Abstract
Angiogenesis (the growth of new blood vessels) is essential in most of the body’s physiological processes, such as in the normal functioning of the endometrium during and after the menstrual cycle. Vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP) are the mostly expressed angiogenic factors, especially, during the process of endometrial degeneration and remodeling. In carcinogenesis, tumor hypoxia-induced factors, through the process of “angiogenic switch”, stimulate the production of angiogenic factors, particularly VEGF and MMP. Subsequently, these angiogenic factors are associated with degradation, differentiation, proliferation, and migration of vascular endothelial cells, enhancing the formation of new blood vessels to supply the tumor with oxygen and nutrients. This process is equally significant for tumor development and metastasis. Hence, like in other cancers, the overexpression of MMP and VEGF in endometrial cancer (EC) seems to play a significant role in its tumorigenesis and metastasis. This research will discuss the influence of MMP and VEGF on angiogenesis, metastasis, and the prognosis of EC as well as the clinical importance of the factors in the diagnosis of EC.
Introduction
Angiogenesis (ie, growth of new blood vessels) is vital for several physiological processes including wound healing and tissue remodeling in the case of ischemic tissue diseases.Citation1,Citation2 It is also crucial for embryo implantation and post-menstruation endometrial repair.Citation3 Angiogenesis has also been associated with disorders such as diabetic retinopathy.Citation4,Citation5 Recently, several angiogenic factors have been studied, which include vascular endothelial growth factor (VEGF)-A, -B, -C, and -D and their receptors (VEGFR-1, VEGFR-2, and VEGFR-3), placental growth factor (PLGF), matrix metalloproteinase (MMP), platelets-derived growth factor (PDGF), fibroblast growth factor (FGF), tumor growth factor (TGF), and angiopoietins. VEGF-A has been associated with the proliferation, differentiation, degradation, and migration of endothelial cells. Consequently, this leads to the formation of new tubes in the extracellular matrix which is necessary for new vessel formation.Citation6 Under normal physiological processes, most of these factors are harmless; however, aberrations during angiogenesis may enhance tumor development.
In some types of cancer such as colorectal cancer, VEGF-associated angiogenesis seems to play a major role contributing to factors associated with unfavorable prognosis.Citation7 However, in other types of cancers, the role of angiogenesis in carcinogenesis and cancer progression is not well understood. This fact has led most physicians and researchers not to conclusively label angiogenesis as an independent factor in the diagnosis and prognosis of these cancers.
Endometrial cancer (EC) starts in the endometrium (the inner lining of the uterus). There are two known clinical pathological variants of EC – Type I and Type II. The most common variant of EC is endometrioid type endometrial carcinoma (EEC), which falls under Type I, and it accounts for about 80%−90% of all ECs. This type of cancer is estrogen dependent and is associated with endometrial hyperplasia, resulted from excessive unopposed estrogen secretion. It mostly affects post-menopausal women of the average age of 60 years. The prognosis of this cancer is good if early detected. In contrast, Type II EC is non-estrogen dependent and it consists of variants such as uterine serous carcinoma, clear cell carcinoma, and mucinous adenocarcinoma. It mostly affects perimenopause women and typically has poor prognosis.Citation8 In the United States alone, about 60,000 new cases of EC have been predicted in 2016, and about 10,000 women would die of the disease.Citation9
The purpose of this review is to evaluate the clinical significance of angiogenic markers, specifically VEGF-A and MMP-2/MMP-9, and their related influence in predicting metastasis and prognosis of the patients with EC. Furthermore, we will research whether these markers can be applied as diagnostic markers and in angiogenic inhibition-based therapy for treatment of this deadly disease.
Overview of angiogenesis in cancer
The essential role of angiogenesis in tumor growth was allegedly first proposed in 1971 by Judah Folkman. Tumor cells are those that have lost their ability to divide in a controlled fashion. Moreover, in order to grow, they need constant nourishment. According to Naumov et alCitation12 and Folkman,Citation13 the ability of tumors to progress from a non-angiogenic to an angiogenic phenotype is central to the progression of cancer and is termed the “angiogenic switch”.Citation10 This phenomenon is a prerequisite for tumor growth and metastasis. Tumor cells can migrate from their primary site to a new site through direct metastasis, blood vessels, or lymphatic system. However, if these tumors are of a microscopic size and angiogenic factors are inhibited, they may remain dormant without further growth. But if the inhibitor is suppressed, they resume rapid growth. Tumors that grow >1–2 mm are angiogenic dependent. Angiogenesis facilitates the escape of cancer cells through the new blood vessels and starts to form a new colony of cancers called metastasis. Tumors that are located in avascular areas (blood vessels scarce areas) mostly remain dormant for longer periods compared to those located in a well-vascularized area; the former is associated with late symptoms and late metastasis compared to the latter.Citation10–Citation13 Arguably, Gelao et alCitation14 found that the tendency of tumor dormancy is multifactorial and does not depend entirely on angiogenesis.
Vascular endothelial growth factor and matrix metalloproteinase in the endometrium and cancers in general
VEGF is a member of six structurally related proteins which include VEGF-B, -C, -D, and the PLGF. VEGF-A was the earliest to be found and the most studied of the members.Citation15 Originally known as vascular permeability factor (VPF), it is a prime angiogenic stimulus for vascular permeability based on its capability to bring on vascular leakage.Citation16,Citation17 Aberration of this factor may contribute to the pathology of the endometrium-like dysfunctional uterine bleeding.Citation18 In line with the process of endometrial remodeling, the release of VEGF is thought to be initiated by the hypoxia or ischemia of tissues. When a tissue is hypoxic, the hypoxia-induced factors are stimulated through a number of pathways to signal the release of different growth factors including VEGF, which then leads to the degradation of the extracellular matrix by the MMP.Citation19
In adults, angiogenesis and biological effects of VEGF, which induce endothelial cell proliferation and migration to form new vessels, are mediated by two tyrosine kinase receptors, VEGF receptor-1 and -2 (VEGFR-1 and VEGFR-2). Of the two tyrosine kinases, VEGFR-2 is thought to be more prominent and more involved in the angiogenic processes than VEGFR-1. VEGF can also bind to a lymphangiogenesis-related tyrosine kinase VEGFR-3.Citation16 According to Lucas et alCitation20 VEGF expression is restricted not only to vascular endothelial blood cells but it can also be expressed in other cells like macrophages.
MMPs, a family of zinc-dependent endoproteinases, are key players in the degradation of extracellular matrix and basement membranes, as well as in vascularization and cell migration.Citation21,Citation22 There are 24 estimated types of MMP genes and 23 types of MMP proteins known so far which have diverse physiological and pathological functions.Citation23–Citation25 The physiological functions of MMPs are controlled by the proteins called tissue inhibitors of the matrix metalloproteinase (TIMPs), which are also expressed in tumor sites.Citation26 MMP-2 and MMP-9 are also known as the gelatinases (gelatinases A and B, respectively).
The angiogenic markers MMP and VEGF are considered to be involved in the remodeling of endometrium after menstruation. Goffin et al using normal endometrial tissues of healthy women with regular menstrual cycles found that the MMP-9 mRNA was highly expressed during the menstrual phase while MMP-2 mRNA remained consistent throughout the whole cycle.Citation27 In another study, Skinner et al using the tissue samples of women with normal, regular menstrual cycle reported that MMP-9 protein was immunolocalized to glandular epithelial cells throughout the menstrual cycle with maximal intensity in the glandular epithelium.Citation28 These constant cyclic changes in the endometrium, degeneration of the superficial layer, and reconstruction of a new one are all associated with angiogenesis and neovascularization.
In patients with anovulatory dysfunctional uterine bleeding, Shan et al found that VEGF, MMP-2, and MMP-9 were equally overexpressed, and silencing VEGF significantly reduced the expression of MMP-2 and MMP-9.Citation29 In the case of implantation, MMP activity is reduced and VEGF activity is increased to prepare the endometrium for placentation. Therefore, the two angiogenic markers work hand in hand to ensure the normal functioning of the endometrium during menstrual cycle and implantation.Citation30
VEGF and MMP, simultaneously, have been found to have significant impacts on either tumor invasion, metastasis, advanced tumor stage, or adverse prognosis. For example, using thyroid cancer cells, Jia et alCitation31 studied the angiogenesis-related protein S100A4 and discovered that its downregulation resulted also in the downregulation of VEGF together with MMP-9. Additionally, the knockdown of both VEGF and MMP-9 resulted in significant inhibition of the thyroid cells invasion, metastasis, and angiogenesis, respectively. Moreover, Zheng et alCitation32 by using 249 gastric cancer tissues found that MMP-2, MMP-9, and VEGF were positively correlated with tumor size, depth of invasion, lymphatic and venous invasion, lymph node metastasis, and microvascular density (MVD) of gastric carcinoma. In addition, VEGF expression was positively linked with levels of MMP-2 and MMP-9. Furthermore, VEGF, MMP-2, and MMP-9 were also found to be significantly related to recurrence of hepatocellular carcinoma in the patients who underwent liver transplant.Citation33
In gynecological cancers, for example in ovarian cancer, the expression of VEGF was closely related to the expression of MMP-2 which in turn resulted in the increased invasion of the epithelial ovarian cancer cells in vitro and in vivo.Citation34,Citation35 In addition, in uterine cervical cancer, MMP-2 and VEGF were found to be correlated with adverse prognosis in young women.Citation36
The mechanism resulting to tumor metastasis has been elucidated using “angiogenic switch” phenomenon. summarizes the process of angiogenic switch and metastasis as previously described by Folkman.Citation13
Figure 1 Influence of VEGF-A in EC.
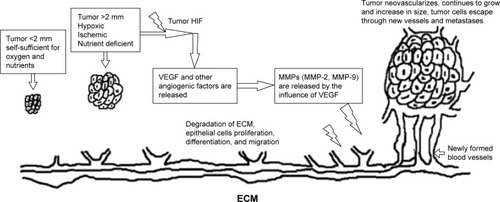
The overexpression of VEGF and its relationship with prognosis and metastasis have been widely studied in different types of solid cancers. For instance, VEGF angiogenesis was found to be an independent prognostic indicator in breast cancer and was associated with poor prognosis and metastasis.Citation37 In non-small cell lung carcinoma, Bremnes et alCitation38 found that VEGF was found to be associated with poor prognosis, disease aggressiveness, and poor survival. In ovarian cancer,Citation39 cervical cancer,Citation40 colon cancers, and melanomas,Citation41,Citation42 the overexpression of serum VEGF was either associated with lower disease-free survival (DFS), metastasis, or poor disease progression. According to the study of Dai et al, Piastowska-Ciesielska et al, and Yang et al,Citation43–Citation45 it is evident that VEGF is overexpressed in endometrial malignancies, and it is thought to be an important angiogenic marker in the carcinogenesis of this type of cancer.
The mechanism in which these tumors induce signals for VEGF expression is said to be due to tumor hypoxia which then signals the production of cellular hypoxia-inducible factor (HIF). According to North et al,Citation46 this factor initiates the overproduction of VEGF and starts the angiogenic process through the tyrosine kinase pathway which stimulates endothelial cell proliferation, migration, and the formation of new vessels to supply oxygen and nutrients to the tumor which facilitate its growth and metastasis.
Saarelainen et al, while assessing the prognostic significance of VEGF in EC from preoperative sera of 98 women presenting with EC, reported that the serum concentrations of VEGF and its receptors were assessed by enzyme-linked immunosorbent assay (ELISA). The results were correlated to the presence of deep (>50%) myometrial invasion and metastasis. The serum concentration of VEGF was higher in the group with metastasis than in the group without metastasis (median [range]: 743 pg/mL [546–1,183 pg/mL] vs 383 pg/mL [31–1,524 pg/mL]) and hence VEGF was related to poor prognosis and metastasis.Citation47 In addition, Topolovec et alCitation48 found that the overexpression of VEGF was also associated with deep myometrial invasion, advanced histological stage, and grade of differentiation.
Contrary to the above findings, Soufla et alCitation49 found that VEGF expression status (whether high or low) corresponded only with the malignant transformation of the endometrium but did not correlate with either tumor stage, myometrial invasion, or grade of differentiation; therefore, its expression was not a significant indicator of prognosis. Dobrzycka et alCitation52 went further to investigate the expression of VEGF in the two pathological types of EC and found that VEGF expression was associated with advanced stage in Type II but not in Type I EC and was not found to be an independent prognostic factor or corresponding with DFS.Citation50,Citation51 In 2010, the same authors evaluated the prognostic significance of VEGF using 84 EEC tissue samples; their results showed that out of 84 cancer tissue samples, strong positive expression of VEGF was observed in 35 (42%) tumors. There was a significant correlation between histological grade, clinical stage, and VEGF overexpression. The 5-year DFS of patients with VEGF overexpression was significantly lower than that of those with a weakly positive or negative tumor.Citation52 Furthermore, Wang et al found that serum VEGF overexpression in ECs was significantly higher in late stage EC than in early stage EC and it increased significantly from well differentiated to poorly differentiated tumors.Citation53 Moreover, Topolovec et alCitation48 and Yang et alCitation45 in two different studies also found that elevated VEGF expression was significantly associated with either deep myometrial invasion, poor differentiation and histologic type, or lymph node metastasis in patients with EC.
Influence of matrix metalloproteinase-2 and matrix metalloproteinase-9 in endometrial cancer
Association of MMPs with carcinogenesis has been studied in different types of solid cancers such as colon cancers,Citation54 ovarian cancer,Citation55 and breast cancer.Citation56 These studies found MMPs to correlate either with cancer progression, disease aggressiveness, metastasis, and poor disease prognosis or with poor DFS. This part of the review will discuss the influence of MMP-2 and MMP-9 on metastasis and the overall prognosis of EC.
There is evidence that MMP-2 and MMP-9 are overexpressed in EC, especially in EEC; however, their expression varies according to the grade of differentiation or histological grades.Citation57–Citation60 There are scarce studies on how these gelatinases influence prognosis and metastasis of EC. However, the limited data already available provide insight into how important gelatinases are in the pathogenesis of EC. Li et alCitation61 examined the correlation of MMP-2 expression with the clinical characteristics and prognosis of the endometrial adenocarcinoma in patients aged 32–80 years in 81 paraffin-embedded samples. They found that the overexpression of MMP-2 was negatively correlated with tumor differentiation and prognosis of endometrial adenocarcinoma.
In determining the prognostic significance of MMP-2 in EC, Honkavuori-Toivola et al used 225 tissue samples of patients with EEC; in their results, they found MMP-2 negative patients had a significantly higher 5-year survival rate compared to MMP-2 positive patients. Also relative risk of death in the latter group was 4.7 times higher compared to the former group of patients.Citation62 Additionally, Yu et al used 128 tissue samples from Chinese women with EC to study the MMP-9 expression and its correlation with survival and clinicopathological features. Their results showed that there was a significantly higher expression of MMP-9 in the tissues; this overexpression was correlated only with lymph node metastasis, histological grade, and myometrial invasion but was not correlated with patient survival.Citation63 However, Bogusiewicz et al, in studying the activities of MMP-2 and MMP-9 in EC, used 28 samples of EC and 15 samples of normal endometrium. They observed significantly higher activity of MMP-9 in EC samples compared to normal endometrium, which was related to EC progression, however there was no changes of activity for MMP-2 in EC compared to the normal endometrium. These same results were found with Yilmaz et al.Citation64,Citation65
In their experiment to further explore whether the stromal MMP-2 and MMP-9 or epithelial MMP-2 and MMP-9 were more significant in determining prognosis in EC patients, Puljiz et al found that in univariate analysis stromal MMP-2 expression was identified as one of the significant determinants of EC recurrence while epithelial MMP-2 expression and epithelial and stromal MMP-9 expressions were not. During multivariate analysis, strong staining of stromal MMP-2 increased the risk of EC recurrence in a subgroup of patients aged ≥63.6 years with endometrioid adenocarcinoma and papillary serous carcinoma, and all FIGO (International Federation of Gynecology and Obstetrics revised version of 2009) stage 1 diseases.Citation66 The study by Karahan et al supported MMP-2 and MMP-9 to significantly correlate with myometrial, vascular, and lymphatic invasions.Citation67 Contrarily, authors like Aglund et al found that the protein overexpression of both MMP-2 and MMP-9 was associated with poor survival and histological grades, and only MMP-9 correlated with clinical stage of EC and not MMP-2. Furthermore, the overexpression of both proteins was not associated with any type of invasion.Citation68 However, Graesslin et al in two different studiesCitation69,Citation70 revealed that only MMP-2 correlated with histological grade and was significant as a prognostic marker for local and distal metastases in endometrial carcinoma.
Concluding remarks for VEGF-A
The prognostic and metastatic effects of VEGF have been associated with the value/number of new blood vessels formed, which is referred to as MVD. This means that the higher the value of MVD, the higher is the likelihood of deep myometrial invasion, vascular metastasis, poor grade, poor prognosis, and consequently, less DFS. According to the study by Horrée et alCitation71 and the above theory, it seems that the more the new blood vessels are formed the higher are the chances of tumor metastasis and poor prognosis.
There is a positive correlation between the overexpression of VEGF and MVD with either increased deep myometrial invasion, poorly differentiated tumors, histological-type FIGO stage, lymphovascular infiltration, or lymph node involvement.Citation48,Citation72,Citation73
In this review, the overexpression of VEGF seems to play a critical role in determining prognosis and metastasis and hence overall survival in EC. It is well established that the uterus is a hormonally controlled organ; therefore, in order to widely grasp the extent to which this angiogenic factor affects prognosis and metastasis of EC, the influence of hormones such as estrogen and progesterone in relation to VEGF expression cannot be neglected.Citation74 In addition, since the introduction of the use of the angiogenesis inhibitors for VEGF, such as bevacizumab, as a single agent or in combination with other agents like chemotherapy drugs for the treatment of EC, many patients have benefited in spite of the claimed major side effects, especially those of cardiovascular and gastrointestinal systems.Citation75,Citation76 In view of the above, serum levels of VEGF should be considered before, during, and after treatment and as a routine checkup for EC patients at a certain interval. This is significant in comparing the patients’ disease progression and evaluating treatment efficiency.
Concluding remarks for MMP-2/MMP-9
In conclusion, both MMP-2 and MMP-9 seem to play significant independent roles in tumor/cancer development, thereby being involved one way or the other as a prognosis and metastasis indicator. However, in EC, there is still a controversy involving which of the MMPs are more involved in metastasis, advanced tumor grade, histological grade, depth of invasion, and poor prognosis. Moreover, most of the cited authors pointed out that the enhanced expression of MMP-2 is more associated with disease progression and aggressiveness and more unfavorable prognosis compared to that of MMP-9 in EC.
In addition, studies concerning the influence of these angiogenic markers on EC variants, histological grade, and the stage are greatly needed as they will give a deeper understanding of their involvement in the progression of EC. However, further studies will determine whether stromal or epithelial MMP-2/MMP-9 has much significance in carcinogenesis of EC. The above concerns may widen the knowledge in the field of metalloproteinase-based therapy which can help reduce the mortality and morbidity and prolong the survival rate in endometrial carcinoma patients.
Finally, understanding the connected pathways that associate VEGFs and MMPs in carcinogenesis and their contributing factors of their enhanced expression in either serum or cancer tissues, and their association with other genes that are involved in apoptotic, migration, and differentiation of cells, may contribute significantly in the establishment of angiogenic-based inhibition therapy for EC.
Disclosure
The authors report no conflicts of interest in this work.
References
- PolveriniPJAngiogenesis and wound healing: basic discoveries, clinical implications, and therapeutic opportunitiesEndod Top2011241130145
- DiPietroLAAngiogenesis and scar formation in healing woundsCurr Opin Rheumatol2013251879123114588
- DemirRYabaAHuppertzBVasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantationActa Histochem2010112320321419481785
- Abu El-AsrarAMNawazMIKangaveDSiddiqueiMMOlaMSOpdenakkerGAngiogenesis regulatory factors in the vitreous from patients with proliferative diabetic retinopathyActa Diabetol201350454555121947384
- ZhouJWangSXiaXRole of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathyCurr Eye Res201237541642022409294
- TammelaTEnholmBAlitaloKPaavonenKThe biology of vascular endothelial growth factorsCardiovasc Res200565355056315664381
- WangYYaoXGeJHuFZhaoYCan vascular endothelial growth factor and microvessel density be used as prognostic biomarkers for colorectal cancer? A systematic review and meta-analysisScientificWorldJournal2014201410273625143961
- BokhmanJVTwo pathogenetic types of endometrial carcinomaGynecol Oncol198315110176822361
- Cancer.org [homepage on the Internet]2017 American Cancer Society, Inc Available from: http://www.cancer.org/cancer/endometrialcancer/detailedguide/endometrial-uterine-cancer-key-statisticsAccessed 4 January 2017
- NaumovGNAkslenLAFolkmanJRole of angiogenesis in human tumor dormancy: animal models of the angiogenic switchCell Cycle20065161779178716931911
- NaumovGNFolkmanJStraumeOTumor dormancy due to failure of angiogenesis: role of the microenvironmentClin Exp Metastasis2009261516018563595
- NaumovGNFolkmanJStraumeOAkslenLATumor-vascular interactions and tumor dormancyApmis20081167–856958518834403
- FolkmanJRole of angiogenesis in tumor growth and metastasisSemin Oncol20026Suppl 161518
- GelaoLCriscitielloCFumagalliLTumour dormancy and clinical implications in breast cancerEcancermedicalscience2013732023717341
- YamazakiYMoritaTMolecular and functional diversity of vascular endothelial growth factorsMol Divers200610451552716972015
- FerraraNGerberHPLeCouterJThe biology of VEGF and its receptorsNat Med20039666967612778165
- LaiTHVlahosNShihIMZhaoYExpression patterns of VEGF and Flk-1 in human endometrium during the menstrual cycleJ Reprod Infertil20151613925717429
- ZhangXQiCLinJEnhanced expressions of matrix metalloproteinase (MMP)-2 and -9 and vascular endothelial growth factors (VEGF) and increased microvascular density in the endometrial hyperplasia of women with anovulatory dysfunctional uterine bleedingFertil Steril20109372362236719249761
- ChristofferssonGVågesjöEVandoorenJVEGF-A recruits a proangiogenic MMP-9–delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissueBlood2012120234653466222966168
- LucasTWaismanARanjanRDifferential roles of macrophages in diverse phases of skin repairJ Immunol201018473964397720176743
- ChangCWerbZThe many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasisTrends Cell Biol20011111S37S4311684441
- ZítkaOKukackaJKrizkovSMatrix metalloproteinasesCurr Med Chem201017313751376820846107
- Hadler-OlsenEFadnesBSylteIUhlin-HansenLWinbergJORegulation of matrix metalloproteinase activity in health and diseaseFEBS J20112781284521087458
- Hadler-OlsenEWinbergJOUhlin-HansenLMatrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targetsTumor Biol201334420412051
- KleinTBischoffRPhysiology and pathophysiology of matrix metal-loproteasesAmino Acids201141227129020640864
- DeryuginaEIQuigleyJPMatrix metalloproteinases and tumor metastasisCancer Metastasis Rev200625193416680569
- GoffinFMunautCFrankenneFExpression pattern of metalloproteinases and tissue inhibitors of matrix-metalloproteinases in cycling human endometriumBiol Reprod200369397698412773401
- SkinnerJLRileySCGebbieAEGlasierAFCritchleyHORegulation of matrix metalloproteinase-9 in endometrium during the menstrual cycle and following administration of intrauterine levonorgestrelHuman Reprod1999143793799
- ShanBLiWYangSYLiZREstrogen up-regulates MMP2/9 expression in endometrial epithelial cell via VEGF-ERK1/2 pathwayAsian Pac J Trop Med201361082683023870474
- FurukawaYKawanoYFukudaJMatsumotoHNaraharaHThe production of vascular endothelial growth factor and metalloproteinase via protease-activated receptor in human endometrial stromal cellsFertil Steril200991253554118314110
- JiaWGaoXJZhangZDYangZXZhangGS100A4 silencing suppresses proliferation, angiogenesis and invasion of thyroid cancer cells through downregulation of MMP-9 and VEGFEur Rev Med Pharmacol Sci201317111495150823771538
- ZhengHTakahashiHMuraiYExpressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinomaAnticancer Res2006265A3579358317094486
- ZhangQChenXZhouJCD147, MMP-2, MMP-9 and MVD-CD34 are significant predictors of recurrence after liver transplantation in hepatocellular carcinoma patientsCancer Biol Ther20065780881416775432
- ZhangAMengLWangQEnhanced in vitro invasiveness of ovarian cancer cells through up-regulation of VEGF and induction of MMP-2Oncol Rep200615483183616525667
- BelottiDCalcagnoCGarofaloAVascular endothelial growth factor stimulates organ-specific host matrix metalloproteinase-9 expression and ovarian cancer invasionMol Cancer Rese200864525534
- NoriyukiMSumiTZhiXVascular endothelial growth factor, matrix metalloproteinases, and cyclooxygenase-2 influence prognosis of uterine cervical cancer in young womenInt J Oncol200731353153617671679
- AliEMShetaMEl MohsenMAElevated serum and tissue VEGF associated with poor outcome in breast cancer patientsAlexandria J Med2011473217224
- BremnesRMCampsCSireraRAngiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and bloodLung Cancer200651214315816360975
- BandieraEFranceschiniRSpecchiaCPrognostic significance of vascular endothelial growth factor serum determination in women with ovarian cancerISRN Obstet Gynecol2012201224575622792477
- DuKGongHYGongZMInfluence of serum VEGF levels on therapeutic outcome and diagnosis/prognostic value in patients with cervical cancerAsian Pac J Cancer Prev2013152087938796
- CrosbyMBYangHGaoWZhangLGrossniklausHESerum vascular endothelial growth factor (VEGF) levels correlate with number and location of micrometastases in a murine model of uveal melanomaBr J Ophthalmol201195111211720819828
- RajabiPNeshatAMokhtariMRajabiMAEftekhariMTavakkoliPThe role of VEGF in melanoma progressionJ Res Med Sci201217653453923626629
- DaiHZhaoSXuLChenADaiSExpression of Efp, VEGF and bFGF in normal, hyperplastic and malignant endometrial tissueOncol Rep201023379579920127022
- Piastowska-CiesielskaAWPłuciennikEWójcik-KrowirandaKBieńkiewiczABednarekAOchędalskiTAnalysis of the expression of angiotensin II type 1 receptor and VEGF in endometrial adenocarcinoma with different clinicopathological characteristicsTumor Biol2012333767774
- YangYLiuHLiWExpression of Mta-1 and VEGF and their correlation in the endometrial cancerXi Bao Yu Fen Zi Mian Yi Xue Za Zhi2010267682684 Chinese20619094
- NorthSMoennerMBikfalviARecent developments in the regulation of the angiogenic switch by cellular stress factors in tumorsCancer Lett2005218111415639335
- SaarelainenSKStaffSPeltonenNEndoglin, VEGF, and its receptors in predicting metastases in endometrial carcinomaTumor Biol201435546514657
- TopolovecZĆoručićABabićDVascular endothelial growth factor and intratumoral microvessel density as prognostic factors in endometrial cancerColl Antropol201034244745320698116
- SouflaGSifakisSPorichisFSpandidosDAPrognostic value of tgfb1 protein in endometrioid adenocarcinomaEur J Clin Invest2013431799023176363
- DobrzyckaBMackowiak-MatejczykBKinalskiMTerlikowskiSJPretreatment serum levels of bFGF and VEGF and its clinical significance in endometrial carcinomaGynecol Oncol2013128345446023206584
- DobrzyckaBTerlikowskiSJKowalczukOKulikowskiMNiklinskiJSerum levels of VEGF and VEGF-C in patients with endometrial cancerEur Cytokine Netw2011221455121411409
- DobrzyckaBTerlikowskiSKwiatkowskiMGarbowiczMKinalskiMChyczewskiLPrognostic significance of VEGF and its receptors in endometrioid endometrial cancerGinekol Pol201081642242520695190
- WangXPanZLiAZhuMStudy on the correlation of Xinjiang endometrial tumor and vascular endothelial growth factorZhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi201024298100 Chinese21110424
- ParkKSKimSJKimKHKimJCClinical characteristics of TIMP2, MMP2, and MMP9 gene polymorphisms in colorectal cancerJ Gas-troenterol Hepatol2011262391397
- HuXLiDZhangWZhouJTangBLiLMatrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasionArch Gynecol Obstet201228661537154322832979
- MinKWKimDHDoSIExpression patterns of stromal MMP-2 and tumoural MMP-2 and -9 are significant prognostic factors in invasive ductal carcinoma of the breastApmis2014122121196120624909183
- GrybosABarJThe relationships between the immunoexpression of KAI1, MMP-2, MMP-9 and steroid receptors expression in endometrial cancerFolia Histochem Cytobiol201452318719425308734
- PlanagumàJLiljeströmMAlamedaFMatrix metalloproteinase-2 and matrix metalloproteinase-9 codistribute with transcription factors RUNX1/AML1 and ETV5/ERM at the invasive front of endometrial and ovarian carcinomaHum Pathol2011421576720970160
- WangXChenXExpression and significance of Twist1 and MMP-2 in endometrial endometrioid adenocarcinomaZhonghua Zhong Liu Za Zhi2012348588591 Chinese23158991
- WeigelMTKrämerJSchemCDifferential expression of MMP-2, MMP-9 and PCNA in endometriosis and endometrial carcinomaEur J Obstet Gynecol Reprod Biol20121601747822056701
- LiSShenXYangZClinical significance of MMP2 overexpression in endometrial adenocarcinomaNan Fang Yi Ke Da Xue Bao2014343423425 Chinese
- Honkavuori-ToivolaMSantalaMSoiniYTurpeenniemi-HujanenTTalvensaari-MattilaACombination of strong MMP-2 and weak TIMP-2 immunostainings is a significant prognostic factor in endometrial carcinomaDis Markers201335426126624344400
- YuFJiangQZhouYAbnormal expression of matrix metalloproteinase-9 (MMP9) correlates with clinical course in Chinese patients with endometrial cancerDis Markers201232532132722674412
- BogusiewiczMStryjecka-ZimmerMRechbergerTActivity of matrix metalloproteinases -2 and -9 (MMP-2 and MMP-9) and content of their tissue inhibitors in endometrial cancer–a preliminary studyGinekol Pol2007785366372 Polish17867327
- YilmazEKoyuncuogluMGörkenİBExpression of matrix metalloproteinase-2 and survivin in endometrioid and nonendometrioid endometrial cancers and clinicopathologic significanceJ Gynecol Oncol2011222899621860734
- PuljizMPuljizZVucemiloTPrognostic significance of matrix metalloproteinases 2 and 9 in endometrial cancerColl Antropol20123641367137223390835
- KarahanNGüneyMBaspinarSOralBKapucuogluNMunganTExpression of gelatinase (MMP-2 and MMP-9) and cyclooxygenase-2 (COX-2) in endometrial carcinomaEur J Gynaecol Oncol200728318418817624083
- AglundKRauvalaMPuistolaUGelatinases A and B (MMP-2 and MMP-9) in endometrial cancer – MMP-9 correlates to the grade and the stageGynecol Oncol200494369970415350361
- GraesslinOCortezAFauvetRLorenzatoMBirembautPDaraïEMetalloproteinase-2, -7 and -9 and tissue inhibitor of metalloproteinase-1 and -2 expression in normal, hyperplastic and neoplastic endometrium: a clinical-pathological correlation studyAnn Oncol200617463764516407419
- GraesslinOCortezAUzanCBirembautPQuereuxCDaraïEEndometrial tumor invasiveness is related to metalloproteinase 2 and tissue inhibitor of metalloproteinase 2 expressionsInt J Gynecol Cancer20061651911191717009991
- HorréeNvan DiestPJvan der GroepPSie-GoDMHeintzAPHypoxia and angiogenesis in endometrioid endometrial carcinogenesisCell Oncol200729321922717452774
- GusetGCostiSLazarEExpression of vascular endothelial growth factor (VEGF) and assessment of microvascular density with CD34 as prognostic markers for endometrial carcinomaRom J Morphol Embryol201051467768221103625
- WangJTaylorAShoweilRExpression profiling and significance of VEGF-A, VEGFR2, VEGFR3 and related proteins in endometrial carcinomaCytokine20146829410024845798
- FerraraNChenHDavis-SmythTVascular endothelial growth factor is essential for corpus luteum angiogenesisNat Med1998433363409500609
- BoglioloSCassaniCGardellaBCurrent opinion on bevacizumab on endometrial cancer treatmentExp Opin Biol Ther2015152299307
- AghajanianCSillMWDarcyKMPhase II trial of bevacizumab in recurrent or persistent endometrial cancer: a gynecologic oncology group studyJ Clin Oncol201129162259226521537039
