Abstract
Background
Protective effects of several chemopreventive agents (CPAs) against colorectal adenomas have been well documented in randomized controlled trials (RCTs); however, there is uncertainty regarding which agents are the most effective.
Methods
We searched for RCTs published up until September 2016. Retrieved trials were evaluated using risk of bias. We performed both pairwise analysis and network meta-analysis (NMA) of RCTs to compare the effects of CPAs on the recurrence of colorectal adenomas (primary outcome). Using NMA, we ranked CPAs based on efficacy.
Results
We identified 20 eligible RCTs enrolling 12,625 participants with a history of colorectal cancer or adenomas who were randomly assigned to receive either a placebo or one of 12 interventions. NMA using all trials demonstrated that celecoxib 800 mg/day (relative risk [RR] 0.61, 95% confidence interval [CI] 0.45–0.83), celecoxib 400 mg/day (RR 0.70, 95% CI 0.55–0.87), low-dose aspirin (RR 0.75, 95% CI 0.59–0.96) and calcium (RR 0.81, 95% CI 0.69–0.96) were significantly associated with a reduction in the recurrence of any adenomas. NMA results were consistent with those from pairwise meta-analysis. The evidence indicated a high (celecoxib), moderate (low-dose aspirin) and low (calcium) Grading of Recommendations, Assessment, Development and Evaluation (GRADE) quality. NMA ranking showed that celecoxib 800 mg/day and celecoxib 400 mg/day were the best CPAs, followed by low-dose aspirin and calcium. Considering advanced adenoma recurrence, only celecoxib 800 mg/day and celecoxib 400 mg/day were demonstrated to have a protective effect (RR 0.37, 95% CI 0.27–0.52 vs RR 0.48, 95% CI 0.38–0.60, respectively).
Conclusion
The available evidence from NMA suggests that celecoxib is more effective in reducing the risk of recurrence of colorectal adenomas, followed by low-dose aspirin and calcium. Since cyclooxygenase-2 (COX-2) inhibitors (eg, celecoxib) are associated with important cardiovascular events and gastrointestinal harms, more attention is warranted toward CPAs with a favorable benefit-to-risk ratio, such as low-dose aspirin and calcium.
Introduction
Colorectal cancer (CRC) is among the most common forms of cancer in the world, with ~1.36 million new cases in 2012Citation1; it is the fourth leading cause of cancer death worldwide.Citation1 The burden of CRC in terms of mortality, morbidity and costs is enormous for the community.Citation2,Citation3 Moreover, CRC-related mortality is increasing owing to the late stage at which many cases present.Citation4 Therefore, effort is required to find effective ways to prevent this condition.
It is widely accepted that adenomas/polyps are precursors of CRC via adenoma–carcinoma sequence.Citation5 Hence, colorectal adenomas are considered as a reasonable surrogate end point for trials in this area, especially in subjects with a history of CRC or adenomas, for whom the incidence rates are known to be higher than those in the general population.Citation6,Citation7 Early detection and removal of pre-cancerous colorectal adenomas by screening, followed by appropriate therapy and continued surveillance, can decrease mortality.Citation8 Although many screening interventions are available for the detection and removal of asymptomatic adenomas and finding the early stages of CRC, their uptake continues to be low.Citation9 Moreover, even after the removal of adenomas, the recurrence rate is reasonably high.Citation10–Citation12 Acceptance of continual screening recommendations involves a large volume of health care resources; its attainment will also depend on a high adherence rate and consistent follow-up. Therefore, increased attention is being given to the possible use of chemopreventive agents (CPAs) as a complement to, or substitute for, screening.
In the light of cyclooxygenase-2 (COX-2) overexpression associated with CRC tissue,Citation13 nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin,Citation14–Citation25 have been the most highly researched drugs in the prevention of recurrent colorectal adenomas. However, many other potential CPAs have been investigated, ranging from calcium with or without vitamin DCitation10,Citation26–Citation29 to micronutrients, such as folic acid and antioxidants.Citation18,Citation30–Citation36
Despite evidence of the effectiveness of COX-2 inhibitors and of aspirin at any dose in preventing colorectal adenomas, these agents are associated with important cardiovascular eventsCitation37–Citation41 and gastrointestinal harms.Citation42,Citation43 Low-dose aspirin used for cardiovascular protection may provide an additional advantage, as the balance of benefits and risks seems to be more favorable.Citation42,Citation44,Citation45 Recent randomized controlled trials (RCTs)Citation16,Citation17 have demonstrated the moderate beneficial effect of low-dose aspirin on the incidence of adenomas. Similarly, evidence from good quality RCTsCitation46–Citation48 suggests a possible protective effect of calcium supplementation on the recurrence of adenomas, without important adverse effects.Citation49 However, evidence of the comparative advantage of low-dose aspirin and calcium with other potential CPAs on adenoma recurrence is necessary to justify the continuous growth of these agents in this era of stagnant screening acceptance,Citation9,Citation50 limited endoscopic capacityCitation51 and rising health care expenditures.Citation52
Choosing the most effective CPA for the prevention of the recurrence of adenomas in subjects with a history of CRC or adenomas remains an important consideration; however, uncertainty remains in the data informing the best choice. Hence, we performed network meta-analysis (NMA) to compare the effects of competing CPAs on the recurrence of colorectal adenomas. The results of our analysis can provide readers with useful information to guide clinical decision-making in this field.
Methods
Study design
This study was conducted as a part of a systematic review and NMA of CPAs for CRC, which has been registered (registration number: CRD42015025849) with International Prospective Register of Systematic Reviews (PROSPERO) previously.Citation53 A complete description of the parent study design and methods has been published elsewhere.Citation54 The reporting followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.Citation55
Search strategy and study selection
We identified relevant studies by a systematic search of MEDLINE, Embase, Cochrane Central Register of Controlled Trials, CINAHL Plus, International Pharmaceutical Abstracts and ClinicalTrials.gov website from January 2008 to September 2016. We developed the search strategy in MEDLINE and modified it for other databases (Table S1). The search was restricted to studies published from 2008 onward because studies published up to 2007 could be identified from the published systematic reviews.Citation40,Citation42,Citation47–Citation49,Citation56–Citation60 To identify studies not captured by database search, we manually checked the reference lists of published systematic reviews and identified articles. In the present study, we considered all RCTs with a follow-up period or duration of treatment at least 1 year and concerned with demonstrating the efficacy of CPAs (low- or high-dose aspirin [80–325 mg/day] with or without folic acid, folic acid, non-aspirin NSAIDs, vitamin D, calcium with or without vitamin D and any antioxidants) compared to placebo for the prevention of colorectal adenomas. We considered studies for inclusion if participants had a history of CRC or adenomas. Our primary outcome measure was the incidence of any recurrent adenomas. In subgroup analysis, the recurrence of advanced adenomas was considered separately.
Data extraction and quality assessment
Requisite data were extracted independently and in duplicate by two reviewers into a data extraction form (SKV and SMC). If multiple publications of the same trial were retrieved, only the most recent and informative publication was included. Two reviewers (SKV and SMC) independently assessed the risk of bias (ROB) within each study by using a Cochrane ROB instrument.Citation61,Citation62 We evaluated sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other sources of bias. Reviewers resolved disagreements by discussion, and one of two arbitrators adjudicated any unsolved disagreements. When ROB varied across included studies, we stratified studies according to ROB and produced two estimates of the intervention effect: from trials at low ROB and from all studies.
Evidence grading
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approachCitation63,Citation64 adapted to NMACitation65 was used to rate the quality of evidence of estimates (high, moderate, low and very low).
Statistical analysis
For direct comparison, a standard pairwise meta-analysis was conducted by using a random-effects model. If a direct comparison was based on two or more studies, heterogeneity between trials was assessed by considering the I2 statistics.Citation62 An I2 estimate ≥50% was interpreted as evidence of substantial levels of heterogeneity.Citation62 The outcome measure was estimated in relative risk (RR), which is the ratio between the incidences of colorectal adenoma in the intervention arm and those in the control arm along with a 95% confidence interval (CI).
A random-effects NMA was carried out by combining direct and indirect evidences.Citation66 A model with either consistency or inconsistency was assessed in Stata version 14.0 (StataCorp, College Station, TX, USA) by contrasting direct and indirect estimates in each triangular loop using the methods described by Veroniki et al.Citation67 Network inconsistency assumption, which refers to a disagreement between the direct and indirect estimates, was evaluated using the loop-specific approach described by Bucher et al.Citation68 We also used the design-by-treatment interaction approach and node-splitting technique to assess the network inconsistency.Citation68 The design-by-treatment interaction approach was used to assess inconsistency globally, while the Bucher et alCitation68 method and node-splitting techniqueCitation67 were used to assess inconsistency locally in all closed loops. To rank the intervention hierarchy in NMA, the rankograms, the surface area under the cumulative ranking (SUCRA) curves as proposed by Salanti et alCitation69 and the mean ranks were estimated. The number-needed-to-treat was calculated in order to provide readers with information on the absolute effect of treatment on patients’ outcome.
Excluding treatments in NMAs can occasionally have the largest potential to change the results and reduce the applicability and usefulness of NMA.Citation70 However, excluding treatment that is unimportant from a clinical point of view is justifiable.Citation70 Although COX-2 inhibitors seem to be more effective in preventing recurrence of adenomas, the balance of benefits to risk does not favor chemoprevention.Citation40 Hence, we performed the sensitivity analyses separately for low-bias risk trials and all trials (low- and high-bias risk trials) concerning the efficacy of CPAs, excluding COX-2 inhibitors, to establish the best evidence for potential interventions other than COX-2 inhibitors for adenoma prevention. Publication bias was examined with a comparison-adjusted funnel plot.
Results
Search findings
A flow diagram depicting the search and selection process is provided in Figure S1. Overall, 3,985 records were identified by searching databases and additional records. Ultimately, we identified 20 potentially eligible RCTs for inclusion in our analysis.Citation10,Citation16–Citation21,Citation26–Citation36,Citation71,Citation72 Another 14 relevant articles were identified for CPAs but did not meet the eligibility criteria and were excluded with reasons (Table S2).Citation14,Citation15,Citation22–Citation25,Citation73–Citation80
Characteristics of the included studies
All included studies were randomized and specific for the prevention of recurrent colorectal adenomas. Descriptions of included studies, interventions and outcomes are summarized in Tables S3–Table S5. A total of 12,625 participants who completed the follow-up colonoscopy and reported any adenoma recurrence were included in the main analysis. All trials included both men and women with a history of adenomas, except in the two-group randomization of the Baron et alCitation28 2015 study, where women were elected to be randomly assigned to receive either calcium or calcium plus vitamin D. The length of follow-up from recruitment to study was up to 2–3 years in ten trials and 4–5 years in six trials, whereas the follow-up of the remaining small trials ranged from ~1.5 to 15 years. Most of the CPA or combinations of CPA involved in comparison with placebo were any antioxidants (six trials), followed by calcium (five trials), high-dose aspirin (four trials), low-dose aspirin (three trials), folic acid (three trials), celecoxib 400 mg/day (two trials) and low-dose and high-dose aspirin plus folic acid (two trials); only one trial was available for the remaining interventions (celecoxib 800 mg/day, vitamin D, calcium plus vitamin D and aspirin plus calcium plus vitamin D). The dose ranges per day for CPAs were as follows: calcium – 500–2,000 mg (elemental calcium), high-dose aspirin – 300–325 mg, low-dose aspirin – 80–160 mg and folic acid – 0.5–1 mg; celecoxib was used in doses of either 400 or 800 mg. All trials of antioxidants used comparisons of one or more antioxidants against placebo, and the doses were highly varied.
Quality of included studies
A summary of ROB of included RCTs and an ROB graph are presented in Table S6 and Figure S2. Among 20 RCTs, 13 had low ROB in most of the criteria and were categorized as RCTs with low ROB in our analysis.Citation16–Citation21,Citation26,Citation28,Citation30,Citation31,Citation34,Citation72,Citation81 The remaining seven trials showed either unclear or high ROB according to most criteria.Citation27,Citation29,Citation32,Citation33,Citation35,Citation36,Citation71 Because of differences in ROB, we carried out a sensitivity analysis by restricting to RCTs with low ROB.
Network consistency
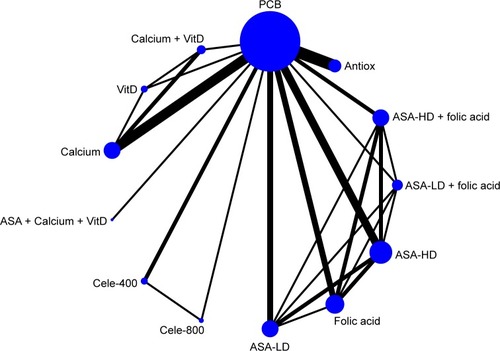
The network of eligible comparisons for any adenoma incidence from all trials is shown in . Graphical representations of all other networks are presented in Figures S3 and S4). An assessment of inconsistency is presented in detail in Tables S7–S9. The test of global inconsistency showed no significant difference between the consistency and inconsistency models (Table S7). Tests of local inconsistency using the loop-specific approach and the node-splitting model showed no significant differences between comparisons in both outcomes (Tables S8 and S9).
Figure 1 Network plot for incidence of any adenomas.
Abbreviations: Antiox, antioxidants; ASA-HD, high-dose aspirin; ASA-LD, low-dose aspirin; Calcium, calcium supplements; Cele-400, celecoxib 400 mg/day; Cele-800, celecoxib 800 mg/day; PCB, placebo; VitD, vitamin D.
Efficacy of CPAs on any adenoma recurrence from pairwise meta-analysis
The results of any adenoma recurrence from single RCTs and standard pairwise meta-analysis of direct comparisons and their corresponding statistical heterogeneity are presented in full in Table S10. Out of 12 interventions, meta-analysis of efficacy was feasible for eight CPAs (any antioxidants, folic acid, calcium, celecoxib 400 mg/day and low- or high-dose aspirin with or without folic acid), for which at least two datasets were available. Among these eight CPAs, a random-effects meta-analysis of all trials showed that only celecoxib 400 mg/day (RR 0.70, 95% CI 0.64–0.76), low-dose aspirin (RR 0.83, 95% CI 0.70–0.99) and calcium (RR 0.83, 95% CI 0.75–0.93) were significantly associated with a reduction in recurrence of any adenomas compared to placebo. Restricting the analysis to trials with low bias risk demonstrated similar results.
In two factorial trials,Citation16,Citation30 comparison of low-dose aspirin versus high-dose aspirin showed that no interventions appeared to be superior in reducing adenomas (RR 0.67, 95% CI 0.37–1.21). However, in one RCT,Citation30 low-dose aspirin plus folic acid demonstrated a significant association in reducing any adenomas compared to high-dose aspirin plus folic acid (RR 0.75, 95% CI 0.58–0.98), but not with placebo (RR 0.80, 95% CI 0.61–1.05). Another trial presented two sets of data for the comparison of calcium alone versus calcium plus vitamin D (factorial arm and two-arm data; provided by the author on request)Citation28 and demonstrated no statistically significant association with the reduction in any adenomas (RR 0.98, 95% CI 0.86–1.12).
Finally, among the remaining CPAs (celecoxib 800 mg/day, vitamin D and calcium plus vitamin D) with only one RCT available, celecoxib 800 mg/day was statistically significantly associated with a reduction in the recurrence of any adenomas (RR 0.62, 95% CI 0.55–0.69) compared to placebo. In a recent RCT,Citation72 the combination of low-dose aspirin, calcium and vitamin D was compared with placebo, but no statistically significant association was observed in the reduction of any adenomas (RR 0.94, 95% CI 0.68–1.29).
Efficacy of CPAs on adenoma recurrence from NMA
In order to compare and rank CPAs based on their effects on adenoma recurrence, we performed the NMA of the relevant RCTs. Network meta-analytic results on adenoma recurrence from all trials were reasonably comparable with those from standard pairwise meta-analysis (). According to the findings from the NMA using all trials, celecoxib 800 mg/day (RR 0.61, 95% CI 0.45–0.83), celecoxib 400 mg/day (RR 0.70, 95% CI 0.55–0.87), low-dose aspirin (RR 0.75, 95% CI 0.59–0.96) and calcium (RR 0.81, 95% CI 0.68–0.97) were statistically significantly associated with the reduction in any recurrent adenomas. The number needed to treat (NNT) was 6, 8, 10 and 13 for celecoxib 800 mg/day, celecoxib 400 mg/day, low-dose aspirin and calcium, respectively. Since we observed varying adenoma recurrence rates in different RCTs, these values do not necessarily reflect the magnitude of the RR associated with the corresponding agents.
Figure 2 Pairwise (upper right portion) and network (lower left portion) meta-analytic results for incidence of any adenomas (all studies).
Abbreviations: Antiox, antioxidants; ASA-HD, high-dose aspirin; ASA-LD, low-dose aspirin; Calcium, calcium supplements; Cele-400, celecoxib 400 mg/day; Cele-800, celecoxib 800 mg/day; CI, confidence interval; NA, not applicable; NMA, network meta-analysis; PCB, placebo; RR, relative risk; VitD, vitamin D.
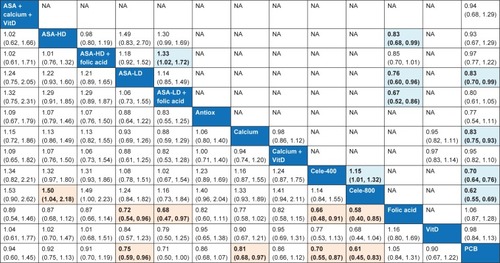
The results of NMA (estimated RR and SUCRA rank) for CPAs on the incidence of adenomas from all trials are given in Table S11 and Figure S5. SUCRA curves for any adenoma incidence are presented in Figure S6.
Subgroup analyses: advanced adenoma recurrence
A meta-analysis of all trials using a random-effects model informing the efficacy on advanced adenomas demonstrated that celecoxib 800 mg/day (RR 0.37, 95% CI 0.26–0.51) and celecoxib 400 mg/day (RR 0.48, 95% CI 0.38–0.60) were statistically significantly associated with a reduction in the recurrence of advanced adenomas compared to placebo; however, low-dose aspirin (RR 0.84, 95% CI 0.46–1.55) and calcium (RR 1.01, 95% CI 0.74–1.38) were not associated with reduced advanced adenoma risk. Restricting the analysis to trials with low bias risk demonstrated similar results. The results of pairwise meta-analyses on advanced adenoma are presented in Table S10.
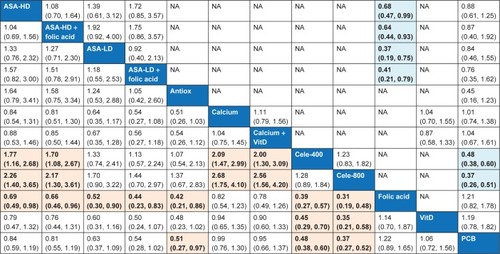
Pairwise and NMA results for the incidence of advanced adenomas from all studies are presented in . According to the NMA results on the advanced adenoma recurrence from all low-bias risk trials, only two CPAs (celecoxib 800 mg/day [RR 0.37, 95% CI 0.27–0.52] and celecoxib 400 mg/day [RR 0.48, 95% CI 0.38–0.60]) demonstrated evidence of efficacy in reducing advanced adenoma recurrence. Meanwhile, low-dose aspirin demonstrated only a moderately significant association in reducing advanced adenoma recurrence (RR 0.63, 95% CI 0.37–1.09). Results of NMA for CPAs on the incidence of advanced adenoma and the corresponding SUCRA curves are presented in Table S12 and Figures S7 and S8, respectively.
Figure 3 Pairwise (upper right portion) and network (lower left portion) meta-analytic results for incidence of advanced adenomas (all studies).
Abbreviations: Antiox, antioxidants; ASA-HD, high-dose aspirin; ASA-LD, low-dose aspirin; Calcium, calcium supplements; Cele-400, celecoxib 400 mg/day; Cele-800, celecoxib 800 mg/day; CI, confidence interval; NA, not applicable; NMA, network meta-analysis; PCB, placebo; RR, relative risk; VitD, vitamin D.
Sensitivity analyses
The results from multiple sensitivity analyses are reported in Table S13 and S14 and Figures S9 and S10. Overall, the results were justifiably robust to the main analysis for each outcome based on excluding RCTs on celecoxib and restricting to RCTs with low ROB with or without celecoxib. The most important sensitivity analysis was the exclusion of seven RCTs, which exhibited either unclear or high ROB in most criteria. Restricting these RCTs did not have a marked effect on the results, although low-dose aspirin plus folic acid now showed a statistically significant reduction (RR 0.74, 95% CI 0.58–0.94) in adenoma recurrence compared to the main analysis (Table S13). Sensitivity analyses after excluding RCTs on celecoxib showed that low-dose aspirin with and without folic acid was ranked first and second, respectively, followed by calcium; however, no statistically significant association was demonstrated for both adenomas (Table S13) and advanced adenomas (Table S14).
GRADE summary of evidence
A summary of findings and strength of evidence from network meta-analyses for important CPAs demonstrating the evidence of efficacy in reducing adenoma recurrence is shown in Table S15. Using all included trials, our application of GRADE methodology that specifically adapted to NMACitation65 led us to conclude that the accumulated evidence for CPAs is as follows: celecoxib (high quality), low-dose aspirin (moderate quality) and calcium (low quality).
Publication bias
Comparison-adjusted funnel plots for each outcome from the network meta-analyses are provided in Figures S11 and S12. Comparison adjusted plots showed no evidence of district asymmetry.
Discussion
To our knowledge, we performed the first NMA of CPAs in the prevention of recurrent colorectal adenomas as a part of our systematic review of CPAs for CRC prevention, which has been registered with PROSPERO previously.Citation53 The currently available best evidence of adenoma prevention is based on the results of RCTs and standard meta-analyses of single CPAs or CPA classes. In the present systematic review, we captured evidence from 20 RCTs evaluating the role of 12 interventions (six CPAs with different doses and combinations) in >12,000 subjects with a history of CRC or adenomas, which makes the present review the largest ever analyzed in this field.
Pairwise meta-analytic results demonstrated that celecoxib 400 mg/day, low-dose aspirin and calcium were associated with a significant reduction in any adenomas. According to the evidence grading system using trials with low ROB, the level of evidence supporting the efficacy of these agents against any adenoma was high for celecoxib 400 mg/day, moderate for low-dose aspirin and low for calcium. The network meta-analytic results in terms of incidence of recurrent adenomas from all trials were justifiably comparable with those from standard pairwise meta-analysis. Using all trials and low-bias risk trials in NMA, we ranked five CPAs for which we found evidence of adenoma prevention (celecoxib 800 mg/day, celecoxib 400 mg/day, low-dose aspirin plus folic acid, low-dose aspirin and calcium). However, folic acid alone or in combination with aspirin (low- or high dose) did not show any significant effects on adenoma incidence in previous studies;Citation18,Citation49,Citation58,Citation82 this suggests that the effect of low-dose aspirin plus folic acid as shown by our NMA could be due to low-dose aspirin alone. Hence, we suggest that evidence of therapeutic activity in terms of efficacy for the secondary prevention of any adenoma recurrence was available for only four CPAs, in which celecoxib 400 mg/day and celecoxib 800 mg/day are the best candidates, followed by low-dose aspirin and calcium. The level of evidence supporting the efficacy of these CPAs using GRADE methodology adapted for NMA was high for celecoxib, moderate for low-dose aspirin and low for calcium.Citation65
COX-2 inhibitors (celecoxib 400–800 mg/day) had a greater protective effect than low-dose aspirin and calcium in reducing the incidence of any recurrent colorectal adenomas compared to placebo. However, the beneficial effect of COX-2 inhibitors as shown in RCTs did not persist during the posttreatment period,Citation20–Citation22 and an increased risk of adenoma incidence was observed ~1–2 years after treatment cessation.Citation22,Citation74 Moreover, the risk of gastrointestinalCitation83,Citation84 and cardiovascularCitation38–Citation40,Citation49,Citation85,Citation86 harms associated with these CPAs as shown in previous reviews does not appear to favor these drugs as CPAs.
To establish the best evidence for potential interventions other than COX-2 inhibitors for adenoma prevention, we excluded celecoxib and analyzed the data separately. A sensitivity analysis showed that low-dose aspirin (with or without folic acid) was ranked first, followed by calcium. Although calcium supplements modestly increase bone densityCitation87 and have a marginal efficacy against fracture,Citation88,Citation89 the risk of cardiovascular events, especially myocardial infarction,Citation90,Citation91 suggests that a reassessment of the role of calcium as a CPA is warranted. Meanwhile, high-quality evidence has shown that aspirin can decrease serious adverse events in patients at increased risk of cardiovascular disease.Citation92 Although aspirin demonstrated a dose-dependent effect relating to the risks of gastrointestinal toxicity and hemorrhagic stroke,Citation41–Citation43 the use of low-dose aspirin in these individuals would result in positive cardiovascular effects and fewer adverse outcomes, and they would obtain the added benefit of fewer colorectal adenomas, as shown by our analysis. However, low-dose aspirin failed to show a significant protective effect on advanced adenomas. Based on the existing good quality RCTs, only celecoxib demonstrated a protective effect against advanced adenomas in our NMA. The nonsignificant effect of low-dose aspirin on advanced adenoma could be due to the small information size (only 373 patients) and inconsistent low control event rate (1.2%–8.5%) in the three conducted trials used for our analysis.Citation16,Citation17,Citation30 More high-quality RCTs comparing low-dose aspirin versus placebo are still needed to conclude the evidence for low-dose aspirin on both adenomas and advanced adenomas.
With regard to CPAs for which no evidence of adenoma prevention is available (antioxidants, folic acid, high-dose aspirin, low-dose aspirin plus calcium plus vitamin D and vitamin D alone), some considerations are needed. For any antioxidant and folic acid, the results from trials having a low ROB were consistent with the previous systematic reviews,Citation49,Citation60,Citation82 which demonstrated no evidence of reduction in any adenoma recurrence. For aspirin, there is no apparent explanation for the absence of a dose–response pattern, as seen in the case of celecoxib (400 mg/day and 800 mg/day), and the surprising lack of efficacy of the high-dose aspirin. A recent RCT demonstrated that the use of high-dose aspirin for 4 years increased the risk of adenomas, but not for 1 year;Citation16 this observation suggests the need for a reassessment of preventive potential based on the duration of use. Although low-dose aspirin and calcium demonstrated a possible protective effect against adenomas, surprisingly, in one RCT, the combination of these agents (low-dose aspirin 75 mg plus elemental calcium 500 mg plus vitamin D) showed no significant effect.Citation72 A possible explanation for this finding could be due to the use of lower doses of aspirin and calcium compared to previous studies (low-dose aspirin 81–160 mg; elemental calcium 1,200–2,000 mg) that showed a possible protective effect for these agents.Citation14,Citation16,Citation17,Citation26,Citation81 Similarly, the lack of any effect of vitamin D observed in the present analysis was consistent with the given results from the recent RCT.Citation28
There are some limitations to this systematic review. There were few good quality RCTs, and the sample sizes of many interventional studies were small. We could not confirm the comparative advantage of all CPAs for which evidence of activity against adenomas is available over other interventions. Moreover, we could not demonstrate a protective effect against advanced adenomas for most CPAs due to insufficient information size from the trials. Furthermore, because the follow-up of studies was not sufficiently long, we could not explore the long-term effects of CPAs on the recurrence of adenomas and the progression to cancer.
Conclusion
The available evidence from NMA suggests that celecoxib is more effective in reducing the risk of recurrence of colorectal adenomas in patients with a previous history of CRC or adenomas, followed by low-dose aspirin and calcium. Since COX-2 inhibitors (eg, celecoxib) are associated with important cardiovascular events and gastrointestinal harms, more attention should be paid to CPAs with favorable benefit to risk ratio, such as low-dose aspirin and calcium. However, cardiovascular adverse effects associated with calcium supplementation in the light of new evidence and the deficiency of data informing the appropriate dose of aspirin (80 or 160 mg/day) in terms of efficacy and acceptability hamper recommendations concerning the use of these agents. More high-quality RCTs for low-dose aspirin and calcium are still needed in order to confirm their efficacy and acceptability in the secondary prevention of the recurrence of colorectal adenomas and advanced adenomas.
Author contributions
SKV drafted the protocol. NC revised the protocol. SKV and KGL coordinated the identification of trials. SKV and SMC conducted the trial selection and the data extraction. SKV and SMC independently assessed the ROB. NT and SKV conducted the standard statistical analyses, which were appraised by SS and NC. SKV and NT drafted the review. NT, KGL, SMC, SS, PP and NC revised the review. All authors participated in the interpretation of analyses, revised and commented on the article and approved the final version of the manuscript.
Acknowledgments
The authors wish to thank Prof Dato’ Dr (Mrs) Kew Siang Tong, School of Medicine, International Medical University, and Dr Muhammad Radzi bin Abu Hassan, Head of Gastroenterology Service, Ministry of Health, Malaysia, for their expertise and advice during the development of the protocol. The authors wish to thank Prof Brian L Furman, Strathclyde Institute of Pharmacy and Biomedical Sciences, Glasgow, UK, for his valuable comments and support, which helped to improve the manuscript. The authors also wish to thank Mr Razman Shah MohdRazali, reference librarian, International Medical University, for providing the full-text articles whenever needed.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- ArnoldMSierraMSLaversanneMSoerjomataramIJemalABrayFGlobal patterns and trends in colorectal cancer incidence and mortalityGut201766468369126818619
- VeettilSKLimKGChaiyakunaprukNChingSMHassanMRAColorectal cancer in Malaysia: its burden and implications for a multiethnic countryAsian J Surg Epub201681
- SiegelRDeSantisCJemalAColorectal cancer statistics, 2014CA Cancer J Clin201464210411724639052
- U.S. Preventive Services Task ForceRoutine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statementAnn Intern Med2007146536136417339621
- LevinBPotential pitfalls in the use of surrogate endpoints in colorectal adenoma chemopreventionJ Natl Cancer Inst2003951069769912759378
- YamajiYMitsushimaTIkumaHIncidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic JapaneseGut200453456857215016753
- LevinBLiebermanDAMcFarlandBScreening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of RadiologyCA Cancer J Clin200858313016018322143
- CDC [webpage on the Internet]Colorectal Cancer Screening Rates Remain LowThe Centers for Disease Control and Prevention (CDC)2013 Available from: http://www.cdc.gov/media/releases/2013/p1105-colorectal-cancer-screening.htmlAccessed December 15, 2016
- Bonithon-KoppCPiardFFengerCColorectal adenoma characteristics as predictors of recurrenceDis Colon Rectum200447332333314991494
- CottetVJoosteVFournelIBouvierA-MFaivreJBonithon-KoppCLong-term risk of colorectal cancer after adenoma removal: a population-based cohort studyGut20126181180118622110052
- StrumWBColorectal adenomasN Engl J Med2016374111065107526981936
- WangDDuBoisRNThe role of COX-2 in intestinal inflammation and colorectal cancerOncogene201029678178819946329
- BaronJAColeBFSandlerRSA randomized trial of aspirin to prevent colorectal adenomasN Engl J Med20033481089189912621133
- BenamouzigRDeyraJMartinADaily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trialGastroenterology2003125232833612891533
- BenamouzigRUzzanBDeyraJPrevention by daily soluble aspirin of colorectal adenoma recurrence: 4-year results of the APACC randomised trialGut201261225526121890814
- IshikawaHMutohMSuzukiSThe preventive effects of low-dose enteric-coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trialGut201463111755175924488498
- LoganRFAGraingeMJShepherdVCArmitageNCMuirKRukCAP Trial GroupAspirin and folic acid for the prevention of recurrent colorectal adenomasGastroenterology20081341293818022173
- SandlerRSHalabiSBaronJAA randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancerN Engl J Med20033481088389012621132
- ArberNEagleCJSpicakJCelecoxib for the prevention of colorectal adenomatous polypsN Engl J Med2006355988589516943401
- BertagnolliMMEagleCJZauberAGCelecoxib for the prevention of sporadic colorectal adenomasN Engl J Med2006355987388416943400
- BaronJASandlerRSBresalierRSA randomized trial of rofecoxib for the chemoprevention of colorectal adenomasGastroenterology200613161674168217087947
- LadenheimJGarciaGTitzerDEffect of sulindac on sporadic colonic polypsGastroenterology19951084108310877698575
- MeyskensFLMcLarenCEPelotDDifluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trialCancer Prev Res (Phila)200811323818841250
- TakayamaTNagashimaHMaedaMRandomized double-blind trial of sulindac and etodolac to eradicate aberrant crypt foci and to prevent sporadic colorectal polypsClin Cancer Res201117113803381121385928
- BaronJABeachMMandelJSCalcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study GroupN Engl J Med199934021011079887161
- HofstadBAlmendingenKVatnMGrowth and recurrence of colorectal polyps: a double-blind 3-year intervention with calcium and antioxidantsDigestion19985921481569586828
- BaronJABarryELMottLAA trial of calcium and vitamin D for the prevention of colorectal adenomasN Engl J Med2015373161519153026465985
- ChuDZJHusseyMAAlbertsDSColorectal Chemoprevention Pilot Study (SWOG-9041), randomized and placebo controlled: the importance of multiple luminal lesionsClin Colorectal Cancer201110431031621782524
- ColeBFBaronJASandlerRSFolic acid for the prevention of colorectal adenomas: a randomized clinical trialJAMA2007297212351235917551129
- MacLennanRMacraeFBainCRandomized trial of intake of fat, fiber, and beta carotene to prevent colorectal adenomasJ Natl Cancer Inst19958723176017667473832
- BonelliLPuntoniMGatteschiBAntioxidant supplement and long-term reduction of recurrent adenomas of the large bowel. A double-blind randomized trialJ Gastroenterol201348669870523065023
- WuKPlatzEAWillettWCA randomized trial on folic acid supplementation and risk of recurrent colorectal adenomaAm J Clin Nutr20099061623163119864409
- GreenbergERBaronJATostesonTDA clinical trial of anti-oxidant vitamins to prevent colorectal adenomaN Engl J Med199433131411478008027
- McKeown-EyssenGHollowayCJazmajiVBright-SeeEDionPBruceWRA randomized trial of vitamins C and E in the prevention of recurrence of colorectal polypsCancer Res19884816470147053293777
- Ponz de LeonMRoncucciLChemoprevention of colorectal tumors: role of lactulose and of other agentsScand J Gastroenterol Suppl199722272759145453
- Gomez CerezoJLubomirov HristovRCarcas SansuánAJVázquez RodríguezJJOutcome trials of COX-2 selective inhibitors: global safety evaluation does not promise benefitsEur J Clin Pharmacol200359216917512698301
- JüniPNarteyLReichenbachSSterchiRDieppePAEggerMRisk of cardiovascular events and rofecoxib: cumulative meta-analysisLancet200436494502021202915582059
- MukherjeeDNissenSETopolEJRisk of cardiovascular events associated with selective COX-2 inhibitorsJAMA2001286895495911509060
- RostomADubéCLewinGNonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task ForceAnn Intern Med2007146537638917339623
- SerebruanyVLSteinhublSRBergerPBAnalysis of risk of bleeding complications after different doses of aspirin in 192,036 patients enrolled in 31 randomized controlled trialsAm J Cardiol200595101218122215877994
- DubéCRostomALewinGThe use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task ForceAnn Intern Med2007146536537517339622
- Massó GonzálezELPatrignaniPTacconelliSGarcía RodríguezLAVariability among nonsteroidal antiinflammatory drugs in risk of upper gastrointestinal bleedingArthritis Rheum20106261592160120178131
- Bibbins-DomingoKU.S. Preventive Services Task ForceAspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. preventive services task force recommendation statementAnn Intern Med20161641283684527064677
- CampbellCLSmythSMontalescotGSteinhublSRAspirin dose for the prevention of cardiovascular disease: a systematic reviewJAMA2007297182018202417488967
- BonovasSFiorinoGLytrasTMalesciADaneseSCalcium supplementation for the prevention of colorectal adenomas: a systematic review and meta-analysis of randomized controlled trialsWorld J Gastroenterol201622184594460327182169
- CarrollCCooperKPapaioannouDHindDPilgrimHTappendenPSupplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysisClin Ther201032578980320685491
- ShaukatAScourasNSchünemannHJRole of supplemental calcium in the recurrence of colorectal adenomas: a metaanalysis of randomized controlled trialsAm J Gastroenterol2005100239039415667497
- CooperKSquiresHCarrollCChemoprevention of colorectal cancer: systematic review and economic evaluationHealth Technol Assess201014321206
- GuptaSSussmanDADoubeniCAChallenges and possible solutions to colorectal cancer screening for the underservedJ Natl Cancer Inst20141064dju03224681602
- SteinwachsDAllenJDBarlowWENIH state-of-the-science conference statement: enhancing use and quality of colorectal cancer screeningNIH Consens State Sci Statements2010271131
- Centers for Medicare and Medicaid Services, Office of the Actuary, National Health Statistics Group [webpage on the Internet]National Health Expenditure Data2012 Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.htmlAccessed December 15, 2016
- VeettilSKChaiyakunaprukNSaokaewSGheeLKMooiCSComparative Effectiveness of Chemopreventive Interventions for Colorectal Cancer: Protocol for a Systematic Review and Network Meta-analysis PROSPERO 2015; CRD420150258492015 Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015025849Accessed December 15, 2016
- VeettilSKSaokaewSLimKGChingSMPhisalprapaPChaiyakunaprukNComparative effectiveness of chemopreventive interventions for colorectal cancer: protocol for a systematic review and network meta-analysis of randomised controlled trialsJ Gastrointest Oncol20167459560227563450
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementAnn Intern Med20091514264269W6419622511
- AsanoTKMcLeodRSNon steroidal anti-inflammatory drugs (NSAID) and Aspirin for preventing colorectal adenomas and carcinomasCochrane Database Syst Rev20042CD004079
- BaronJAEpidemiology of non-steroidal anti-inflammatory drugs and cancerProg Exp Tumor Res20033712412795046
- ColeBFLoganRFHalabiSAspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trialsJ Natl Cancer Inst2009101425626619211452
- GaoFLiaoCLiuLTanACaoYMoZThe effect of aspirin in the recurrence of colorectal adenomas: a meta-analysis of randomized controlled trialsColorectal Dis200911989390119055515
- BjelakovicGNagorniANikolovaDSimonettiRGBjelakovicMGluudCMeta-analysis: antioxidant supplements for primary and secondary prevention of colorectal adenomaAliment Pharmacol Ther200624228129116842454
- HigginsJPTAltmanDGGøtzschePCThe Cochrane Collaboration’s tool for assessing risk of bias in randomised trialsBMJ2011343d592822008217
- HigginsJPTGreenS webpage on the InternetCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [Updated March 2011]The Cochrane Collaboration2011 Available from: http://handbook.cochrane.org/Accessed December 15, 2016
- AtkinsDBestDBrissPAGrading quality of evidence and strength of recommendationsBMJ20043287454149015205295
- GuyattGHOxmanADVistGEGRADE: an emerging consensus on rating quality of evidence and strength of recommendationsBMJ2008336765092492618436948
- PuhanMASchünemannHJMuradMHA GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysisBMJ2014349g563025252733
- HoaglinDCHawkinsNJansenJPConducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2Value Health201114442943721669367
- VeronikiAAVasiliadisHSHigginsJPTSalantiGEvaluation of inconsistency in networks of interventionsInt J Epidemiol201342133234523508418
- BucherHCGuyattGHGriffithLEWalterSDThe results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trialsJ Clin Epidemiol19975066836919250266
- SalantiGAdesAEIoannidisJPAGraphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorialJ Clin Epidemiol201164216317120688472
- MillsEJKantersSThorlundKChaimaniAVeronikiA-AIoannidisJPAThe effects of excluding treatments from network meta-analyses: surveyBMJ2013347f519524009242
- RoncucciLDi DonatoPCaratiLAntioxidant vitamins or lactulose for the prevention of the recurrence of colorectal adenomas. Colorectal Cancer Study Group of the University of Modena and the Health Care District 16Dis Colon Rectum19933632272348449125
- PommergaardH-CBurcharthJRosenbergJRaskovHAspirin, calcitriol, and calcium do not prevent adenoma recurrence in a randomized controlled trialGastroenterology2016150111412226404953
- GrauMVSandlerRSMcKeown-EyssenGNonsteroidal anti-inflammatory drug use after 3 years of aspirin use and colorectal adenoma risk: observational follow-up of a randomized studyJ Natl Cancer Inst2009101426727619211442
- ArberNSpicakJRáczIFive-year analysis of the prevention of colorectal sporadic adenomatous polyps trialAm J Gastroenterol201110661135114621503000
- BaronJABeachMMandelJSCalcium supplements and colorectal adenomas. Polyp Prevention Study GroupAnn N Y Acad Sci199988913814510668490
- BertagnolliMMEagleCJZauberAGFive-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib TrialCancer Prev Res (Phila)20092431032119336730
- PalmieriYReduction of the Incidence of Metachronous Adenomas of the Large Bowel by Means of AntioxidantsBrusselsSe-Te Press1998
- LiZYGuJLZengZShiWClinical study of aspirin in the prevention of recurrence of colorectal adenoma in the elderlyChinese J Med Guide20111389
- BenamouzigRUzzanBDeyraJMartinAChaussadeSPrevention by aspirin of colorectal adenoma recurrence: some advances and latest results of the APACC trialCurr Colorectal Cancer Rep2010713341
- JaszewskiRMisraSTobiMFolic acid supplementation inhibits recurrence of colorectal adenomas: a randomized chemoprevention trialWorld J Gastroenterol200814284492449818680228
- Bonithon-KoppCKronborgOGiacosaARäthUFaivreJCalcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study GroupLancet200035692381300130611073017
- Castillo-LancellottiCTur MaríJAUauy DagachRSuplementación con ácido fólico y prevención de recurrencia de adenomas colorrectales; revisión sistemática [Folic acid supplementation and colorectal adenoma recurrence: systematic review]Nutr Hosp20122711321 Spanish22566300
- OfmanJJMacleanCHStrausWLMeta-analysis of dyspepsia and nonsteroidal antiinflammatory drugsArthritis Rheum200349450851812910557
- OfmanJJMacLeanCHStrausWLA metaanalysis of severe upper gastrointestinal complications of nonsteroidal antiinflammatory drugsJ Rheumatol200229480481211950025
- CaldwellBAldingtonSWeatherallMShirtcliffePBeasleyRRisk of cardiovascular events and celecoxib: a systematic review and meta-analysisJ R Soc Med200699313214016508052
- ZhangJDingELSongYAdverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events: meta-analysis of randomized trialsJAMA2006296131619163216968832
- TaiVLeungWGreyAReidIRBollandMJCalcium intake and bone mineral density: systematic review and meta-analysisBMJ2015351h418326420598
- BollandMJLeungWTaiVCalcium intake and risk of fracture: systematic reviewBMJ2015351h458026420387
- TangBMPEslickGDNowsonCSmithCBensoussanAUse of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysisLancet2007370958865766617720017
- BollandMJAvenellABaronJAEffect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysisBMJ2010341c369120671013
- BollandMJGreyAAvenellAGambleGDReidIRCalcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysisBMJ2011342d204021505219
- Antithrombotic Trialists’ CollaborationCollaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patientsBMJ20023247329718611786451
