Abstract
Background
Accumulating data have reported that GSTM1 polymorphism may be related to nasopharyngeal cancer (NPC) and laryngeal cancer (LC). This meta-analysis was performed to investigate the relationship between GSTM1 polymorphism and risks of NPC and LC.
Methods
Pubmed, Embase, and China National Knowledge Infrastructure (CNKI) databases were searched for potential articles. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to evaluate the relationship of GSTM1 polymorphism with the risks of NPC and LC. I2>50% or P<0.05 indicates significant heterogeneity. When heterogeneity existed, the random-effects model was used to pool data, otherwise, the fixed-effects model was adopted. Publication bias was detected by Begg’s funnel plot and Egger’s regression. Quality of each study was evaluated by Newcastle-Ottawa Scale.
Results
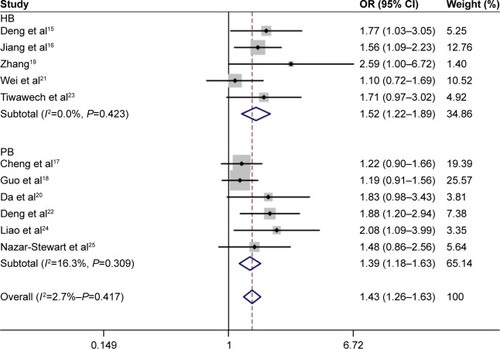
Thirty-two eligible articles were included. Pooled outcome suggested the significant relationship of GSTM1 null genotype with increased risk of LC (OR =1.28, 95% CI =1.05–1.54). Compared with hospital-based (HB) population, GSTM1 null genotype was also related to increased risk of LC (OR =1.38, 95% CI =1.06–1.80). Positive relationship of GSTM1 null genotype with enhanced risk of NPC was observed (OR =1.43, 95% CI =1.26–1.63). A similar trend was also observed in the subgroup analysis by source of control (population-based [PB]: OR =1.39, 95% CI =1.18–1.63; HB: OR =1.52, 95% CI =1.22–1.89).
Conclusion
GSTM1 null genotype is related to increased risk of NPC and LC.
Keywords:
Introduction
Nasopharyngeal cancer (NPC) is a fast-growing tumor which features distant metastasis and frequent nodal at diagnosis time.Citation1 Epstein-Barr virus (EBV) infection has been demonstrated as a major risk factor for NPC.Citation2 Besides, alcohol consumption and cigarette smoking could also increase the risk of NPC.Citation3 Laryngeal cancer (LC) is a common malignancy in the head and neck region.Citation4 Evidences have indicated that cigarette smoking and alcohol consumption play important roles in the development of the cancer.Citation5 Recent studies indicate that carcinogen-metabolizing genes could modulate individual susceptibility to cancers. Polymorphisms of these genes may influence carcinogen activation/detoxification by altering the expression and function of the genes.
Xenobiotics could be detoxified by the GSTM1 and GSTT1 enzymes. These phase II enzymes are involved in the detoxification of benzopyrene and polycyclic aromatic hydrocarbons (PAHs).Citation6 In addition, GSTM1 and GSTT1 serve as important factors in metabolizing carcinogens derived from tobacco smoke.Citation7 It has been observed that homozygous deletions of GSTM1 and GSTT1 genes bring about phenotypic absence of glutathione S-transferases (GSTs) activity.Citation8,Citation9 GSTM1 and GSTT1 null genotypes show an association with susceptibility to lung cancer or bladder cancer, which are induced by environmental factors.Citation10,Citation11
GSTM1 products are responsible for catalyzing the conjugation of glutathione to epoxide derivatives of PAHs, which are the major carcinogens in tobacco smoke.Citation12 Three different polymorphisms are observed in GSTM1 gene.Citation13 Among them, the most important polymorphism (GSTM1 null genotype) causes the inactivation of GSTM1 enzyme. The frequency of the null genotype ranges from 23% to 62% among different populations.Citation14
This present meta-analysis aimed to investigate the association of GSTM1 polymorphism with NPC and LC. The obtained outcome contributes to uncovering the pathogenesis of the cancers. Meanwhile, it contributes to clinical diagnosis of high-risk individuals for NPC and LC.
Methods
Article search
Pubmed, Embase, and China National Knowledge Infrastructure (CNKI) databases were searched for potential articles without language limitation. The search date was limited to May 2017. The keywords used in the search were: GSTM1, polymorphism or mutation or variant, nasopharyngeal carcinoma or nasopharynx cancer, LC or laryngocarcinoma. The references of obtained articles were also checked for additional articles.
Inclusion and exclusion criteria
The eligible articles had to meet the following criteria: (1) case-control studies; (2) articles investigating the relationship of GSTM1 polymorphism with NPC or LC; (3) articles providing the genotype data in case and control groups. The articles would be excluded if they were: (1) review articles; (2) animal or in vitro experiments; (3) GSTM1 polymorphism and risk of other cancers rather than NPC or LC.
Data extraction
Two authors were responsible for data extraction. The work was performed independently and any disagreements were resolved by discussion with a third author. The extracted information included: name of first author, publication year, country, ethnicity, experimental method, sample size, and genotypes’ distribution in case and control groups. Quality of each study was evaluated by the method of Newcastle-Ottawa Scale (NOS).
Statistical analysis
All the analyses were performed with Stata 12.0 software. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to evaluate the relationship of GSTM1 polymorphism with NPC or LC. I2 and P-value were used to assess the inter-study heterogeneity. I2>50% or P<0.05 indicates the presence of heterogeneity. If heterogeneity existed, the random-effects model was performed to pool data, otherwise, the fixed-effects model was used. Subgroup analyses based on ethnicity and source of control were initiated as well. Sensitivity analysis was conducted to assess the robustness of pooled results. Begg’s funnel plot and Egger’s regression analysis were used to evaluate the potential publication bias.
Results
Article selection
During the search, a total of 346 relevant articles were obtained. After screening the titles and abstracts, 213 records were excluded for review articles (n=82), GSTM1 polymorphism and other cancers (n=67), and other genes and NPC or LC (n=64). The remaining 133 articles were evaluated for eligibility. During the evaluation, 101 articles were excluded for unavailable data (n=35), case studies (n=37), GSTM1 polymorphism and pathological condition (n=29). Finally, 32 eligible articles were selected for the present meta-analysis.Citation15–Citation46 The detailed selection process was shown in . The basic information of included articles was listed in . The results about the quality assessment was shown in as well.
Figure 1 Article selection process. 32 eligible articles were included into the present meta-analysis.
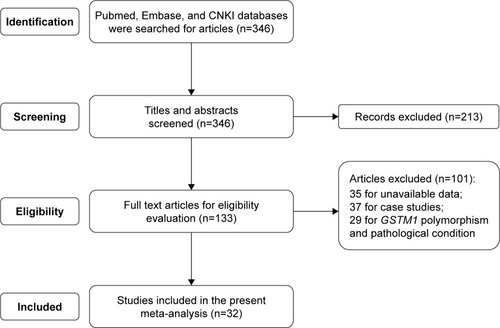
Table 1 Basic information of included articles
Relationship of GSTM1 polymorphism with LC
Random-effects model was used to analyze the association between GSTM1 polymorphism and risk of LC (P=0.000). The pooled results indicated that GSTM1 null genotype was related to increased risk of LC (OR =1.28, 95% CI =1.05–1.54). Subgroup analyses by ethnicity and source of control were performed as well (). The outcome indicated that GSTM1 null genotype was correlated with enhanced risk of LC, compared with hospital-based (HB) population (OR =1.38, 95% CI =1.06–1.80) (). No positive results were observed in the analysis of ethnicity.
Figure 2 Subgroup analysis by source of control about the association between GSTM1 null genotype and risk of LC.
Abbreviations: LC, laryngeal cancer; OR, odds ratio; CI, confidence interval; PB, population-based; HB, hospital-based.
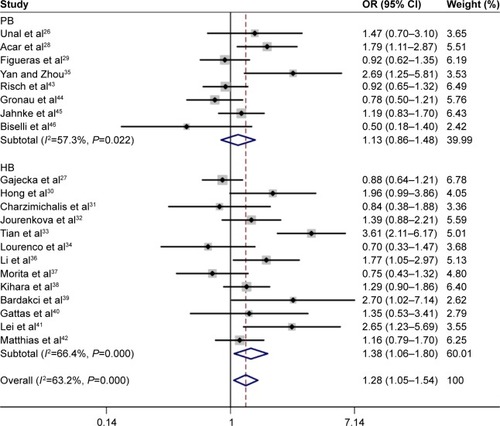
Table 2 Pooled results of the present meta-analysis
Relationship of GSTM1 polymorphism with NPC
Fixed-effects model was adopted to analyze the relationship of GSTM1 polymorphism with NPC (P=0.417). Overall results indicated that GSTM1 null genotype could increase the risk of NPC (OR =1.43, 95% CI =1.26–1.63). In the subgroup analysis by source of control, we found that GSTM1 null genotype was still related to increased risk of NPC (population-based: OR =1.39, 95% CI =1.18–1.63; HB: OR =1.52, 95% CI =1.22–1.89) ().
Sensitivity analysis
Sensitivity analysis was performed by deleting one study at a time. The analysis indicated that the pooled results were robust.
Publication bias detection
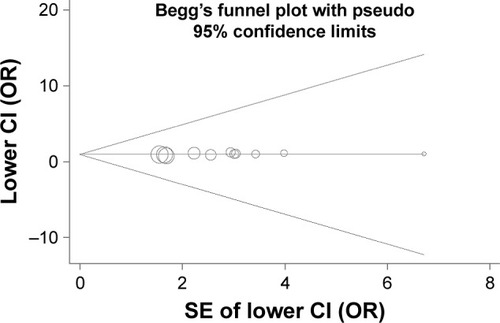
Begg’s funnel plot and Egger’s regression analysis were performed to detect the potential publication bias. The funnel plot seemed to be symmetrical (P=0.436) (). Egger’s analysis also suggested the absence of publication bias (P=0.097).
Discussion
GSTs, member of a super-family of detoxification enzymes, show important effects in resisting various environmental toxicants and chemical carcinogens. For the phase II detoxification enzymes, more than five classes (mu, sigma, pi, alpha, theta) of GSTs have been confirmed. Among these enzymes, only enzymes of GST-M (mu), GST-T (theta) and GST-P (pi) play important roles in the detoxification of carcinogenic electrophiles. Null mutations of GSTM1 are linked with complete loss of enzyme activities for binding with genotoxic substrates, such as epoxides.Citation47 Cumulative data have confirmed that individuals with null genotype of GSTM1 are more likely to develop various cancers such as colorectal cancer, prostate cancer, gastric cancer, lung cancer, liver cancer, bladder cancer, breast cancer, ovarian cancer, skin cancer, oral cancer, NPC, and LC.Citation25,Citation27,Citation48–Citation57
Accumulating data suggest that EBV infection, carcinogen exposure, and genetic susceptibility play an important role in NPC tumorigenesis. EBV infection is confirmed as a causal factor.Citation58 However, not all EBV-infected individuals would develop NPC, which indicates that other factors may be involved in the pathogenesis of the cancer, such as tumor promotion, lifestyle, and exposure to carcinogens.Citation59–Citation61 Besides, susceptibility genes such as interferon-alpha, HLA-regions and p53 alleles, and certain polymorphic genes encoding enzymes involved in metabolic activation and detoxification of xenobiotics have been regarded as risk factors.Citation62,Citation63 The null genotype of GSTM1 is linked with the loss of enzyme activity for binding with genotoxic substrates, therefore, the individuals with GSTM1 null are believed to be more likely to suffer NPC than individuals with normal genotype of GSTM1. The fact is that frequency of GSTM1 null genotype is different among different ethnic groups, such as 45%–56% in Asians, 40%–58% in Caucasians, and 29%–30% in African-Americans.Citation64–Citation67 In addition, the opinions on the relationship of GSTM1 null genotype with risk of NPC were inconsistent among the published studies. Therefore, this meta-analysis was initiated to obtain a more accurate outcome. The outcome indicated that GSTM1 null genotype could increase the risk of NPC (OR =1.43, 95% CI =1.26–1.63). A similar outcome was also observed in the subgroup analysis by source of control.
As for LC, smoking tobacco has been regarded as its main risk factor and only 1% of LC occurs among nonsmokers. However, not all smokers would develop LC. The incidence of LC is different among different countries and ethnic groups. Besides, LC cases exhibit geographic variations in distribution. These evidences indicate the important role of genetic susceptibility in the pathogenesis of LC. Tobacco contains aldehydes, nitrosamines, aromatic amines, and PAHs. These components can cause genetic mutations. There are many protective enzymes functioning in the deactivation or degradation of carcinogenic compounds, such as phase I enzymes (cytochrome p450, alcohol dehydrogenase) and phase II enzymes (N-acetyl transferases, GSTs). These enzymes, known as xenobiotic-metabolizing enzymes, commonly exist in the liver and have been found in the mucosa of the upper aerodigestive tract.Citation68 Phase II enzymes are involved in most metabolic detoxification processes of chemical carcinogens. Products of GSTM1 gene contribute to conjugating glutathione to epoxide derivatives of PAHs. The associations of GSTM1 null genotypes with tobacco-related cancers have been extensively reported, however, the role of GSTM1 polymorphism in LC is still controversial. Our results, based on a meta-analysis, suggested that GSTM1 null genotype was related to increased risk of LC (OR =1.28, 95% CI =1.05–1.54). The subgroup analysis by source of control also indicated that GSTM1 null genotype was correlated with enhanced risk of LC.
This meta-analysis was performed with 32 eligible articles. The sample size was 10,185. The outcome showed certain priority in accuracy compared with other studies. However, several limitations existed in the analysis. Only GSTM1 genetic polymorphism was analyzed, phase I and other phase II enzymes were not considered. Future analysis should focus much more on genes encoding these enzymes, which will contribute to uncovering the pathogenesis of NPC and LC. In addition, the occurrences of NPC and LC involve many risk factors. The analysis only considered the genetic factor and other environmental factors and genes should be investigated to get a much more complete outcome. Besides, significant heterogeneity existed in the analysis of LC, which might affect the accuracy of pooled results.
Subgroup analyses based on ethnicity and source of control were performed to identify the source of heterogeneity.
Conclusion
GSTM1 null genotype was related to increased risk of NPC and LC. The outcome will contribute to screening high-risk populations for NPC and LC.
Acknowledgments
We are indebted to the authors of the primary studies.
Disclosure
The authors report no conflicts of interest in this work.
References
- LinCLLoWFLeeTHImmunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinomaCancer Res200262236952695812460912
- O’NeilJDOwenTJWoodVHEpstein-Barr virus-encoded EBNA1 modulates the AP-1 transcription factor pathway in nasopharyngeal carcinoma cells and enhances angiogenesis in vitroJ Gen Virol200889Pt 112833284218931081
- ChengYJHildesheimAHsuMMCigarette smoking, alcohol consumption and risk of nasopharyngeal carcinoma in TaiwanCancer Causes Control199910320120710454065
- CannCIFriedMPRothmanKJEpidemiology of squamous cell cancer of the head and neckOtolaryngol Clin North Am1985183367388
- KoskinenWJBrøndboKMellin DahlstrandHAlcohol, smoking and human papilloma-virus in laryngeal carcinoma: a Nordic prospective multicenter studyJ Cancer Res Clin Oncol2007133967367817486368
- SchneiderJBerngesUPhilippMWoitowitzHJGSTM1, GSTT1, and GSTP1 polymorphism and lung cancer risk in relation to tobacco smokingCancer Lett20042081657415105047
- RebbeckTRMolecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibilityCancer Epidemiol Biomarkers Prev1997697337439298582
- SeidegardJVorachekWRPeroRWPearsonWRHereditary difference in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletionProc Natl Acad Sci U S A19888519729372973174634
- PembleSSchroederKRSpencerSRHuman glutathione S-transferase θ(GSTT1): cDNA cloning and the characterization of genetic polymorphismBiochem J1994300Pt 12712768198545
- SaarikoskiSTVohoAReinikainenMCombined effect of polymorphic GST genes on individual susceptibility to lung cancerInt J Cancer19987745165219679751
- BellDATaylorJAPaulsonDFGenetic risk and carcinogen exposure: a common inherited defect of the carcinogen-metabolism gene glutathione S-transferase M1 (GSTM1) that increases susceptibility to bladder cancerJ Natl Cancer Inst19938514115911648320745
- HannaEMacLeodSVuralELangNGenetic deletions of glutathione-S-transferase as a risk factor in squamous cell carcinoma of the larynx: A preliminary reportAm J Otolaryngol200122212112311283827
- BeckettGJHayesJDGlutathione S-transferases: biomedical applicationsAdv Clin Chem1993302813808237562
- CottonSCSharpLLittleJBrocktonNGlutathione S-transferase polymorphisms and colorectal cancer: A HuGe reviewAm J Epidemiol2000151173210625170
- DengZLWeiYPMaYFrequent genetic deletion of detoxifying enzyme GSTM1 and GSTT1 genes in nasopharyngeal carcinoma patients in Guangxi Province, ChinaZhonghua Zhong Liu Za Zhi20042610598600 Chinese15634519
- JiangYLiNDongPPolymorphisms in GSTM1, GSTT1 and GSTP1 and nasopharyngeal cancer in the east of China: a case-control studyAsian Pac J Cancer Prev201112113097310022393996
- ChengYJChienYCHildesheimANo association between genetic polymorphisms of CYP1A1, GSTM1, GSTT1, GSTP1, NAT2, and nasopharyngeal carcinoma in TaiwanCancer Epidemiol Biomarkers Prev200312217918012582034
- GuoXCO’BrienSJZengYNelsonGWWinklerCAGSTM1 and GSTT1 gene deletions and the risk for nasopharyngeal carcinoma in Han ChineseCancer Epidemiol Biomarkers Prev20081771760176318628429
- ZhangGCYP1A1 及GSTM1 基因多态性与鼻咽癌发病风险关系的 研究To investigate the relationship between CYP1A1 and GSTM1 gene polymorphisms and nasopharyngeal carcinoma (thesis)DaliDali University, People’s Republic of China2012 China
- DaSJLiangBWuHLGuanLLGSTM1 基因多态性与鼻咽癌遗传 易感性的关系研究 [Relationship between GSTM1 gene polymorphism and genetic susceptibility in nasopharyngeal carcinoma]The Practical Journal of Cancer2002176617618626 Chinese
- WeiYPLongXDLiuZGMaYDengZL肝细胞癌、鼻咽癌患 者谷胱甘肽硫转移酶M1和T1基因型分布 [Genetic polymorphism of Glutathione-S-transferase M1 and T1 in hepatocellular carcinoma and nasopharyngeal carcinoma]Cancer Research on Prevention and Treatment2010371011621165 Chinese
- DengZLWeiYPLuoWLiaoZLMaYGlutathione S-transferase M1 and T1 gene deletion associated with increased susceptibility to nasopharyngeal carcinomaThe Chinese-German Journal of Clinical Oncology200545276278
- TiwawechSrivatanakulPKaralakAIshidaTGlutathione S-transferase M1 gene polymorphism in Thai nasopharyngeal carcinomaAsian Pac J Cancer Prev20056327027516235985
- LiaoZDengZLWeiYPGSTT1, GSTM1基因缺失多态性 与鼻咽癌的发病关系 [Relationships of GSTT1, GSTM1 deletion polymorphisms with risk of nasopharyngeal carcinoma]Journal of Guangxi Medical University2005223372374 Chinese
- Nazar-StewartVVaughanTLBurtRDChenCBerwickMSwansonGMGlutathione S-Transferase M1 and susceptibility to nasopharyngeal carcinomaCancer Epidemiol Biomarkers Prev19998654755110385146
- UnalMTamerLAtesNAGlutathione S-transferase M1, T1, and P1 gene polymorphism in laryngeal squamous cell carcinomaAm J Otolaryngol200425531832215334395
- GajeckaMRydzaniczMJaskula-SztulRKujawskiMSzyfterWSzyfterKCYP1A1, CYP2D6, CYP2E1, NAT2, GSTM1 and GSTT1 polymorphisms or their combinations are associated with the increased risk of the laryngeal squamous cell carcinomaMutat Res20055741–211212315914211
- AcarHOzturkKMuslumanogluMHRelation of glutathione S-transferase genotypes (GSTM1 and GSTT1) to laryngeal squamous cell carcinoma riskCancer Genet Cytogenet20061692899316938565
- To-FiguerasJGeneMGomez-CatalanJMicrosomal epoxide hydrolase and glutathione S-transferase polymorphisms in relation to laryngeal carcinoma riskCancer Lett20051871–295101
- HongYJLeeJKLeeGHHongSIInfluence of Glutathione S-transferase M1 and T1 genotypes on larynx cancer risk among Korean smokersClin Chem Lab Med200038991791911097350
- CharzimichalisMXenellisJTzagaroulakisAGSTT1, GSTM1, GSTM3 and NAT2 polymorphisms in laryngeal squamous cell carcinoma in a Greek populationJ Laryngol Otol2010124331832319922706
- JourenkovaNReinikainenMBouchardyCDayerPBenhamousSHirvonenALarynx cancer risk in relation to glutathione S-transferase M1 and T1 genotypes and tobacco smokingCancer Epidemiol Biomarkers Prev19987119239456238
- TianSZZhangJGXiaoQThe association between genetic polymorphisms of GSTM1, GSTT1, GSTP1 and susceptibility to laryngeal carcinoma from the Han people in Guangdong zoneLin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi2011255204210 Chinese21604464
- LourencoGJSilvaEFRinck-JuniorJAChoneCTLimaCSCYP1A1, GSTM1 and GSTT1 polymorphisms, tobacco and alcohol status and risk of head and neck squamous cell carcinomaTumour Biol20113261209121521870186
- YanYBZhouWR谷胱甘肽S转移酶M1基因缺失与喉癌易患性 的相关性研究 [Glutathinoe S-transferase M1 null genotypes and susceptibility to laryngeal carcinoma]Journal of Nantong University (Medical Sciences)200323255257 Chinese
- LiLLinPDengYFZhuZLLuHHRelationship between susceptibility and prognosis of laryngeal cancer and genetic polymorphisms in CYP1A1 and GSTM1Zhonghua Er Bi Yan Hou Ke Za Zhi200439127 Chinese15127559
- MoritaSYanoMTsujinakaTGenetic polymorphisms of drug-metabolizing enzymes and susceptibility to head-and-neck squamous-cell carcinomaInt J Cancer199980568568810048967
- KiharaMKiharaMKubotaAFurukawaMKimuraHGSTM1 gene polymorphism as a possible marker for susceptibility to head to neck cancers among Japanese smokersCancer Lett199711222572629066737
- BardakciFCanbayEDegerliNCobanLCanbayEIRelationship of tobacco smoking with GSTM1 gene polymorphism in laryngeal cancerJ Cell Mol Med20037330731214594555
- GattasGJde CarvalhoMBSiraqueMSGenetic polymorphisms of CYP1A1, CYP2E1, GSTM1, and GSTT1 associated with head and neck cancerHead Neck200628981982616721740
- LeiDPPanXLGuoCHGSTM1空白基因型与喉癌遗传 易感性的研究 [The study about GSTM1 null genotype and genetic susceptibility of laryngeal cancer]Chinese Journal of Medical Genetics200219534535 Chinese
- MatthiasCBockmuhlUJahnkeVPolymorphism in cytochrome P450 CYP2D6, CYP1A1, CYP2E1 and glutathione S-transferase, GSTM1, GSTM3, GSTT1 and susceptibility to tobacco-related cancers: studies in upper aerodigestive tract cancersPharmacogenetics1998829110010022746
- RischARamrothHRaedtsVLaryngeal cancer risk in Caucasians is associated with alcohol and tobacco consumption but not modified by genetic polymorphisms in class I alcohol dehydrogenases ADH1B and ADH1C, and glutathione-S-transferases GSTM1 and GSTT1Pharmacogenetics200313422523012668919
- GronauSKoenig-GregerDJergMRiechelmannHGene polymorphisms in detoxification enzymes as susceptibility factor for head and neck cancerOtolaryngol Head Neck Surg2003128567468012748560
- JahnkeVStrangeRMatthiasCFryerAGlutathione S-transferase and cytochrome P450 genotypes as risk factors for laryngeal carcinomaEur Arch Otorhinolaryngol1997253Suppl 1S147S149
- BiselliJMde Angelo Calsaverini LealRCRuizMTGSTT1 and GSTM1 polymorphism in cigarette smokers with head and neck squamous cell carcinomaBraz J Otorhinolaryngol200672565465817221058
- HayesJDPulfordDJThe glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistanceCrit Rev Biochem Mol Biol19953064456008770536
- Gawronska-SzklarzBLubinskiJKlandyJPolymorphism of GSTM1 gene in patients with colorectal cancer and colonic polypsExp Toxicol Pathol1999514–532132510445390
- AutrupJLThomassenLHOlsenJHWolfHAutrupHGlutathione S-transferases as risk factors in prostate cancerEur J Cancer Prev19998652553210643942
- SetiawanVWZhangZFYuGPGSTT1 and GSTM1 null genotypes and risk of gastric cancer: a case-control study in a Chinese populationCancer Epidemiol Biomarkers Prev200091738010667466
- SweeneyCNazar-StewartVStapletonPLEatonDLVaughanTLGlutathione S-transferase M1, T1, and P1 polymorphisms and survival among lung cancer patientsCancer Epidemiol Biomarkers Prev200312652753312814998
- DengZLWeiYPMaYPolymorphism of glutathione S-transferase mu 1 and theta 1 genes and hepatocellular carcinoma in southern Guangxi, ChinaWorld J Gastroenterol200511227227415633230
- SrivastavaDSKumarAMittalBMittalRDPolymorphism of GSTM1 and GSTT1 genes in bladder cancer: a study from North IndiaArch Toxicol200478843043415057507
- van der HelOLPeetersPHHeinDWNAT2 slow acetylation and GSTM1 null genotypes may increase postmenopausal breast cancer risk in long-term smoking womenPharmacogenetics200313739940712835615
- SpurdleABWebbPMPurdieDMChenXGreenAChenevix-TrenchGPolymorphisms at the glutathione S-transferase GSTM1, GSTT1 and GSTP1 loci: risk of ovarian cancer by histological subtypeCarcinogenesis2001221677211159743
- HeagertyAHFitzgeraldDSmithAGlutathione S-transferase GSTM1 phenotypes and protection against cutaneous tumoursLancet199434388922662687905099
- KietthubthewSSriplungHAuWWGenetic and environmental interactions on oral cancer in Southern ThailandEnviron Mol Mutagen200137211111611246217
- ZongYSShamJSNgMHImmunoglobulin A against viral capsid antigen of Epstein-Barr virus and indirect mirror examination of the nasopharynx in the detection of asymptomatic nasopharyngeal carcinomaCancer1992691371309307
- MirabelliMCHoppinJTolbertPEOccupational exposure to chlorophenol and the risk of nasal and nasopharyngeal cancers among U.S. men aged 30 to 60Am J Ind Med200037553254110723047
- VaughanTLStewartPATeschkeKOccupational exposure to formaldehyde and wood dust and nasopharyngeal cancerOccup Environ Med200057637638410810126
- HoCKLoWCHuangPHWuWTChristianiDCLinCTSuspected nasopharyngeal carcinoma in three workers with long term exposure to sulphuric acid vapourOccup Environ Med199956642642810474541
- GolovlevaIBirganderRSjalanderALundgrenEBeckmanLInterferon-alpha and p53 alleles involved in nasopharyngeal carcinomaCarcinogenesis19971846456479111194
- KongruttanachokNSukdikulSSetavarinSCytochrome P450 2E1 polymorphism and nasopharyngeal carcinoma development in Thailand: a correlative studyBMC Cancer20011411389775
- FordJGLiYO’SullivanMMGlutathione S-transferase M1 polymorphism and lung cancer risk in African-AmericansCarcinogenesis200021111971197511062156
- GaoYZhangQPolymorphisms of the GSTM1 and CYP2D6 genes associated with susceptibility to lung cancer in ChineseMutat Res1999444244144910521684
- KiyoharaCShirakawaTHopkinJMGenetic polymorphism of enzymes involved in xenobiotic metabolism and the risk of lung cancerEnviron Health Prev Med200272475921432264
- StrangeRCJonesPWFryerAAGlutathione S-transferase: genetics and role in toxicologyToxicol Lett2000112–113357363
- ScullyCFieldJKTanzawaHGenetic aberrations in oral or head and neck squamous cell carcinoma (SCCHN): 1. Carcinogen metabolism, DNA repair and cell cycle controlOral Oncol200036325626310793327