Abstract
Background
Studies on the association between two single nucleotide polymorphisms (SNPs) in estrogen receptor α (ERα), PvuII (rs2234693 T>C) and XbaI (rs9340799 A>G), and the prostate cancer risk are inconsistent. Therefore, we performed a meta-analysis to derive a more accurate estimation of this relationship.
Methods
A literature search of PubMed, Embase, Web of Science databases until October 1, 2016, was conducted. Crude odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to assess the strength of this association.
Results
Eighteen case-control studies, with a total of 3,317 prostate cancer patients and 8,324 controls, were included. Results showed that both PvuII and XbaI polymorphisms were significantly associated with a higher prostate cancer risk in overall populations. To derive a more accurate estimation, subgroup analysis stratified by ethnicity revealed that this relation-ship existed only in Caucasians, but not in Asians. Furthermore, PvuII polymorphism was significantly associated with high Gleason grade (Gleason score ≥7) cancers.
Conclusion
The current meta-analysis demonstrates that ERα PvuII and XbaI polymorphisms are associated with a higher prostate cancer risk in Caucasians, but not in Asians, and PvuII polymorphism is significantly associated with high Gleason grade tumors, indicating the probability of inherited susceptibility to prostate cancer arising from different genomic ERα SNPs, which may help us understand the pathogenesis of prostate cancer in Caucasians.
Introduction
Prostate cancer is the most common malignancy in men and a major cause of cancer-related deaths.Citation1 Since prostate-specific antigen (PSA)-based screening regime for prostate cancer remains controversial because of the high rate of overdiagnosis and overtreatment, a validated biomarker to complement PSA for screening and prognostic biomarkers with clinical utility remain an unmet need.Citation2
In addition to androgens, estrogens also affect prostatic growth and carcinogenesis.Citation3 The cellular effects of estrogens are mediated by two estrogen receptors (ERs), ERα and ERβ. The human ERα encoding gene locates on chromosome 6q25.1 and consists of eight exons and seven introns.Citation4 Previous studies showed that the expression of ERα is gradually increased from prostate intraepithelial neoplasia, locally invasive cancers to metastatic lesions at both mRNA and protein levels.Citation5 Furthermore, in vivo studies using ERα knockout mice revealed that ERα is an important determinant of prostatic carcinogenesis.Citation3
Although several single nucleotide polymorphisms (SNPs) have been identified in the ERα gene, only a few have been extensively studied in prostate cancer. Furthermore, substantial controversial conclusions have been drawn by these studies. To address these issues, we chose two common polymorphisms in the ERα gene, PvuII (rs2234693 T>C) and XbaI (rs9340799 A>G), and conducted a meta-analysis on case-control studies between prostate cancer patients and prostate cancer-free controls. The aim of this study was to investigate the potential role of ERα PvuII and XbaI polymorphisms in the prostate cancer risk stratification.
Methods
The current meta-analysis was designed and reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (Table S1).Citation6
Literature search
Relevant papers published before October 1, 2016, were identified through a literature search in PubMed, Embase, and Cochrane databases using the following strategy: [(“gene mutation” OR “polymorphism”) OR (“SNP”) OR (“genetic variants”)] AND [(“prostate carcinoma”) OR (“prostate neoplasms”) OR (“prostate cancer”)] AND [(“estrogen receptor”) OR (“estradiol receptor”) OR (“ER”) OR (“ESR”)]. References from eligible articles were also manually searched to identify other potential publications.
Study eligibility
Studies were eligible for inclusion if, 1) studies focused on the association between ERα PvuII and XbaI polymorphisms and the prostate cancer risk; 2) they were case-control clinical studies on human subjects; 3) all diagnoses with prostate cancer were confirmed by pathological or histological examinations and controls were confirmed to be cancer free; 4) sufficient data were provided to estimate crude odds ratios (ORs) with 95% confidence intervals (CIs).
Two authors independently completed the screening process according to the Cochrane Collaboration guidelines.Citation7 The methodological quality of each retrieved study was assessed using the Strengthening the Reporting of Genetic Association Studies (STREGA) quality score system.Citation8 Forty-nine assessment items related to the quality appraisal were used in this score system with scores ranging from 0 to 49. Scores of 0–25, 26–37, and 38–49 were defined as low, moderate, and high quality, respectively (Table S2).
Data extraction
Detailed data from each publication were independently extracted by two authors using a standardized data extraction sheet and were checked by a third author (Table S3). The following information was extracted from each article: first author, year of publication, SNP ID and alternate name, ethnicity, country, language, study design, number of subjects, source of cases and controls, detecting sample, genotype method, allele and genotype frequencies, minor allele frequency (MAF), and evidence of Hardy–Weinberg equilibrium (HWE) in controls. Clinical parameters of prostate cancer patients were also collected, including tumor stage, serum PSA level at prostate cancer diagnosis, age at prostate cancer diagnosis, and smoking status. Risk of bias in each individual study was also evaluated.
Statistical analysis
The association strength between both polymorphisms and the prostate cancer risk was measured by ORs with 95% CIs under five genetic models: allele model, dominant model, recessive model, homozygous model, and heterozygous model. The statistical significance of the pooled OR was examined by the Z test. Heterogeneity between studies was estimated using the I2 test. I2<50% indicated that heterogeneity among studies was acceptable, and the fixed effects model (the Mantel–Haenszel method) was used. Otherwise, the random effects model (DerSimonian Laird method) was used. Subgroup analyses through ethnicity, country, source of cases and controls, genotype method, and whether HWE was in control or not were also conducted. We also stratified prostate cancer patients according to selected clinical param-eters. HWE was evaluated by the χ2 test in controls. Sensitivity analysis was performed by omitting each study in order to assess the stability of pooled results. Begger’s funnel plots and Egger’s linear regression test were used to evaluate publication bias. If significant publication bias existed, the trim and fill method was used to adjust pooled estimates.Citation9 All tests were two sided, and P<0.05 was considered statistically significant. Data analysis was performed using the STATA software (version 12.0; Stata Corp, College Station, TX, USA).
Results
Characteristics of included studies
According to the inclusion criteria, 18 case-control studies were included in the current meta-analysis.Citation10–Citation27 The flow chart presenting the selection process is shown in . A total of 3,317 prostate cancer patients and 8,324 controls were included in the synthesis. Years of publications ranged from 2001 to 2015. Genotype frequencies among controls were consistent with the HWE test, except for three studies.Citation11,Citation24,Citation26 STREGA scores ranged from 29 to 36, which suggested that all of these studies were qualified to the quantitative synthesis. The characteristics and methodological quality of the included studies are summarized in and S4.
Figure 1 Flow of information through different phases of the present meta-analysis.
Table 1 Characteristics and methodological quality of included studies
ERα PvuII (rs2234693 T>C) polymorphism and the prostate cancer risk
Eighteen studies, comprising 3,317 cases and 8,324 controls, were involved in the synthesis. The results showed that PvuII polymorphism was related to an increased prostate cancer risk in overall populations under four genetic models (allele model OR: 1.16, 95% CI: 1.04–1.29, P<0.01; dominant model OR: 1.24, 95% CI: 1.06–1.47, P=0.01; recessive model OR: 1.16, 95% CI: 1.04–1.30, P<0.01; homozygous model OR: 1.37, 95% CI: 1.09–1.72, P<0.01; and S5). To derive a more accurate estimation, subgroup analyses stratified by ethnicity were conducted. The results indicated that PvuII polymorphism was significantly correlated with a higher prostate cancer risk in Caucasians (allele model OR: 1.12, 95% CI: 1.03–1.21, P<0.01; dominant model OR: 1.19, 95% CI: 1.05–1.35, P<0.01; recessive model OR: 1.13, 95% CI: 1.03–1.29, P=0.04; homozygous model OR: 1.25, 95% CI: 1.06–1.46, P=0.01; and S5), but not in Asians. Furthermore, when grouped according to Gleason grades, PvuII polymorphism was found to be significantly correlated with high Gleason grade cancers (Gleason score ≥7) under the allele model (OR: 1.90, 95% CI: 1.44–2.50, P<0.01, ).
Table 2 Meta-analysis findings on the association between ERα PvuII (rs2234693 T>C) polymorphism and prostate cancer risk
Table 3 Subgroup analysis by Gleason score of the association between ERα PvuII (rs2234693 T>C) polymorphism and prostate cancer risk under the allele model (T vs C)
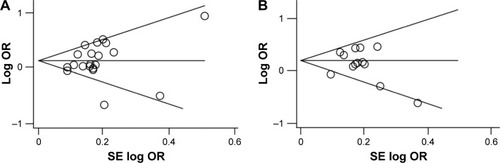
Sensitivity analysis suggested that individual studies did not affect pooled ORs (Figure S1). Begger’s funnel plot and Egger’s linear regression test did not show any statistical evidence of publication bias (). In addition, we excluded one study that deviated significantly from HWE, as the results showed that this non-HWE study had no effect on pooled ORs ().
Figure 2 Begg’s funnel plot for the publication bias test of the meta-analysis.
Abbreviations: ERα, estrogen receptor α; OR, crude odds ratio; SE, standard error.
ERα XbaI (rs9340799 A>G) polymorphism and the prostate cancer risk
The correlation between XbaI polymorphism and the prostate cancer risk was investigated in 13 studies with 1,946 cases and 2,744 controls. The results showed that XbaI polymorphism had a positive association with the risk of prostate cancer in overall populations under four genetic models (allele model OR: 1.23, 95% CI: 1.06–1.43, P<0.01; dominant model OR: 1.38, 95% CI: 1.11–1.72, P<0.01; recessive model OR: 1.24, 95% CI: 1.03–1.49, P=0.03; homozygous model OR: 1.44, 95% CI: 1.17–1.76, P<0.01; and S6). Similarly, we performed a sub-group analyses based on ethnicity to derive a more accurate estimate, and the results showed that XbaI polymorphism was significantly correlated with an increased risk of prostate cancer in Caucasians (allele model OR: 1.31, 95% CI: 1.07–1.61, P<0.01; dominant model OR: 1.43, 95% CI: 1.07–1.92, P=0.01; recessive model OR: 1.36, 95% CI: 1.09–1.69, P<0.01; homozygous model OR: 1.48, 95% CI: 1.17–1.88, P<0.01; and S6), but not in Asians.
Table 4 Meta-analysis findings on the association between ERα XbaI (rs9340799 A>G) polymorphism and prostate cancer risk
Sensitivity analysis suggested that individual studies did not affect pooled ORs (Figure S2). Begger’s funnel plot and Egger’s linear regression test did not show any statistical evidence of publication bias (). Moreover, we excluded two studies that deviated significantly from the HWE test, and the results showed that these two non-HWE studies had no effect on pooled ORs ().
Discussion
The role of PSA as a biomarker in prostate cancer remains unsatisfactory, leading to considerable overdiagnoses and overtreatment. This study on novel biomarkers in prostate cancer represents a long-standing hotspot in biomedical research.Citation28 Since ERα is an important determinant of prostatic carcinogenesis,Citation3 various studies have focused on SNPs in the ERα gene to determine their possible associations with the prostate cancer susceptibility. In the current study, we chose two common SNPs in the ERα gene, PvuII (rs2234693 T>C) and XbaI (rs9340799 A>G), and conducted a meta-analysis to evaluate their associations with prostate cancer susceptibility.
To our knowledge, the present work represents the largest meta-analysis performed to estimate the association of ERα SNPs with the risk of prostate cancer. The results showed that both PvuII and XbaI polymorphisms were correlated with an increased risk of prostate cancer in Caucasians, but not in Asians, and this disparity might be attributable to discrepancies in racial backgrounds and geography.Citation14,Citation29 Furthermore, there was a significant positive correlation of PvuII polymorphism and high Gleason grade cancers (Gleason score ≥7), which indicated its potential role in prostate cancer malignant transformation.
Both PvuII and XbaI polymorphisms lie in intron 1 of the ERα gene, which is part of the A/B domain, the trans-activating factor 1. This domain is an important site for stimulating transcription from certain estrogen-responsive promoters.Citation25 Among the possible explanations as to how these intronic polymorphisms affected prostate cancer risk are that, 1) both polymorphisms may be in linkage disequilibrium with other unknown variants in the gene, which may affect the gene expression or function;Citation24,Citation25,Citation30 2) the alteration of another unidentified gene was adjacent to the ERα gene;Citation30 and 3) intronic changes may have an impact on the expression of receptors by influencing the transcription through alternative splicing of the mRNA transcript.Citation24 Although the susceptibility of Caucasians to prostate cancer is known to be much higher than that of Asians, the underlying mechanism remains unknown.Citation28 Our findings demonstrated the different roles of two ERα SNPs in prostate cancer risk estimation between Caucasians and Asians, indicating the probability of inherited susceptibility to prostate cancer arising from different genomic ERα SNPs, which may help elucidate the pathogenesis of prostate cancer.
Several limitations in this meta-analysis should be noted when interpreting our findings. First, the statistical power was still limited in the stratified analysis. Second, heterogeneity between studies was observed, although subgroup analyses were conducted to minimize the perturbation. Third, only published studies in English were included. Published studies in other languages, ongoing studies, and unpublished data were not obtained. Given these limitations, our conclusions should be interpreted cautiously.
Conclusion
In summary, the current meta-analysis demonstrates the different roles of ERα PvuII and XbaI polymorphisms in prostate cancer risk stratification between Caucasians and Asians, indicating the probability of inherited susceptibility to prostate cancer arising from different genomic ERα SNPs, a finding that may help elucidate the pathogenesis of prostate cancer in Caucasians.
Author contributions
Yining Zhao, Xi Zheng, and Xiang Zhang conceived and designed the experiment; Yining Zhao performed the experiment; Lijie Zhang and Hua Jiang screened the studies; Lijie Zhang, Qiang Hu, and Xi Zheng extracted the data; Yining Zhao, Xi Zheng, and Shennan Shi performed the data analysis; and Yining Zhao and Xiang Zhang wrote the paper. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No 81372335).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer Statistics, 2017CA Cancer J Clin201767173028055103
- QinZLiXTangJAssociation between insulin-like growth factor-binding protein-3 polymorphism-202 A/C and the risk of prostate cancer: a meta-analysisOnco Targets Ther201695451545927660462
- JiaMDahlman-WrightKGustafssonJAEstrogen receptor alpha and beta in health and diseaseBest Pract Res Clin Endocrinol Metab201529455756826303083
- ZhaoXZLiuYZhouLJWangZQWuZHYangXYRole of estrogen in lung cancer based on the estrogen receptor-epithelial mesenchymal transduction signaling pathwaysOnco Targets Ther201582849286326491358
- RickeWAMcPhersonSJBiancoJJCunhaGRWangYRisbridgerGPProstatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signalingFASEB J20082251512152018055862
- KnoblochKYoonUVogtPMPreferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication biasJ Craniomaxillofac Surg2011392919221145753
- HigginsJPTGreenSCochrane Handbook for Systematic Reviews of Interventions2011 Available from: http://www.cochrane-handbook.orgAccessed July 1, 2014
- LittleJHigginsJPIoannidisJPSTrengthening the REporting of Genetic Association studies (STREGA) – an extension of the STROBE statementEur J Clin Invest200939424726619297801
- PetersJLSuttonAJJonesDRAbramsKRRushtonLPerformance of the trim and fill method in the presence of publication bias and between-study heterogeneityStat Med200726254544456217476644
- ModugnoFWeissfeldJLTrumpDLAllelic variants of aromatase and the androgen and estrogen receptors: toward a multigenic model of prostate cancer riskClin Cancer Res20017103092309611595700
- SuzukiKNakazatoHMatsuiHGenetic polymorphisms of estrogen receptor alpha, CYP19, catechol-O-methyltransferase are associated with familial prostate carcinoma risk in a Japanese populationCancer20039871411141614508827
- TanakaYSasakiMKaneuchiMShiinaHIgawaMDahiyaRPolymorphisms of estrogen receptor alpha in prostate cancerMol Carcinog200337420220812891629
- FukatsuTHirokawaYArakiTGenetic polymorphisms of hormone-related genes and prostate cancer risk in the Japanese populationAnticancer Res20042442431243715330195
- HernándezJBalicIJohnson-PaisTLAssociation between an eestrogen receptor alpha gene polymorphism and the risk of prostate cancer in black menJ Urol2006175252352716406987
- LowYLTaylorJIGracePBPhytoestrogen exposure, polymorphisms in COMT, CYP19, ESR1, and SHBG genes, and their associations with prostate cancer riskNutr Cancer2006561313917176215
- BerndtSIChatterjeeNHuangWYVariant in sex hormone-binding globulin gene and the risk of prostate cancerCancer Epidemiol Biomarkers Prev200716116516817220347
- KjaergaardADEllervikCTybjaerg-HansenAEstrogen receptor alpha polymorphism and risk of cardiovascular disease, cancer, and hip fracture: cross-sectional, cohort, and case-control studies and a meta-analysisCirculation2007115786187117309937
- OnsoryKSobtiRCAl-BadranAIHormone receptor-related gene polymorphisms and prostate cancer risk in North Indian populationMol Cell Biochem20083141–2253518483761
- SobtiRCGuptaLSinghSKSethAKaurPThakurHRole of hormonal genes and risk of prostate cancer: gene-gene interactions in a North Indian populationCancer Genet Cytogenet20081852788518722876
- GuptaLThakurHSobtiRCSethASinghSKRole of genetic polymorphism of estrogen receptor-alpha gene and risk of prostate cancer in north Indian populationMol Cell Biochem20103351–225526119904497
- BalistreriCRCarusoCCarrubaGMiceliVCandoreGGenotyping of sex hormone-related pathways in benign and malignant human prostate tissues: data of a preliminary studyOMICS201115636937421348640
- SissungTMDanesiRKirklandCTEstrogen receptor alpha and aromatase polymorphisms affect risk, prognosis, and therapeutic outcome in men with castration-resistant prostate cancer treated with docetaxel-based therapyJ Clin Endocrinol Metab2011962E368E37221106711
- SzendroiASpeerGTabakAThe role of vitamin D, estrogen, calcium sensing receptor genotypes and serum calcium in the pathogenesis of prostate cancerCan J Urol20111835710571621703046
- SafarinejadMRSafarinejadSShafieiNSafarinejadSEstrogen receptors alpha (rs2234693 and rs9340799), and beta (rs4986938 and rs1256049) genes polymorphism in prostate cancer: evidence for association with risk and histopathological tumor characteristics in Iranian menMol Carcinog201251Suppl 1E104E11722228197
- JurecekovaJSivonovaMKEvinovaAKlimentJDobrotaDThe association between estrogen receptor alpha polymorphisms and the risk of prostate cancer in Slovak populationMol Cell Biochem20133811–220120723737135
- PazarbasiAYilmazMBAlptekinDGenetic polymorphisms of estrogen receptor alpha and catechol-O-methyltransferase genes in Turkish patients with familial prostate carcinomaIndian J Hum Genet201319440841124497704
- LuXYamanoYTakahashiHAssociations between estrogen receptor genetic polymorphisms, smoking status, and prostate cancer risk: a case-control study in Japanese menEnviron Health Prev Med201520533233726251204
- AttardGParkerCEelesRAProstate cancerLancet201638710013708226074382
- FeiXLiuNLiHShenYGuoJWuZPolymorphisms of vitamin D receptor gene TaqI susceptibility of prostate cancer: a meta-analysisOnco Targets Ther201691033104527042096
- SundermannEEMakiPMBishopJRA review of estrogen receptor alpha gene (ESR1) polymorphisms, mood, and cognitionMenopause201017487488620616674
