Abstract
Lung adenocarcinoma is the most common pathological pattern of lung cancer. During the past decades, a number of targeted agents have been explored to treat advanced lung adenocarcinoma. In the present clinical practice, antagonists of the epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF)-directed therapies are widely used. In the former category, the agent erlotinib (tyrosine kinase inhibitor) has shown obvious advantages over cytotoxic therapy. Anti-VEGF therapy bevacizumab used for lung adenocarcinoma was recommended in NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) as first-line therapy. Similarly, apatinib is speculated to response by selectively inhibiting the vascular endothelial growth factor receptor-2. The patient with unknown EGFR status benefited 5-month progressive free survival (PFS) from erlotinib, and then another 5.1-month PFS with combined treatment of apatinib, which suggested a new option for lung adenocarcinoma. However, when dabigatran was used to cancer-related venous thromboembolism during apatinib therapy, extensive subcutaneous bleeding occurred, warning us against the risks of bleeding. Besides, hypertension and anorexia were observed, causing dosage adjustment.
Introduction
Lung cancer is one of the leading causes of morbidity and mortality among cancer patients, posing a major threat to public health.Citation1 In China, lung adenocarcinoma makes the maximum proportion. In Asian non-small cell lung cancer (NSCLC) patients, about 40%–60% epidermal growth factor receptor (EGFR) mutations are detected, for which anti-EGFR agents, erlotinib (F Hoffmann-La Roche Ltd, Basel, Switzerland), are proposed to use. Nowadays, EGFR-tyrosine kinase inhibitor (TKI) resistance is a popular research hotspot.
Apatinib (Hengrui Pharmaceutical Co. Ltd, Jiangsu, People’s Republic of China) is a novel tyrosine kinase inhibitor that selectively inhibits the VEGF-2 and inhibits VEGF-mediated endothelial cell migration and proliferation, thus blocking new blood vessel formation in tumor tissue.Citation2,Citation3 Currently, phase II/III clinical trials in China have approved the indication of chemotherapy-refractory advanced metastatic gastric cancer.Citation4,Citation5 The phase I clinical trial showed a potential targeted treatment for advanced lung cancer, metastatic breast cancer, and advanced hepatocellular carcinoma.Citation6
We here report a case of apatinib’s response in an advanced NSCLC patient with EGFR-TKI resistance, who died of severe pneumonia with disease progression. As far as we know, this is the first report of the combination treatment with apatinib and erlotinib combating acquired EGFR-TKI resistance.
Case report
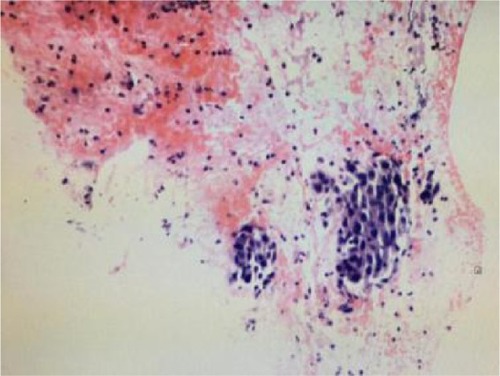
In August 2014, the male patient had a sudden chest distress and cough but without phlegm. Computed tomography (CT) of chest in Beijing Chest Hospital showed a mass in the left upper lung sized ~41×27 mm, with segmented edge, metastasis in mediastinal 4R,7 regions and ipsilateral hilar lymph nodes, and right pulmonary pleura with micronodulaire. Cranial magnetic resonance imaging (MRI), abdominal CT, and bone scans were found normal. On September 28, 2014, adenocarcinoma was diagnosed by fine-needle aspiration biopsy under ultrasonic bronchoscope (). EGFR test was unable to perform because of the small size of specimens. The patient was diagnosed with lung adenocarcinoma on the left upper lobe with poorly differentiated stage IIIb.
From October 3, 2014, to March 3, 2015, the patient was treated with the first-line chemotherapy (gemcitabine 2.2 g days 1 and 8, 8 plus cisplatin 50 mg days 1–3, every 21 days) at Beijing Chest Hospital. The overall efficacy evaluation by thoracic CT was partial response (PR) after six therapy circles. The partial radiotherapy of mediastinum and mass in the left lobe was from April 20 to June 1, 2015, in Cancer Hospital, Chinese Academic of Medical Sciences, and the efficacy evaluation was PR. Left headache with blurred vision occurred on August 26, 2015. Cranial MRI showed metastasis in the left occipital lobe. Whole-brain radiotherapy and dehydration treatment eliminated the syndrome, and MRI showed edema, which was obviously alleviated, but the tumor in the left lobe was increased. Because of unbearable adverse events (AEs), especially gastrointestinal side effects, the patient asked for targeted therapy despite the unknown EGFR status. On September 30, 2015, the patient refused to receive re-biopsy and started taking erlotinib (150 mg/day) as the second-line therapy. Imageological examination demonstrated stable disease, verifying erlotinib was effective to the patient. However, cranial MRI on December 27, 2015, revealed edema aggravated. The patients received gamma knife radiation for brain metastasis in Beijing Tian Tan Hospital on December 30, 2015. Chest CT showed that the solid mass in the left upper lobe was slightly enlarged on February 26, 2016 (). A bone scan suggested bone metastases and the encephaledem slightly deteriorated (), a possible indication of progressive disease (PD) and erlotinib resistance. Combined use of erlotinib and apatinib (250 mg/day) was chosen as the third-line therapy from March 17, 2016 () in China-Japan Friendship Hospital. After approximately a month’s treatment, the chest CT on April 25, 2016, showed cavitation formed in the solid mass of the left lobe, and the solid mass was significantly reduced with stable mediastinal lymph node (). Cranial MRI on April 27, 2016, showed edema in the left occipital lobe alleviated (). Monthly imageological examination revealed the effectiveness of the combination treatment ( and ). Circulation tumor cell from the patient’s plasma reported 3.87 FU/3 mL within normal limits on July 7, 2016, whereas 30.1 FU/3 mL on August 29, 2016, beyond normal limits. The contrast might imply disease progression. Ultimately, another 5.1-month PFS was achieved and severe pneumonia took away the patient’s life with disease progression on August 31, 2016.
Figure 2 Thoracic computed tomography (CT) showing a mass in the left upper lobe before taking apatinib (A), an obvious cavitation in the mass (B), mass increasing after apatinib suspension for 6 days (C), cavitation extending gradually with added dose of apatinib via monthly CT examination (D and E), and an overall increase of mass despite the larger cavitation (F). The red arrows indicate specific region of tumor.
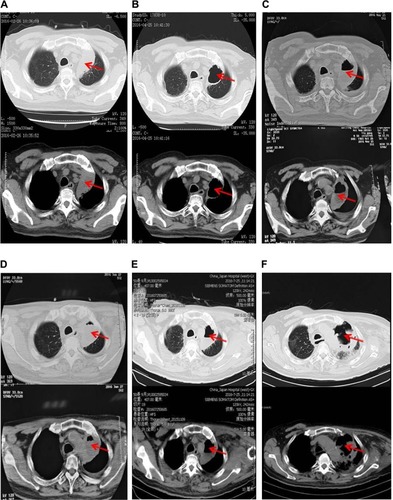
Figure 3 Cranial MRI showing the encephaledem in the occipital lobe of the head (A), encephaledem obviously alleviating (B), slightly worsening after suspension of apatinib for 6 days (C), the encephaledem basically under control as shown by CT every 2 months (D and E).
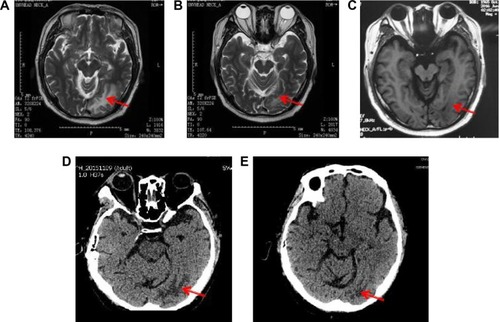
Table 1 Medication of apatinib combined with erlotinib
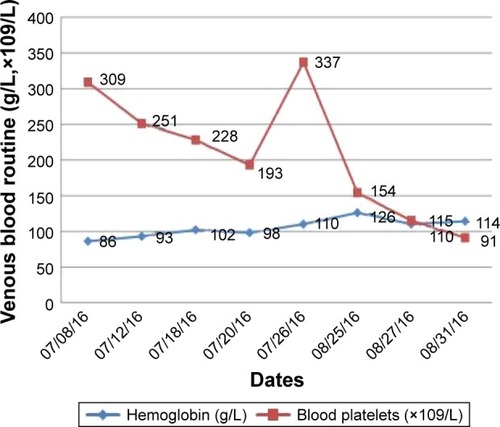
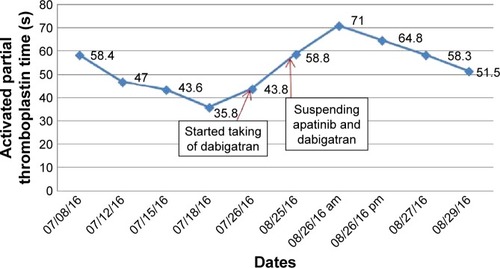
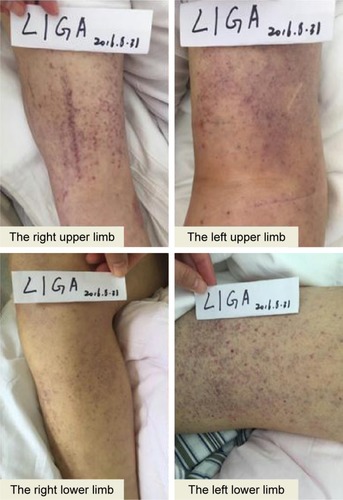
The left upper limb was found swelling on July 5, 2016, and ultrasonography showed venous thromboembolism (VTE). Fraxiparine, low molecular weight heparin (LMWH) calcium, was applied to anticoagulant therapy for the monitoring coagulation function from July 8, 2016. On July 26, 2016, ultrasonography showed the sign of vascular recanalization and reduction of swelling. Considering the difficulty complying with INR monitoring, the patient requested medication on discharge, so dabigatran (Boehringer Ingelheim Pharmaceutical Co., Ingelheim, Germany), a new oral anticoagulants, was chosen. On August 21, 2016, the patient suddenly felt dizzy with spraying vomit, and extensive subcutaneous hemorrhage occurred. On August 25, the patient was hospitalized and treated with hemostasis and plasma supplement as the blood routine (), and activated partial thromboplastin time () was monitored, stopping taking dabigatran. Ultrasonography presented no thrombus despite recurred swelling in the left upper limb () until his death. Written informed consent was obtained from the patient’s wife for publication of the details and accompanying images for this study.
Discussion
The patient was initially diagnosed with left lung adenocarcinoma via pathology tests. Platinum-based doublets chemotherapy was recommended as the first-line therapy. Systemic chemotherapy and local radiotherapy were given to the patient, and disease recurrence was observed after 2 months. Due to the small size of specimens, gene test cannot be performed. However, considering the high rate of EGFR mutation in Asians, erlotinib was chosen as the second-line therapy without EGFR test when the patient refused to receive chemotherapy. Evaluation after application of erlotinib for 1 month was effective, suggesting the patient might be harboring EGFR mutation. After 5 months, drug resistance occurred, which in most patients after 9–13 months of treatment.Citation7–Citation9 The following therapeutic strategy is to develop third-generation drugs against T790M resistance mutation or to investigate new combination strategies to overcome this resistance. However, plasma test (cfDNA) was not introduced by China-Japan Friendship Hospital then. Neither was osimertinib against T790M-positive NSCLC in Mainland China.Citation10 Herein, the patient would like to accept combination targeted treatment.
Angiogenesis plays a significant role in carcinoma growth and metastasis, while the VEGF signaling is the key pathway in vascular endothelial cells.Citation11 In vitro study has verified that the co-treatment with anti-VEGF therapy could enhance antitumor activity of anti-EGFR therapy.Citation12 Targeting angiogenesis by inhibition of VEGFs was shown to be effective for lung cancer,Citation13 including bevacizumab and apatinib.
Bevacizumab, a humanized monoclonal antibody against VEGF, has been recommended as the first-line therapy for lung adenocarcinoma until disease progression in NCCN Guidelines.Citation14 In the randomized open-label phase III BEVERLY study, the combined treatment of erlotinib and bevacizumab was significantly better than erlotinib alone in terms of PFS in patients with nonsquamous NSCLC with activating EGFR mutations, suggesting the possible combination strategy to combat EGFR-TKI resistance.Citation15 Furthermore, a systematic review and meta-analysis validated the benefits of PFS and overall response rate from the second-line co-treatment of NSCLC with bevacizumab and erlotinib.Citation16 Case reports also indicated that the combination of bevacizumab and erlotinib enhanced the efficacy against central nervous system metastasis, especially leptomeningeal carcinomatosis after failure of erlotinib.Citation17,Citation18 Like bevacizumab, apatinib is a kind of vascular endothelial growth inhibitor, while the oral agent ensures the ease in use and is affordable to the patient, considering the patient’s choice of having targeted treatment at home. Apatinib, is also a novel oral anti-angiogenesis agent, potently suppressing the c-kit and c-src, and inhibiting the cellular phosphorylation of VEGFR-2, c-kit, and PDGFR beta,Citation2 achieved certain antitumor drug effect and clinical benefits. Median overall survival and PFS were also significantly improved in the apatinib group compared with the placebo group in phase III trial.Citation5 In light of the above, it should be considered to use apatinib to combat TKI resistance. The patient benefited another 5.1-month PFS after confronting erlotinib resistance.
During the treatment of apatinib, the patient suffered from AEs, causing dosage adjustment. In phase II trial, the dosing regimen of 850 mg once daily was recommended for the treatment of gastric cancer.Citation4 Hypertension, hand-food syndrome, and proteinuria were known to be the most common AEs of antiangiogenic agents in the phase I/II/III trials, and the incidence of hematologic toxicities was low.Citation4–Citation6 After this patient taking the minimum dosage of 250 mg once daily for ~1 month, thoracic CT and cranial MRI showed marked benefits, although facing the challenge of hypertension as well as bleeding when dabigatran was used. The dosage was constantly adjusted in accordance with the disease changes, reaching a maximum of 250 mg once daily. Dosing of apatinib requires adjustments in different cancers and populations to optimize efficacy and benefits to patients.
Cancer patients are more vulnerable to VTE. A recent study showed that a cumulative VTE incidence was as low as 3.2% over a follow-up of 2.5 years, a much lower risk compared with new diagnosis.Citation19 The patient had cancer-related VTE after 23 months of cancer diagnosis. According to the current guidelines, patients are usually treated with anticoagulation for at least 3 months,Citation20 especially those with cancer-related thrombosis who would benefit a lot from anticoagulation therapy, while the therapeutic regimen should adjust with cancer status.Citation21 The current standard of care in patients with cancer and VTE is LMWH.Citation22 Fraxiparine was given for 18 days and dabigatran was given for 27 days as outpatient treatment for the patient before wide-spread subcutaneous hemorrhage occurred.
Dabigatran, a new oral anticoagulant, is the direct thrombin inhibitor. In the sister trials RE-COVER and RE-COVER II,Citation23,Citation24 treatment with dabigatran for VTE in patients with cancer offers a similarly effective, safe, and more convenient alternative to VKA in terms of fixed drug dosing. Renal and hepatic functions of this patient were found normal after dabigatran was taken; however, subcutaneous hemorrhage could not be well controlled. The patient had lower blood platelet and higher APTT. No medication can effectively neutralize the anticoagulant effect in China at present. Probably, apatinib might worsen the bleeding by blocking new blood vessel formation or having interactions with dabigatran. Current studies showed that apatinib was metabolized primarily by CYP3A4/5;Citation25 however, dabigatran was metabolized by esterases in the plasma and liver without significant involvement of CYP3A4.Citation26,Citation27 They might have a low potential for drug–drug interactions though other more specific profile is unknown.
Conclusion
Apatinib may be a recommendation for lung cancer patients and such studies are ongoing. To date, no evidence has been published on the use of apatinib against EGFR-TKI resistance in patients. Our study indicates that apatinib could be a potential option to combat EGFR-TKI resistance. However, further studies are required to verify these findings as well as more exploration on pharmacological dosage and AEs is needed.
Author contributions
YP, HC, ZL, and DL was responsible for collection and assembly of the patient’s data. YP, FL, YS, HD, YQ, and QL performed data analysis and literature search. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This case report was supported by China-Japan Friendship Hospital and Beijing Chest Hospital. We would like to thank Professor Qing Wu (English Department, School of Humanities, Beijing University of Chinese Medicine, Beijing, China) for English language editing. The authors thank the patient and his wife.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLieuleutJJemalAGlobal Cancer Statistics, 2012CA Cancer J Clin20156528710825651787
- TianSQuanHXieCYN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivoCancer Sci201110271374138021443688
- DingJChenXGaoZMetabolism and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor apatinib in humansDrug Metab Dispos20134161195121023509226
- LiJQinSXuJApatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trialJ Clin Oncol201331263219322523918952
- LiJQinSXuJTrial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junctionJ Clin Oncol20163413144826884585
- LiJZhaoXChenLSafety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignanciesBMC Cancer20101052920923544
- ZhouCWuYLChenGErlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, pen-label, randomised, phase 3 studyLancet Oncol201112873574221783417
- RosellRCarcerenyEGervaisRErlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomized phase 3 trialLancet Oncol20121323924622285168
- WuYLZhouCLiamCKFirstline erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE studyAnn Oncol2015261883188926105600
- MokTSWuYLAhnMJPlanchard D. Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung CancerN Engl J Med201737662964027959700
- ChatterjeeSHeukampLCSiobalMTumor VEGF: VEGFR2 autocrine feed forward loop triggers angiogenesis in lung cancerJ Clin Invest201312341732174023454747
- LiHTakayamaKWangSAddition of bevacizumab enhances antitumor activity of erlotinib against non-small cell lung cancer xenografts depending on VEGF expressionCancer Chemother Pharmacol20147461297130525344762
- FolkmanJAngiogenesis in cancer, vascular, rheumatoid and other diseaseNat Med19951127317584949
- EttingerDSWoodDEAkerleyWNCCN Guidelines Insight: non-small cell lung cancer, version 4. 2016J Natl Compr Canc Netw201614325526426957612
- GridelliCRossiACiardielloFBEVERLY: rationale and design of a randomized open-label phase III trial comparing bevacizumab plus erlotinib versus erlotinib alone as first-line treatment of patients with EGFR-mutated advanced nonsquamous non-small-cell lung cancerClin Lung Cancer201617546146527209164
- ZhangSMaoXDWangHTCaiFXuJEfficacy and safety of bevacizumab plus erlotinib versus bevacizumab or erlotinib alone in the treatment of non-small-cell lung cancer: a systematic review and meta-analysisBMJ Open201666e011714
- AriyasuRHoriikeAKoyamaJEfficacy of bevacizumab and erlotinib combination for leptomeningeal carcinomatosis after failure of erlotinibAnticancer Drugs2017
- SakataYKawamuraKShinquNIchikadoKErlotinib plus bevacizumab as an effective treatment for leptomeningeal metastases from EGFR mutation-positive non-small cell lung cancerLung Cancer20169912012227565925
- PrandoniPCasigliaEPiccioliAThe risk of cancer in patients with venous thromboembolism does not exceed that expected in the general population after the first 6 monthsJ Thromb Haemost2010851126112720149076
- O’ConnellCHow I treat incidental pulmonary embolismBlood2015125121877188225533036
- KearonCAklEADuration of anticoagulant therapy for deep vein thrombosis and pulmonary embolismBlood2014123121794180124497538
- LeeAYLevineMNBakerRILow-molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancerN Engl J Med2003349214615312853587
- SchulmanSKearonCKakkarAKDabigatran versus warfarin in the treatment of acute venous thromboembolismN Engl J Med2009361242342235219966341
- SchulmanSKakkarAKGoldhaberSZTreatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysisCirculation2014129776477224344086
- DingJFChenXYGaoZWMetabolism and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor apatinib in humansDrug Metab Dispos20134161195121023509226
- ScaglioneFNew oral anticoagulants: comparative pharmacology with vitamin K antagonistsClin Pharmacokinet2013522698223292752
- ShortNJConnorsJMNew oral anticoagulants and the cancer patientOncologist2014191829324319019