Abstract
Objective
The aim of this study was to evaluate the prognostic value of both platelet to lymphocyte ratio (PLR) and metabolic syndrome (MetS) in colorectal cancer (CRC) patients.
Patients and methods
We retrospectively enrolled 1,163 CRC patients. Preoperative values of PLR were stratified into three groups according to cut-off values of 120 and 220. The Kaplan–Meier analysis was used to calculate cumulative survival rate related to PLR and MetS. Cox proportional hazard regression models were used to analyze potential risk factors and the prognosis associated with PLR and MetS in CRC patients.
Results
PLR was significantly higher in the MetS(+) group as compared to MetS(−) group (P=0.039). An elevated PLR was significantly associated with mortality (P=0.014), but not the existence of MetS (P=0.235). In multivariate regression analysis, PLR was an independent risk factor for overall survival (OS) (P=0.046). For the subgroup with a PLR >220, MetS was an independent predictor for both OS and disease-free survival (P=0.039 and P=0.047, respectively) by multivariate analysis adjusting for confounding covariates. In addition, the presence of MetS was associated with a 2-fold increased risk of mortality and tumor recurrences (hazard ratio [HR] =2.0 and HR =1.9, P<0.05, respectively).
Conclusion
Preoperative PLR was associated with MetS in CRC patients. Testing for the combined presence of PLR and MetS could potentially improve the predictive accuracy of CRC prognosis.
Introduction
Colorectal cancer (CRC) is the third most diagnosed malignant tumor worldwide with over 600,000 related deaths a year.Citation1 In People’s Republic of China, an estimated 376,300 new cases and 191,000 deaths from CRC occurred in 2015.Citation2 Understanding the risk factors for CRC may improve the development of pro-active precision medicine.
Metabolic syndrome (MetS) is characterized by a cluster of metabolic disturbances, including high blood pressure, obesity, atherogenic dyslipidemia, and impaired glucose metabolism.Citation3,Citation4 In the last recent decade, epidemiological and clinical studies have demonstrated a close link between MetS and an increased risk of CRC.Citation5,Citation6 However, the correlation between MetS and quantitative analysis of CRC outcome remained unclear.Citation7–Citation10 One possible explanation might be related to the inter-individual and time-related differences in host systemic inflammatory status, and the quantification of an elevated inflammatory response which is associated with the progression and prognosis of CRC.Citation11–Citation13 Furthermore, chronic low-grade inflammation leads to metabolic disturbances, which in turn lead to insulin resistance,Citation14 altered glucose and lipid metabolism triggering inflammation.Citation15
Systemic inflammation can easily be assessed by means of peripheral blood markers such as serum white blood cells, neutrophils, lymphocytes and platelets, and acute-phase proteins. Platelet to lymphocyte ratio (PLR) has been reported to be associated with the prognosis of CRC.Citation16–Citation18 Previous studies indicated that increased platelet counts were associated with MetS in adults.Citation19,Citation20 Akboga et al reported that increased PLR was significantly associated with MetS in a Turkish population.Citation21 Whether the presence of MetS, combined with the different levels of PLR, could improve the ability to predict prognosis of CRC remains unknown. As such, we aimed to evaluate the prognosis of the association of PLR with MetS in CRC patients.
Patients and methods
Study patients
In this cross-sectional study, data were retrospectively collected from hospital records of 1,163 patients diagnosed with colorectal adenocarcinoma admitted to the First Affiliated Hospital of Wenzhou Medical University between April 2005 and April 2011. Exclusion criteria were as follows: clinical evidence of infection, hematological disease, enterobrosis, intestinal obstruction, neoadjuvant therapy, and patients with familial adenomatous polyposis syndrome. The study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University and all study patients signed a written informed consent.
Diagnostic criteria of MetS
MetS is considered a cluster of metabolic disturbances.Citation3 In this study, we adopted the criteria proposed by the Chinese Diabetes Society in 2004.Citation22 MetS is defined as the presence of three or more of the following criteria: 1) body mass index (BMI) ≥25 kg/m2; 2) anti-hypertensive drug administration and (or) systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg; 3) triglycerides ≥1.7 mmol/L and (or) high-density lipoprotein (HDL) cholesterol <0.9 mmol/L (male), <1.0 mmol/L (female); and 4) fasting plasma glucose ≥6.1 mmol/L or 2 h postprandial glucose ≥7.8 mmol/L.
Clinical–pathological and laboratory data
Demographic information including date of birth, gender, age at CRC diagnosis, tobacco use, history of hypertension and diabetes, and family cancer history were recorded. Detailed clinical data such as body weight, height, and blood pressure were recorded within 1 week before surgery. Preoperative blood values including white blood cell, neutrophil, lymphocyte, monocyte and platelet counts were collected. PLR was calculated as the absolute platelet count divided by the absolute lymphocyte count. BMI was calculated as the weight in kg divided by the square of height in m (kg/m2).
Patients with CRC were treated primarily with surgical resection with adjuvant treatment according to the National Comprehensive Cancer Network guidelines. Tumor staging was performed according to the seventh edition of the American Joint Committee on Cancer staging manual.Citation23 In addition, information related to tumor location, histological differentiation, and vascular invasion was obtained from pathology reports.
Follow-up evaluation
Follow-up evaluation was conducted every 3–6 months for the first 2 years after hospital discharge, every 6 months thereafter for a total of 5 years, and every year thereafter. Colonoscopy and computed tomography (CT) were performed at postoperative follow-up appointments in addition to blood analysis, including carcinoembryonic antigen (CEA) measurements. Tumor recurrence indicated by elevated CEA, abnormal findings on colonoscopy or CT scans, was defined as an earlier follow-up event. Information on death was obtained either from the patient’s social security death index, outpatient medical records, or notifications from the relatives of the patients. The deadline of follow-up was August 1, 2016. Overall survival (OS) was defined as the time from the date of surgery to the date of death or the date of last follow-up. Disease-free survival (DFS) was defined as the time from the date of surgery to the time of recurrence or date of last follow-up.
Statistical analysis
All continuous data were expressed as the mean ± standard deviation and compared using Student’s t-test or Mann– Whitney U test according to the data distribution. Categorical data were expressed in numbers (%) and compared by using the Chi-square test or Kruskal–Wallis test. Based on the optional cut-off values of our previous study,Citation16 the distribution of PLR, and the size of the study population with MetS, patients were stratified into three groups according to the two cut-off values (120, 220). The Kaplan–Meier survival function and log-rank tests were used to assess differences in OS and DFS. The prediction of different variables for the risks of CRC was calculated by Cox proportional hazard regression analyses. The risk effect-size estimates were expressed as hazard ratio (HR) with 95% confidence interval (CI). Variables with P≤0.1 from univariate Cox regression analysis were used in multivariate analysis by forward stepwise selection. All P-values were two-sided and a P-value <0.05 was considered as statistically significant. Statistical analysis were performed using the SPSS statistical software package, version 19.0 (IBM Corporation, Armonk, NY, USA) and MedCalc version 13.0 (MedCalc Software, Mariakerke, Belgium).
Results
Baseline characteristics
Demographic and clinical characteristics are shown in . A total of 234 (20.1%) patients were identified to meet the criteria of MetS. The mean age of patients was 65 years, and the majority were male (60.2%). Six hundred and thirty-eight patients (54.9%) were diagnosed with rectal cancer. The majority of tumors were histologically well and moderately differentiated (74.6%). At initial diagnosis, 16.3% of the CRC patients presented with stage I, 38.2% with stage II, 38.0% with stage III, and 7.5% with stage IV.
Table 1 Characteristics of CRC patients treated with surgical resection according to PLR
The median preoperative PLR was 153. There were statistically significant differences between the groups with respect to total cholesterol, triglycerides, HDL, albumin, and uric acid (each parameter with P<0.05). In addition, patients with PLR >220 were significantly associated with higher incidence of stage IV and a tumor location at the right side. The tumors were also significantly associated with the clinical variable of vascular invasion (P<0.05). There were no statistically significant differences in other clinical or pathological features. Although there is no difference in MetS between the PLR subgroup (P=0.719), further analyses showed a significant difference between the PLR subgroup, comparing the MetS subgroups stratified by the metabolic risk factors (P=0.016). PLR was also significantly higher in the MetS(+) group compared with MetS(−) (162.0±99.8 vs 150.6±68.3, P=0.039, ), however, there was a graded tendency between increasing number of MetS components and PLR (146.3±66.2, 149.3±65.1, 153.8±72.1, 158.3±106.6, 169.5±84.9, P=0.150, respectively), as illustrated in .
Figure 1 The graded relationship between increasing number of MetS components and PLR.
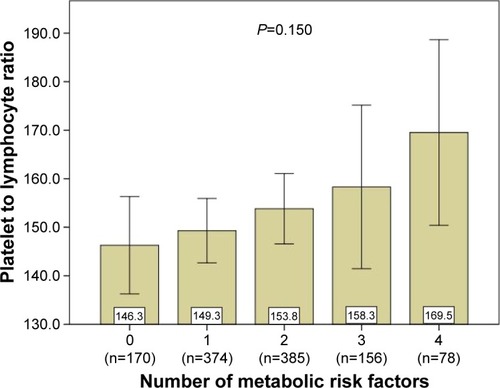
Table 2 Baseline characteristics of CRC patients stratified by MetS
Survival estimates according to PLR and MetS
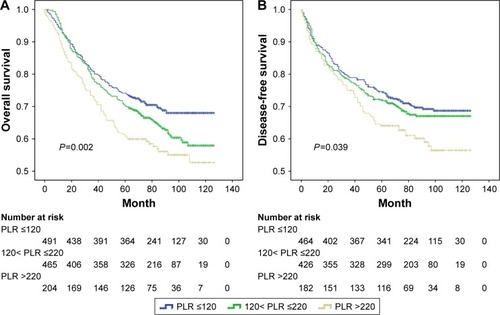
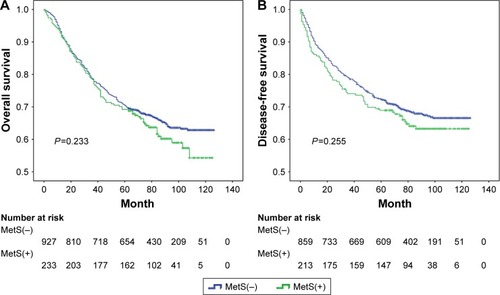
The mean follow-up time was 71.2 months. Kaplan–Meier analysis of OS and DFS demonstrated a progressively lower OS (P=0.002; ) and DFS (P=0.039; ) in elevated PLR groups. As shown in , there was a trend of better OS for patients with MetS(−) compared to MetS(+), but the difference between the two survival curves was not statistically significant (P=0.233). Similar results were noticed for DFS (P=0.255, ).
Risk estimates of PLR and MetS
Cox proportional hazard models were used to identify variables associated with OS and DFS and the results are illustrated in . The patients with a PLR >220 revealed a 59% increase in mortality risk and 43% increased risk for the recurrence of disease compared with patients with a PLR <120 HR =1.594; 95% CI 1.227–2.070, P<0.001 and HR =1.434; 95% CI 1.082–1.902, P=0.012, respectively). Gender, age, HDL, albumin, triglycerides, uric acid, tumor-node-metastasis (TNM) stage, tumor differentiation, the presence of vascular invasion, and CEA were also significantly associated with the risk of death based on univariate analysis (P<0.05 for all measurements). In the multivariate analysis, PLR remained significantly associated with OS (HR =1.511; 95% CI 1.103–2.070, P=0.010). However, only HDL and TNM stage were independent predictors in multivariate Cox analysis for DFS (P<0.05 for all measurements, ).
Table 3 Cox proportional hazard regression models of risk factors associated with prognosis among CRC patients
Subgroup analyses associated with PLR and MetS
In the MetS(+) subgroup, Kaplan–Meier analysis of OS showed significantly progressively worse OS with elevated PLR (P=0.004; ), compared with the MetS(−) subgroup (P=0.064), and PLR remained as an independent predictor for OS in the univariable and multivariable analysis (P=0.006, P=0.047, respectively, ), but not for DFS (P=0.110, P=0.323, respectively). Considering the impact of different ranges of PLR, multivariate analysis showed that the subgroups with PLR values ≤220 were not associated with the prognosis after adjustment for MetS and PLR, even including other covariates. However, in the subgroup of patients with PLR values >220, the difference between the two survival curves stratified by MetS was statistically significant in OS (P=0.043, ), and the coexistence of MetS was associated with a twofold increased risk of CRC mortality and recurrence (HR =2.0, HR =1.9 P<0.05, respectively, ).
Figure 4 Kaplan–Meier survival curves showing overall survival stratified by PLR in CRC patients with MetS (A) and overall survival stratified by MetS in CRC patients with PLR >220 (B).
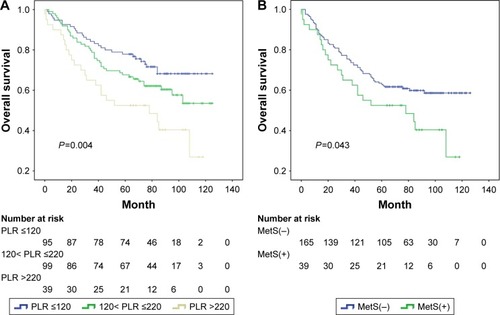
Table 4 Cox proportional hazard regression analysis of prognosis stratified by MetS
Table 5 Cox proportional hazard regression analysis of the prognosis of the subgroups of PLR
Discussion
The main findings of this study are as follows. PLR levels were significantly higher in CRC patients with MetS compared to patients not diagnosed with MetS. Also, no correlation was found with severity of MetS. We revealed that in the subgroup of patients with PLR values >220, MetS was an independent predictor of the prognosis, and the presence of MetS was associated with a twofold increased risk of mortality and recurrence. To the best of our knowledge, there are no comparable reports describing the impact of both PLR values and MetS on the prognosis of patients with CRC.
MetS is defined as a cardiometabolic condition that increases the risk of type 2 diabetes mellitus, cardiovascular disease, and all-cause mortality including cancer.Citation24–Citation26 Previous studies demonstrated that MetS was associated with an increased risk of mortality and recurrences in CRC patients.Citation9,Citation27 However, the mechanisms by which MetS affects the prognosis are not fully understood, although several hypotheses have been proposed, including inflammation and insulin resistance.Citation28–Citation30 A low-grade inflammatory condition in MetS, termed as “metabolic inflammation” or “metaflammation”, has been regarded as a vital factor that revealed a correlation with impaired demand and supply of oxygen, which results in hypoxia and subsequent inflammation.Citation31,Citation32 As such, an interaction between hypoxic and inflammatory signaling pathways and blood coagulation disorders have been related to CRC patients.Citation33–Citation35
In recent studies, PLR was considered a predictor of systemic inflammation and was correlated with the prognosis of CRC. Moreover, a higher PLR level was correlated with adverse postoperative survival in CRC patients.Citation16–Citation18 In this study, we also found that there is an association of high PLR with right-sided colon cancer (RCC), but not left-sided colorectal cancer (LCRC) (P=0.001). Compared with LCRC, RCC had a delay in the diagnosis, due to the more subtle symptoms. To some extent, this leads to RCC having more active immune cells promoting immunogenicity and producing more inflammatory factors.Citation36 Several studies have indicated that patients with RCC have a worse prognosis compared to those with LCRC.Citation37,Citation38 This is consistent with the impact of PLR on the survival of CRC patients. In terms of stage distribution, it is well known that with the advanced TNM stage, malignant solid tumors had higher incidence of inducing a hypercoagulable state,Citation39,Citation40 which may gradually lead to thrombocytosis and high PLR. This can partly explain why PLRs are different in TNM stage distribution. Increased PLR levels were significantly associated with both the presence and severity of MetS in cardiology patients.Citation21 It has been shown that platelets are activated in case of MetS by linking inflammation and thrombosis.Citation41 Activated platelets also release pro-inflammatory mediators which interfere in the pathophysiology of MetS.Citation42 The latest meta-analysis reported that elevated platelet count and thrombocytosis prior to treatment, was related with a poor prognosis for patients with CRC.Citation43,Citation44 Lymphocytes play a vital role in cancer immune surveillance and suppress tumor maturation.Citation45 Based on the previous studies on PLR, peripheral platelets, lymphocytes or their ratio are indicators for the inflammatory process induced by tumor cells. A high level of platelets may promote tumor growth by increasing angiogenesis through the production of vascular endothelial growth factor (VEGF). It has been shown that the overexpression of VEGF was negatively associated with disease prognosis and metastasis in patients with various cancers, including CRC.Citation46
There are some limitations to our study. First, this study has a retrospective cross-sectional design with single-center data and a relatively small number of MetS patients (n=234). Second, we chose the Chinese Diabetes Society (CDS) criteria to define MetS.Citation22 The CDS criteria used BMI rather than waist circumference as index to define “overweight” or “obese”, because the application of the National Cholesterol Education Program-Adult Treatment Panel III criteria or the International Diabetes Federation criteria for Caucasians to East Asians would seriously underestimate the Chinese populations at risk of MetS.Citation47,Citation48 Third, all the CRC patients were enrolled between 2005 and late 2011, during the 7-year period, remarkable advances in surgical techniques and postoperative adjuvant treatment options might potentially have caused a bias. What is more, MetS is a reversible condition associated with a western lifestyle,Citation49 which could have underestimated the impact of MetS on CRC mortality.
In summary, we found that PLR is associated with MetS in CRC patients. PLR might be a useful marker to monitor an increased thrombotic status and inflammatory response in management of MetS, with respect to predicting the prognosis of CRC patients, and identifying novel treatment strategies. In daily clinical practice, much more attention should be paid to evaluating the presence and severity of MetS in CRC patients, especially with high PLR. Future studies are required to elucidate the relationship between PLR and MetS in CRC patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- BrennerHKloorMPoxCPColorectal cancerLancet201438399271490150224225001
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- MalikSWongNDFranklinSSImpact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adultsCirculation2004110101245125015326067
- Expert panel on detection, evaluation, and treatment of high blood cholesterol in adultsExecutive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III)JAMA2001285192486249711368702
- AhmedRLSchmitzKHAndersonKERosamondWDFolsomARThe metabolic syndrome and risk of incident colorectal cancerCancer20061071283616721800
- EspositoKChiodiniPColaoALenziAGiuglianoDMetabolic syndrome and risk of cancer: a systematic review and meta-analysisDiabetes Care201235112402241123093685
- YangYMauldinPDEbelingMEffect of metabolic syndrome and its components on recurrence and survival in colon cancer patientsCancer201311981512152023280333
- StocksTLukanovaABjorgeTMetabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can)Cancer2011117112398240724048787
- YouJLiuWYZhuGQMetabolic syndrome contributes to an increased recurrence risk of non-metastatic colorectal cancerOncotarget2015623198801989026082438
- PengFHuDLinXPreoperative metabolic syndrome and prognosis after radical resection for colorectal cancer: The Fujian prospective investigation of cancer (FIESTA) studyInt J Cancer2016139122705271327560834
- MaedaKShibutaniMOtaniHInflammation-based factors and prognosis in patients with colorectal cancerWorld J Gastrointest Oncol20157811111726306143
- MantovaniAAllavenaPSicaABalkwillFCancer-related inflammationNature2008454720343644418650914
- ColottaFAllavenaPSicaAGarlandaCMantovaniACancer-related inflammation, the seventh hallmark of cancer: links to genetic instabilityCarcinogenesis20093071073108119468060
- Fernandez-RealJMRicartWInsulin resistance and chronic cardiovascular inflammatory syndromeEndocr Rev200324327830112788800
- ShoelsonSELeeJGoldfineABInflammation and insulin resistanceJ Clin Invest200611671793180116823477
- YouJZhuGQXieLPreoperative platelet to lymphocyte ratio is a valuable prognostic biomarker in patients with colorectal cancerOncotarget2016718255162552727027440
- TanDFuYSuQWangHPrognostic role of platelet-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysisMedicine (Baltimore)20169524e383727310960
- SzkanderaJPichlerMAbsengerGThe elevated preoperative platelet to lymphocyte ratio predicts decreased time to recurrence in colon cancer patientsAm J Surg2014208221021424581872
- FangKCChengYLSuCWHigher platelet counts are associated with metabolic syndrome independent of fatty liver diagnosisJ Chin Med Assoc201780312513227686501
- ChenYLHungYJHeCTPlatelet count can predict metabolic syndrome in older womenPlatelets2015261313724512307
- AkbogaMKCanpolatUYukselMPlatelet to lymphocyte ratio as a novel indicator of inflammation is correlated with the severity of metabolic syndrome: A single center large-scale studyPlatelets201627217818326196312
- Chinese Metabolic Syndrome Study CooperationSuggestions about metabolic syndrome of Chinese diabetes societyChin J Diab200412156161
- EdgeSBComptonCCThe American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNMAnn Surg Oncol20101761471147420180029
- O’NeillSBohlMGregersenSHermansenKO’DriscollLBlood-based biomarkers for metabolic syndromeTrends Endocrinol Metab201627636337427150849
- WuSHLiuZHoSCMetabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studiesEur J Epidemiol201025637538420425137
- ArbelYHavakukOHalkinARelation of metabolic syndrome with long-term mortality in acute and stable coronary diseaseAm J Cardiol2015115328328725499926
- JaggersJRSuiXHookerSPMetabolic syndrome and risk of cancer mortality in menEur J Cancer200945101831183819250819
- IshinoKMutohMTotsukaYNakagamaHMetabolic syndrome: a novel high-risk state for colorectal cancerCancer Lett20133341566123085010
- HirabaraSMGorjaoRVinoloMARodriguesACNachbarRTCuriRMolecular targets related to inflammation and insulin resistance and potential interventionsJ Biomed Biotechnol2012201237902423049242
- LiuJJDrutaMShibataDMetabolic syndrome and colorectal cancer: is hyperinsulinemia/insulin receptor-mediated angiogenesis a critical process?J Geriatr Oncol201451404824484717
- DespresJPAbdominal obesity and cardiovascular disease: is inflammation the missing link?Can J Cardiol201228664265222889821
- KlotingNBluherMAdipocyte dysfunction, inflammation and metabolic syndromeRev Endocr Metab Disord201415427728725344447
- Muller-EdenbornKLegerKGlaus GarzonJFHypoxia attenuates the proinflammatory response in colon cancer cells by regulating IkappaBOncotarget2015624202882030125978030
- MarianiFSenaPRoncucciLInflammatory pathways in the early steps of colorectal cancer developmentWorld J Gastroenterol201420299716973125110410
- NielsenVGNfonsamVNMatikaRWColon and pancreas tumors enhance coagulation: role of hemeoxygenase-1Blood Coagul Fibrinolysis201425543543824509340
- LeeGHMalietzisGAskariAIs right-sided colon cancer different to left-sided colorectal cancer? – a systematic reviewEur J Surg Oncol201541330030825468456
- BenedixFKubeRMeyerFComparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survivalDis Colon Rectum2010531576420010352
- MeguidRASlidellMBWolfgangCLChangDCAhujaNIs there a difference in survival between right- versus left-sided colon cancers?Ann Surg Oncol20081592388239418622647
- IshizukaMNagataHTakagiKIwasakiYKubotaKPreoperative thrombocytosis is associated with survival after surgery for colorectal cancerJ Surg Oncol2012106788789122623286
- SasakiKKawaiKTsunoNHSunamiEKitayamaJImpact of preoperative thrombocytosis on the survival of patients with primary colorectal cancerWorld J Surg201236119220022045447
- van RooyMJPretoriusEMetabolic syndrome, platelet activation and the development of transient ischemic attack or thromboembolic strokeThromb Res2015135343444225601172
- WangYYLinSYLiuPHCheungBMLaiWAAssociation between hematological parameters and metabolic syndrome components in a Chinese populationJ Diabetes Complications200418632232715531181
- LongYWangTGaoQZhouCPrognostic significance of pretreatment elevated platelet count in patients with colorectal cancer: a meta-analysisOncotarget2016749818498186127833087
- ZhaoJMWangYHYaoNPoor prognosis significance of pretreatment thrombocytosis in patients with colorectal cancer: a meta-analysisAsian Pac J Cancer Prev20161794295430027797233
- DunnGPOldLJSchreiberRDThe immunobiology of cancer immunosurveillance and immunoeditingImmunity200421213714815308095
- WiesnerTBuglSMayerFHartmannJTKoppHGDifferential changes in platelet VEGF, Tsp, CXCL12, and CXCL4 in patients with metastatic cancerClin Exp Metastasis201027314114920182908
- TanCEMaSWaiDChewSKTaiESCan we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians?Diabetes Care20042751182118615111542
- XiBHeDHuYZhouDPrevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009Prev Med201357686787124103567
- van DuijnhovenFJBueno-De-MesquitaHBCalligaroMBlood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and NutritionGut20116081094110221383385