Abstract
Aim
To explore the expression and clinical significance of ecotropic viral integration site-1 (EVI1) of lung squamous cell cancer (SCC).
Methods
The expression of EVI1 in SCC was detected by immunohistochemistry and the validation cohort was divided into EVI1 high-expression group and low-expression group according to the cutoff of immunohistochemical score. The correlations between EVI1 expression and the clinicopathological factors were analyzed by χ2 test. The relation between EVI1 expression and overall survival rate was evaluated by univariate analysis with Kaplan–Meier method. The independent prognostic factor was identified by multivariate analysis with Cox regression model.
Results
In this study, the EVI1 high-expression percentage was 32.32% (53/164). EVI1 high expression was significantly associated with a poorer overall 5-year survival rate of SCC (P=0.021). Moreover, EVI1 high expression was identified as an independent prognostic factor of SCC, predicting the unfavorable prognosis (P=0.013).
Conclusion
High expression of EVI1 was significantly associated with a poorer prognosis and it was identified as an independent prognostic factor of SCC.
Introduction
Lung cancer is becoming a more and more critical public health problem and medical insurance burden, resulting in the largest number of cancer-related deaths worldwide.Citation1 Approximately 1,590,000 patients die of lung cancer in the world every year.Citation2 Due to severe air pollution, the mortality and morbidity of lung cancer are much more critical in developing countries like China and India. Pathologically, non-small cell lung cancer (NSCLC) takes up ~80%–85% in lung cancer, including two main subtypes, the adenocarcinoma (AC) and squamous cell cancer (SCC). The former type occupies ~40% and the latter type accounts for 25%–30% in all kinds of lung cancers.Citation3 Although surgical techniques and adjuvant therapies have improved remarkably, the 5-year overall survival rate of lung cancer is still very unsatisfactory, remaining 10%–15%.Citation1 There is an urgent need for effective biomarkers of lung cancer, because of the ability to help identify high-risk patients, facilitate the application of optimal treatment, and improve the overall survival rate.
Ecotropic viral integration site-1 (EVI1) is identified as an oncoprotein mostly in hematological malignancies such as acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). Frequent activation of EVI gene could be observed for intrachromosomal or interchromosomal rearrangements in patients with AML or MDS. EVI1 was also demonstrated to regulate lots of cellular processes in tumorigenesis and progression, including cell proliferation, transformation, differentiation, and survival, especially in leukemic cells.Citation4–Citation8 In solid tumors, such as glioblastoma and prostate cancer, EVI1 was also reported to be involved in progression or prognosis.Citation7,Citation9 In SCC, the amplification of chromosome 3q26.2–q29 was frequently observed.Citation10 This region (3q26.2–q29) included the location of EVI1 gene, indicating the possible oncogenic role of EVI1 in lung cancer, especially in SCC. But that study focused on the gene level and could not provide the direct evidence of EVI1 protein as an oncoprotein in SCC.Citation10 In conclusion, the expression, intracellular location, and function of EVI1 have not been explored in SCC.
Although SCC and AC are included in NSCLC with a similar treatment strategy and outcome, their biological features are different.Citation11 Based on the previous indication that the region containing EVI1 gene was amplified in SCC,Citation10 it was suspected that EVI1 might be involved in SCC progression or prognosis. Therefore, the expression of EVI1 was detected in 164 cases of SCC with immunohistochemistry (IHC) and the clinical and prognostic significance of EVI1 in SCC was evaluated further.
Patients and methods
Patients and follow-ups
This study was reviewed and approved by the Ethics Committee of Linyi People’s Hospital. The primary cohort consisted of 461 patients diagnosed with SCC in the medical center of Linyi People’s Hospital, and the validation cohort was selected from the primary cohort based on the following criteria: 1) available paraffin-embedded tissues; 2) no adjuvant chemotherapy or radiation therapy before or after surgery; and 3) radical section of the tumor. According to the above criteria, a total of 164 SCC patients were selected for the validation cohort. All the samples were obtained from the pathology department with prior written informed consent of patients’ family for this study. The tissue samples were sliced and stained with hematoxylin-eosin for double confirmation of diagnosis and selection of IHC area. The pathological tumor-node-metastasis (pTNM) stage of SCC was identified by the seventh American Joint Committee on Cancer/Union for International Cancer Control staging system (2009).
IHC
The protocol of IHC staining was performed according to previous studies.Citation12,Citation13 Briefly, the paraffin-embedded SCC samples were deparaffinized with xylene and graded ethanol. Then the slides were incubated in citrate buffer for 30 minutes for antigen retrieval. Blockage of endogenous peroxidase enzyme was achieved by incubation in 3% hydrogen peroxide in methanol for 20 minutes. Primary anti-EVI1 antibody (Novus Biologicals, Littleton, CO, USA) with dilution at a ratio of 1:100 was used to incubate the tissues at 4°C overnight, followed by a wash of phosphate-buffered saline. The corresponding biotinylated secondary antibody and streptavidin-peroxidase complex were used to incubate at 37°C for 30 minutes. Finally, the slides were visualized by incubation with 3,3′-diaminobenzidine solution.
Evaluation of IHC results
According to previous studies, the IHC results were evaluated by the IHC score, which was defined as the product of score of positive cell percentage and the score of staining intensity.Citation14,Citation15 The score of positive cell percentage was defined as follows: 0 for 10% positive cells; 1 for 10%–30% positive cells; 2 for 30%–50% positive cells; and 3 for ≥50% positive cells. The score of staining intensity was described as follows: 0 for negative staining; 1 for weak staining; 2 for moderate staining; and 3 for strong staining. The total score of IHC was defined as the product of score for staining intensity and the score for positive cells. The total IHC score ranged from 0 to 9, and the cutoff was selected with a receiver operating characteristic curve with the highest sensitivity plus specificity.Citation16 The cohort was divided into EVI1 high-expression group and EVI1 low-expression group, according to the cutoff of EVI1 IHC score.
Statistical analysis
SPSS 17.0 software (IBM Corp., Armonk, NY, USA) was used to analyze all the data. The χ2 test was used to analyze the correlation between EVI1 expression and the clinicopathological factors. The Kaplan–Meier method was performed for the survival curve and the log-rank test was used to compare the statistical difference between the subtypes. The Cox regression hazard model was used for multivariate analysis. A P-value of <0.05 was considered to be statistically significant.
Results
Information of validation cohort
A total of 164 patients diagnosed with SCC were enrolled into our cohort, including 106 male and 58 female patients (). The average age of patients was 64.5 years. The median postoperational survival time was 22 months and the average survival time was 34 months. As previously described in the “Patients and methods” section, the cohort was divided into EVI1 high-expression group and EVI1 low-expression group according to the cutoff of EVI1 IHC score. In this study, the percentage of EVI1 high-expression group was 32.32% (53/164). And EVI1 was mainly observed in the nucleus of SCC cells, but in some cases it was also expressed in cytoplasm. Representative images of low-expression and high-expression EVI1 are displayed in .
Figure 1 Representative images of high-expression and low-expression EVI1 detected with IHC.
Abbreviations: EVI1, ecotropic viral integration site-1; IHC, immunohistochemistry.
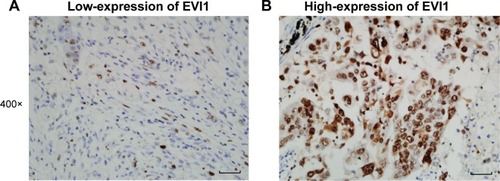
Table 1 Characteristics of patients with SCC
Correlation between the clinicopathological factors and EVI1 expression
After the cohort was divided into EVI1 high-expression group and EVI1 low-expression group, the correlation between EVI1 expression and other clinicopathological factors was analyzed by χ2 test. The clinicopathological factors included the age, gender, lymphatic status, tumor size, smoking status, tumor differentiation, and pTNM stage of patients (). Considering that the patients of stage IV already have a distant metastasis and the radical surgery cannot be achieved as usual, the pTNM stage only included stages I–III in this cohort. The analyses of correlation between the clini-copathological factors and EVI1 expression could help find the potential association of EVI1 with the progression of SCC. However, there was no significant clinicopathological factor identified to be significantly associated with EVI1 expression in this study.
Table 2 Correlation between EVI1 expression and the clinicopathological parameters
Prognostic value of EVI1 in SCC
In previous study,Citation10 the expression of EVI1 was demonstrated to be much higher in SCC compared with AC. It was suspected that EVI1 expression might affect the prognosis of SCC and therefore its prognostic significance was evaluated with univariate and multivariate analyses (). The univariate analysis with Kaplan–Meier method was performed first to analyze the relation between EVI1 expression and the overall 5-year survival rate. In the univariate analysis, positive lymphatic invasion (P<0.001, 5-year survival rate: 13.0 vs 38.4 months) and advanced TNM stage (P<0.001, 5-year survival rate: 0 vs 22.5 vs 32.4 months) were proved to be significantly associated with the poorer prognosis of SCC (). Notably, the high expression of EVI1 could also predict the unfavorable prognosis of SCC (P=0.021, 18.5 vs 26.6 months) (). No other clinicopathological factor was defined as the prognostic factor in this study.
Figure 2 Overall survival curves of the lymphatic status, pTNM stage, and EVI1 expression.
Abbreviations: EVI1, ecotropic viral integration site-1; pTNM, pathological tumor-node-metastasis.
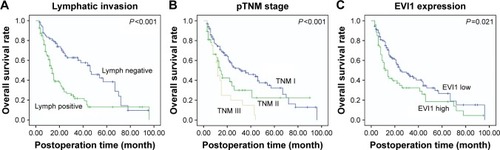
Table 3 Prognostic significance of EVI1 in SCC
The multivariate analysis with the Cox regression model was further performed to detect the independent prognostic factors. All the clinicopathological factors were enrolled into the Cox regression model except TNM stage because of its obvious interaction with lymphatic status. In the multivariate analysis, the high expression of EVI1 was identified as an independent prognostic factor of SCC (hazard ratio [HR] =1.66, 95% confidence interval [CI] =1.11–2.47, P=0.013), indicating that the high expression of EVI1 alone was enough to predict the poorer prognosis of SCC. Besides EVI1, lymphatic status was also defined as an independent prognostic factor in this study (HR =2.45, 95% CI =1.60–3.76, P<0.001).
Discussion
SCC and AC are always classified into one type-NSCLC because of their similar treatment strategy. However, they are different histological types and have significantly different phenotypic properties. In previous studies, the most valuable study inspiring us to carry out the experiments of this study was conducted by Kang et al.Citation10 They performed high-resolution array-comparative genomic hybridization and identified the differences in the patterns of genomic imbalances between SCC and AC. As a result, they demonstrated that SCC instead of AC has an amplified region of gene in 3q26.2–q29, where the EVI1 gene is located.Citation10 Many previous studies have proved the oncogenic role of EVI1 in tumors, especially tumors of hematopoietic system.Citation17 More and more emerging evidence has indicated the oncogenic role of EVI1 in solid tumors, including prostate cancer, ovarian cancer, glioblastoma, hepatocellular carcinoma, and so on.Citation7,Citation9,Citation18 The underlying molecular mechanism of how EVI1 promotes cancer progression and affects prognosis has not been well elucidated. Yasui et al proved that EVI1 could antagonize transforming growth factor-β-mediated growth inhibition in hepatocellular carcinoma, which promotes the proliferation of hepatocellular carcinoma cells.Citation18 However, some sporadic studies pointed out the dual role of EVI1 in cancer progression. For example, Nanjundan et alCitation19 pointed out that amplification of EVI1 is associated with a favorable prognosis in ovarian cancer. Although that study was based on the detection of DNA copy number, it still reflects the controversial role of EVI1 in some special cancer types.Citation19 In conclusion, many studiesCitation20,Citation21 identified EVI1 as a protein promoting cancer progression, but consensus has not been reached. More experiments need to be performed for validation if new cancer types are involved, such as SCC.
Nowadays, the trend of the precise treatment is becoming more and more prevalent. The theoretical basis of the precise treatment is the individual heterogeneity and diversity of patients. Different cancers may exhibit different varieties and abundance of oncogenes or oncoproteins. In post-operational patients with cancer, detection of these oncogenes or oncoproteins is the first step to direct chemotherapy or target therapy. A large number of predicting or prognostic biomarkers have been explored in lung cancer, but only a few have been demonstrated to be clinically relevant. The unionization of medical centers is necessary to enlarge the sample size and reduce diversity, which would help in getting more solid results. Moreover, the detection of candidate proteins in situ may be more valuable for prediction. Detecting the expression of proteins in tumor samples directly could reduce the confounding factors from gene modification to messenger RNA transcription. In tumor treatment therapy, the finding of an effective biomarker could be revolutionary, leading to a targeted drug, or even a potential therapy, just like the exploration of human epidermal growth factor receptor 2 (HER2) function in breast cancer resulted in the development of trastuzumab and the target therapy to patients with breast cancer. The finding of this study that the high expression of EVI1 is an independent prognostic factor could help more specifically stratify SCC high-risk and low-risk patients and help them get different treating therapies. Moreover, along with the elucidation of molecular mechanisms of how EVI1 leads to the poorer prognosis of SCC, EVI1 may become a potent drug target, like the revolution triggered by HER2 in breast cancer treatment. The finding of clinical significance of EVI1 in SCC could inspire more interest on EVI1 and encourage more experiments to explore the underlying mechanisms of EVI1 downstream pathways.
In conclusion, the expression of EVI1 was detected for the first time in 164 cases of SCC samples with IHC, and the cohort was divided into EVI1 high-expression and low-expression groups according to the score. Furthermore, it was demonstrated that the high expression of EVI1 was significantly associated with the unfavorable prognosis of SCC and that EVI1 was an independent prognostic factor of SCC. The findings could trigger interest in the study on the molecular mechanism of EVI1 as an oncoprotein in SCC and help identify the substrate regulated by EVI1. The results could help more specifically stratify SCC high-risk and low-risk patients and help find a potential drug target in SCC treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRMaJZouZJemalACancer statistics, 2014CA Cancer J Clin201464192924399786
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- EttingerDSWoodDEAkerleyWNon-small cell lung cancer, version 6. 2015J Natl Compr Canc Netw201513551552425964637
- NuciforaGLaricchia-RobbioLSenyukVEVI1 and hematopoietic disorders: history and perspectivesGene200636811116314052
- HellerGRommerASteinleitnerKEVI1 promotes tumor growth via transcriptional repression of MS4A3J Hematol Oncol201582825886616
- WangHSchaeferTKonantzMProminent oncogenic roles of EVI1 in breast carcinomaCancer Res2017772148216028209621
- QueisserAHagedornSWangHEcotropic viral integration site 1, a novel oncogene in prostate cancerOncogene2017361573158427617580
- SatoTGoyamaSKataokaKEvi1 defines leukemia-initiating capacity and tyrosine kinase inhibitor resistance in chronic myeloid leukemiaOncogene201433425028503824747972
- HouAZhaoLZhaoFExpression of MECOM is associated with unfavorable prognosis in glioblastoma multiformeOnco Targets Ther2016931532026834490
- KangJUKooSHKwonKCParkJWKimJMIdentification of novel candidate target genes, including EPHB3, MASP1 and SST at 3q26.2-q29 in squamous cell carcinoma of the lungBMC Cancer2009923719607727
- HirschFRSudaKWiensJBunnPAJrNew and emerging targeted treatments in advanced non-small-cell lung cancerLancet2016388100481012102427598681
- XuYFYangXQLuXFFibroblast growth factor receptor 4 promotes progression and correlates to poor prognosis in cholangiocarcinomaBiochem Biophys Res Commun20144461546024565842
- XuYYangXLiZSprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progressionOncotarget201784888490028002800
- XuYFGeFJHanBHigh-mobility group box 1 expression and lymph node metastasis in intrahepatic cholangiocarcinomaWorld J Gastroenterol201521113256326525805932
- LiuHZhangQLiKPrognostic significance of USP33 in advanced colorectal cancer patients: new insights into β-arrestin-dependent ERK signalingOncotarget20167812238124027835898
- LiuHXuYZhangQCorrelations between TBL1XR1 and recurrence of colorectal cancerSci Rep201774427528295012
- FenouilleNBassilCFBen-SahraIThe creatine kinase pathway is a metabolic vulnerability in EVI1-positive acute myeloid leukemiaNat med Epub2017213
- YasuiKKonishiCGenYEVI1, a target gene for amplification at 3q26, antagonizes transforming growth factor-β-mediated growth inhibition in hepatocellular carcinomaCancer Sci2015106792993725959919
- NanjundanMNakayamaYChengKWAmplification of MDS1/EVI1 and EVI1, located in the 3q26.2 amplicon, is associated with favorable patient prognosis in ovarian cancerCancer Res20076773074308417409414
- HinaiAAValkPJReview: Aberrant EVI1 expression in acute myeloid leukaemiaBr J Haematol2016172687087826729571
- WieserRThe oncogene and developmental regulator EVI1: expression, biochemical properties, and biological functionsGene2007396234635717507183
