZH2 has been reported to have a high expression in several tumors and promote cancer development. Here, co-IP and GST pull-down analysis demonstrated that USP21 interacted with EZH2. As a DUB, USP21 deubiquitinated and stabilized several proteins. Next, we detected whether USP21 regulated EZH2 through its DUB activity. The results showed that the ectopic expression of wt-USP21 could stabilize EZH2, but the overexpression of mut-USP21/C221A with inactive catalytic had no effect. Moreover, the inhibition of USP21 could decrease the expression of EZH2; while adding MG132, the expression of EZH2 was revived. Overall, our study suggests that USP21 stabilized EZH2 through its DUB activity. Meanwhile, USP21-facilitated EMT may also depend on its DUB activity.
In summary, our study demonstrated that USP21 was upregulated in bladder carcinoma and USP21 played an important function in tumor proliferation and metastasis. USP21 should be considered as a potential biomarker for BC.
Disclosure
The authors report no conflicts of interest in this work.
References
- SylvesterRJNatural history, recurrence, and progression in superficial bladder cancerScientific World Journal200662617262517619739
- CrawfordJMThe origins of bladder cancerLab Invest200888768669318475256
- KaufmanDSShipleyWUFeldmanASBladder cancerLancet2009374968523924919520422
- BellmuntJGuixMNew agents for bladder cancerAnn Oncol201021Suppl 7vii56vii5820943643
- ResnickMJChangSSOptimizing outcomes for octogenarians with invasive bladder cancer: one size does not fit allUrol Oncol20133111423544191
- HershkoACiechanoverAThe ubiquitin systemAnnu Rev Biochem1998674254799759494
- SunSCDeubiquitylation and regulation of the immune responseNat Rev Immunol20088750151118535581
- CaoJYanQHistone ubiquitination and deubiquitination in transcription, DNA damage response, and cancerFron Oncol2012226
- KomanderDClagueMJUrbeSBreaking the chains: structure and function of the deubiquitinasesNat Rev Mol Cell Biol200910855056319626045
- Reyes-TurcuFEVentiiKHWilkinsonKDRegulation and cellular roles of ubiquitin-specific deubiquitinating enzymesAnnu Rev Biochem20097836339719489724
- LiMChenDShilohADeubiquitination of p53 by HAUSP is an important pathway for p53 stabilizationNature2002416688164865311923872
- YuanJLuoKZhangLChevilleJCLouZUSP10 regulates p53 localization and stability by deubiquitinating p53Cell2010140338439620096447
- BrummelkampTRNijmanSMDiracAMBernardsRLoss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaBNature2003424695079780112917690
- WertzIEO’RourkeKMZhouHDe-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signallingNature2004430700069469915258597
- TzimasCMichailidouGArsenakisMKieffEMosialosGHatzivassiliouEGHuman ubiquitin specific protease 31 is a deubiquitinating enzyme implicated in activation of nuclear factor-kappaBCell Signal2006181839216214042
- SchweitzerKBozkoPMDubielWNaumannMCSN controls NF-kappaB by deubiquitinylation of IkappaBalphaEMBO J20072661532154117318178
- EnesaKZakkarMChaudhuryHNF-kappaB suppression by the deubiquitinating enzyme Cezanne: a novel negative feedback loop in pro-inflammatory signalingJ Biol Chem2008283117036704518178551
- XuGTanXWangHUbiquitin-specific peptidase 21 inhibits tumor necrosis factor alpha-induced nuclear factor kappaB activation via binding to and deubiquitinating receptor-interacting protein 1J Biol Chem2010285296997819910467
- YeYAkutsuMReyes-TurcuFEnchevRIWilkinsonKDKomanderDPolyubiquitin binding and cross-reactivity in the USP domain deubiquitinase USP21EMBO Rep201112435035721399617
- NakagawaTKajitaniTTogoSDeubiquitylation of histone H2A activates transcriptional initiation via trans-histone cross-talk with H3K4 di- and trimethylationGenes Dev2008221374918172164
- ZhangJChenCHouXIdentification of the E3 deubiquitinase ubiquitin-specific peptidase 21 (USP21) as a positive regulator of the transcription factor GATA3J Biol Chem2013288139373938223395819
- PengLHuYChenDJiaoSSunSUbiquitin specific peptidase 21 regulates interleukin-8 expression, stem-cell like property of human renal cell carcinomaOncotarget2016727420074201627259257
- YuHSimonsDLSegallIPRC2/EED-EZH2 complex is up-regulated in breast cancer lymph node metastasis compared to primary tumor and correlates with tumor proliferation in situPLoS One2012712e5123923251464
- YuMAKiangAWang-RodriguezJNicotine promotes acquisition of stem cell and epithelial-to-mesenchymal properties in head and neck squamous cell carcinomaPLoS One2012712e5196723300583
- Martinez-FernandezMRubioCSegoviaCLopez-CalderonFFDuenasMParamioJMEZH2 in bladder cancer, a promising therapeutic targetInt J Mol Sci20151611271072713226580594
- WarrickJIRamanJDKaagMEnhancer of zeste homolog 2 (EZH2) expression in bladder cancerUrol Oncol2016346258.e1e6
- FanYMaoRYuYUSP21 negatively regulates antiviral response by acting as a RIG-I deubiquitinaseJ Exp Med2014211231332824493797
- HerideCRigdenDJBertsoulakiEThe centrosomal deubiquitylase USP21 regulates Gli1 transcriptional activity and stabilityJ Cell Sci2016129214001401327621083
- PannuJBelleJIForsterMUbiquitin specific protease 21 is dispensable for normal development, hematopoiesis and lymphocyte differentiationPLoS One2015102e011730425680095
- DuZTongXYeXCyclin D1 promotes cell cycle progression through enhancing NDR1/2 kinase activity independent of cyclin-dependent kinase 4J Biol Chem201328837266782668723897809
- LeontievaOVBlagosklonnyMVCDK4/6-inhibiting drug substitutes for p21 and p16 in senescence: duration of cell cycle arrest and MTOR activity determine geroconversionCell Cycle201312183063306923974099
- BendrisNLemmersBBlanchardJMCell cycle, cytoskeleton dynamics and beyond: the many functions of cyclins and CDK inhibitorsCell Cycle201514121786179825789852
