?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim of the study
Inflammasome, a large complex of NOD-like receptors (NLRs), drives tumor growth and progression. The present study aimed at exploring the alteration in expression of urinary inflammasome-related microRNAs (miRNAs) in bladder cancer (BC). Our previous report demonstrated the up-regulation of NLRs genes (NLRP3, NLRP4, NLRP9 and NAIP) in urine sediments of patients harboring BC. The expression levels of miRNAs targeting these NLRs (miR-146a-5p, miR-106a-5p, miR-17-5p, miR-223-3p, miR-141-3p, miR-19a-3p, miR-145-5p, miR-185-5p) were assayed in the same patient cohort.
Materials and methods
Forty-six subjects affected by BC, 28 healthy controls (CTR0) and 31 subjects with histologically confirmed bladder inflammation (CTR1) were recruited. Total RNA was extracted from urine sediment and resulting cDNA was used for amplification by real-time polymerase chain reaction. MiRNA expression levels were evaluated and compared among selected groups. Patients were further stratified according to tumor stage, grade and risk of recurrence and progression. Moreover, non-muscle invasive low-grade and high-grade (HG) BC patients were compared.
Results
MiR 141-3p and miR-19a-3p expression decreased in CTR1 with respect to both BC and CTR0. In contrast, miR-146a-5p was up-regulated in BC compared with CTR0. MiR106a-5p, miR17-5p and miR19a-5p were significantly up-regulated in HG, high-risk (HR) and non-muscle invasive HG BC patients, while miR-185-5p was significantly higher in muscle invasive tumors, according to T stage stratification.
Conclusion
The increased expression of miRNAs targeting NLRs in HG and HR BC patients is in accordance with the decrease in NLR mRNAs observed in our previous report. These data corroborate the direct role of NLR genes and respective regulatory miRNAs in BC making these inflammasome-related molecules a reliable non-invasive tool for BC diagnosis.
Introduction
Bladder cancer (BC) is the second most frequent urological malignancy and it is the fourth and ninth most common cancer in men and women, respectively, in the Western world.Citation1
About 75% of newly diagnosed BC cases are non-muscle invasive (NMI): of these, ~70% are confined to the mucosa (stage Ta), 20% involves submucosa (stage T1) and 10% are carcinoma in situ (CIS) lesions.Citation2 Regarding prognosis, 30%–80% of NMIBC will recur and 1%–45% of cases will progress to muscle invasive form within 5 years.Citation3 The risk of recurrence and progression increases with the stage, the grade of malignancy, the size and number of lesions and the presence of the CIS.Citation4 Cancer mortality is significantly affected by progression rates of high-risk (HR) NMIBC and by cure rates of muscle invasive ones. BC is diagnosed by cystoscopy in addition to urinary cytology and histological evaluation of the resected tissue. However, cystoscopy is an invasive, painful and potentially infectious procedure. Although voided urine cytology represents the gold standard for the non-invasive diagnosis of BC, its low sensitivity (16%) for low-grade (LG) tumors is the main limitation; on the other hand, sensitivity rises up to 84% for high-grade (HG) BC.Citation5 Among various malignancies, BC is intimately linked to inflammation. Transitional cells carcinoma owns high immunogenic potential and this intrinsic feature lends BC to immunotherapy.Citation6 Tumor microenvironment is regulated by several factors such as NOD-like receptors (NLRs) that play a pivotal role in the cytokine production.Citation6 NLR family accounts for the recognition of intracellular ligandsCitation7 by pathogen-associated molecular patterns. They are responsible for the formation of inflammasomesCitation8 that regulate the production of inflammation-related cytokines with direct effects on tumor formation and progression.Citation9 Many efforts have been made to confirm the involvement of NLR inflammasomes in tumorigenesis.Citation10,Citation11 Other factors involved in the development and maintenance of the neoplastic state are the microRNAs (miRNAs).Citation12 MiRNAs are non-coding RNAs of about 22 nucleotides, regulating genes by pairing to target regions within the 3′UTR of messenger RNAs.
MiRNA expression is altered in several urologic malignancies, validating their pivotal role to cancer development, progression and metastasis.Citation13 As an example, the expression of miR-200 has been found down-regulated in urine sediments from patients with BC and the ablation of tumor tissue restored the same expression levels of healthy controls.Citation14 Similar findings were obtained for miR-125b and miR-126 that discriminate at the urine level BC subjects from healthy controls with high sensitivity and specificity.Citation15 Expression profiling studies of miRNAs reported a significant deregulation of miR-21 and miR-205 in tissue samples from patients with BC; moreover, a miR-21/miR-205 ratio differentiates an invasive from noninvasive bladder tumor phenotype.Citation16 The differential expression of miRNAs between BCa and normal bladder tissue leads to the elucidation of miRNA signatures as promising prognostic markers of BCa.Citation17 For example, a miR-9, miR-182 and miR-200b signature was demonstrated to correlate with tumor aggressiveness in muscle invasive BC.Citation18 Similar tumor features have been associated with four miRNAs signature (let-7c, miR-125b-1, miR-193a and miR-99a).Citation19 MiRNAs exert a transcriptional regulation on NRLP3 inflammasome, and their crucial role in modulating inflammasome activity has been widely estabilished.Citation20 We assayed the expression of NLRP3, NLRP4, NLRP9 and NAIP in urine sediments from patients harboring BC, subjects affected by bladder inflammation and healthy subjects in a previous work.Citation11 We found an overexpression of NLRP3, NLRP4, NLRP9 and NAIP in patients with BC when compared with controls. Further stratification according to tumor stage, grade and risk of recurrence and progression showed NLRP up-regulations in patients with early-stage cancer. NAIP was overexpressed in HR patients compared with controls and in HG patients compared with CTR0 and CTR1. The aim of the present study is to evaluate a panel of miRNAs targeting those NLRs showing altered expression in BCa.Citation11 Our interest was focused in the analysis of correlation between NLRs, their respective miRNAs and BC histology. Evaluating miRNA expression as well as their molecular targets in BC represents the starting point for a new miRNA-based diagnostic and prognostic tools.
Materials and methods
Patients
Seventy-seven patients with macroscopic or microscopic hematuria and/or irritative symptoms with negative urine culture were enrolled. All patients underwent transurethral bladder resection and histological confirmation of BC was obtained for 46 subjects (BC group), whereas a diagnosis of bladder inflammation was achieved for the remaining 31 patients (CTR1 group). Bladder inflammation was established according to the presence of lymphocytes and neutrophils infiltration without tumoral cells. A third group consisting of 28 healthy controls was included (CTR0). BC patients were stratified according to the degree of bladder wall infiltration, as reported by tumor, node, metastasis classification system (Ta, T1, T2), to the degree of histological differentiation according to the World Health Organization Grading 2004 (LG, HG)Citation21 and to the risk of recurrence and progression according to the European Organization for Research and Treatment of Cancer risk criteria (LG, low-grade; HG, high-grade).Citation22 Specifically, LR tumors included primary, solitary, Ta, papillary urothelial neoplasm of low malignant potential, LG, smaller than 3 cm, no CIS; HR tumors included T1 tumor or HG tumor or CIS or multiple and recurrent and large (>3 cm) Ta, LG tumors; intermediate risk were considered all tumors not defined in the two previous categories. Patients with NMIBC were further stratified in NMI LG (TaLG and T1LG) and NMI HG subtypes (TaHG and T1HG). The Ethics Committee of the University of Perugia approved the study protocols and all subjects signed an informed consent. The investigation conformed to the principles outlined in the Declaration of Helsinki (1997).
Sample collection and processing
Voided urine was collected in sterile cups. Urine was kept at 4°C and processed within 4 h. Samples were centrifuged (2,000× g, 10 min, 4°C) and cell pellets were washed twice with 1X calcium-free phosphate-buffered saline. 300 µL lysis and stabilization buffer (Total RNA Extraction Kit; Norgen Biotek Corp, Thorold, ON, Canada) were added to lyse cells and stabilize nucleic acids before RNA extraction.
RNA extraction
Total RNA including miRNAs was extracted with Total RNA Extraction Kit according to the manufacturer’s instructions. RNA was quantified with Qubit RNA HS Assay Kit (Life Technologies, Carlsbad, CA). RNA was stored at −80°C until use.
Reverse transcription and real-time PCR
Total RNA 7.5 ng were reverse transcribed with miR-CURY LNA™ Universal RT miR polymerase chain reaction (PCR), polyadenylation and complementary DNA (cDNA) synthesis kit (Exiqon, Vedbaek, Rudersdal, Denmark) according to the manufacturer’s protocol. RNA spike-in control UniSp6 was added to the RT mix (total volume 10 µL) as a positive cDNA synthesis control. Real-time PCR assays were performed with miRCURY LNA specific PCR primer set and ExiLENT SYBR® Green Master Mix (Exiqon system) on a Stratagene Mx3005P instrument (Agilent Technology, Santa Clara, CA, USA). cDNA 4 µL diluted 1:20 with nuclease-free water were used in a total reaction volume of 10 µL. UniSp6 positive control was amplified for each sample and outliers (samples with UniSp6 Ct beyond a value of 26) were discarded. Melting curve analysis was carried out, each sample was run in triplicate and results were averaged; no-template controls were included in the analysis. RNU6 was used to normalize data. The −ΔCt method was used to calculate the relative expression of the target genes as follows: −ΔCtmiRNA=− (CtmiRNA − CtRNU6).
Selection of candidate miRNAs
miRNAs predicted to target NLRs analyzed in our previous study (NLRP3, NLRP4, NLRP9, NAIP) were selected using PicTar, TargetScan, miRanda and DIANA microT algorithms. MiR-146a-5p, miR-106a-5p, miR-17p, miR-223-3p, miR-141-3p, miR-19a-3p, miR-145-5p, miR-185-5p were selected after further matching with literature data on cancer-related miRNAs. Experimentally validated and computationally predicted miRNA targets are reported in . Validated targets are marked with asterisks.
Table 1 Summary of miRNAs and related mRNA targets
Statistical analysis
GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA) was employed to calculate statistical significance among groups. A P<0.05 was considered statistically significant. Logistic regression analysis was performed to evaluate the association of selected miRNAs with BC. Prediction models were based on miRNA expression values (−ΔCts). Univariate logistic regression analysis was made for each miRNA and thereafter, multivariate logistic models were built. The association between miRNAs and disease-free survival of patients was evaluated through Kaplan–Meier analysis. Groups were compared using the log-rank test.
Results
Expression levels of selected miRNAs in BC, CTR0 and CTR1 groups\
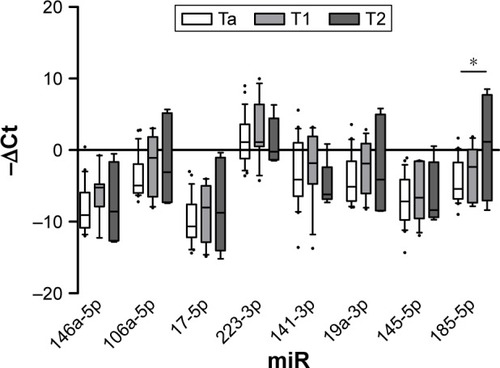
Patient data and hematological parameters are reported in . As can be seen, the absence of statistically significant differences allowed us to exclude other inflammatory processes in our cohort of patients. Median follow-up was 25.3 months (ranged from 18 to 38 months). Analysis of miRNA expression in urine sediment showed decreased levels of miR-141-3p in CTR1 with respect to both BC and CTR0 (fold changes 3.26 and 0.21, respectively). Similar results were observed for miR-19a-3p (fold changes 3.25 and 0.4, ). In contrast, the expression of miR-146a-5p significantly increased in BC compared with CTR0 (fold change 3.78, P=0.005) (). Finally, a slight decrease of miR-146-5p was noticed in CTR1 compared with BC (fold change 3.22, ). The other miRNAs did not change in expression in the groups under analysis ().
Figure 1 Expression levels expressed as −ΔCt (median with range and interquartile range) of selected miRNAs.
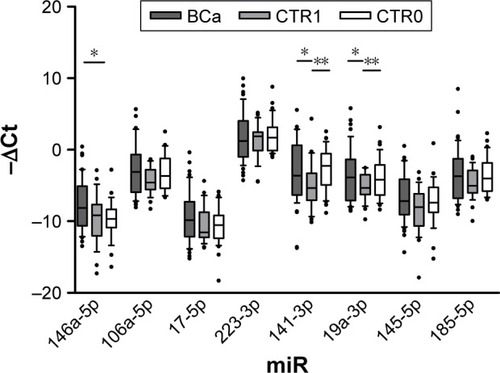
Table 2 Hematological and clinical parameters of enrolled subjects
Stratification of patients with BC
To investigate whether the expression levels of selected miRNAs were related to histological differentiation of BC, patients were stratified according to tumor stage, grade, risk of recurrence and progression. Moreover, miRNA expression in urine sediment from patients with HG BC was compared with NMI LG and NMI HG BC.
Tumor stage
Expression analysis of urine sediment form patients stratified according to T stage showed miR-185-5p significantly up-regulated in muscle invasive (T2) with respect to NMI cancer (Ta, fold change 15.1). Data are shown in .
Tumor grade
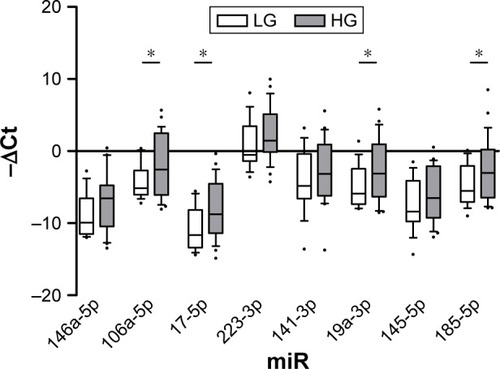
According to tumor grade, expression levels of miR-106a-5p, miR-17-5p, miR-19a-3p and miR-185-5p were significantly increased in urine sediment from patients with HG tumors (fold changes 4.05, 6.01, 4.53 and 4.98, respectively, ).
Risk of recurrence and progression
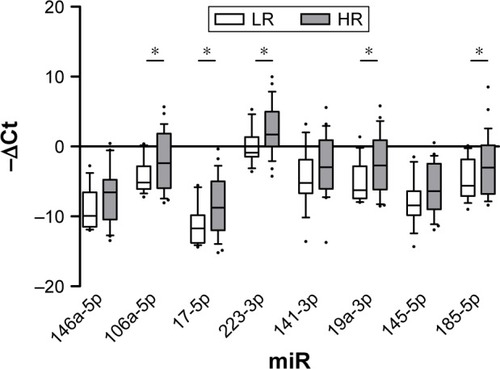
MiRNA expression analysis after stratification according to risk of recurrence and progression reported increased levels of miR-106a-5p, miR-17-5p, miR-19a-3p and miR-185-5p in HR versus LR tumors, as observed in HG versus LG tumors (fold changes 4.47, 5.9, 5.14 and 4.51, respectively). Moreover, miR-223-3p expression increased concomitantly to increasing risk of recurrence and progression (fold change 5.59, ).
NMI LG vs NMI HG cancer
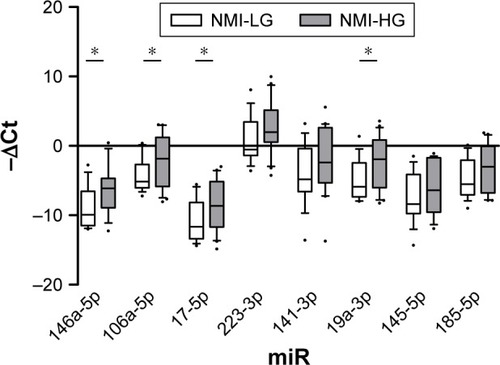
Given the peculiar expression level of miRNAs in HG and muscle invasive tumor, we set out to examine whether miRNAs targeting NLRs inflammasomes distinguish patients with NMI LG from those harboring NMI HG BC (). Results confirmed the up-regulation of miR-106a-5p, miR-17-5p and miR-19a-3p highlighted in both grade and risk-based stratifications (fold changes 3.96, 4.8 and 4.56, respectively). Moreover, miR-146a-5p showed an up- regulation in NMI HG tumors (fold change 6.15, ).
Evaluation of prognostic power of miRNAs
MiRNAs from urine sediment displayed the expression level based on histological differentiation of BC. Based on previous results, miRNAs seemed to have the prognostic power to predict histological outcome. On the basis of these premises, logistic regression analysis was performed and results are shown in .
Table 3 Univariate and multivariate logistic regression analyses of variables predicting the outcome of disease
HG vs LG tumor
According to the grade of histological differentiation, miR17-5p showed statistical significance in the univariate analysis. Sensitivity and accuracy were 76.92% and 57.14%, respectively (). In the multivariate logistic regression analysis, the combination of variables under analysis did not show any relevant finding (data not shown).
HR vs LR BC
Logistic regression analysis was performed to verify the ability of selected miRNAs in discriminating HR from LR BC. Results from the univariate analysis displayed high sensitivity and accuracy in predicting high risk of recurrence and progression for miR-17-5p (81.48% sensitivity, 61.90% accuracy) and for miR-19a-3p (82.24% sensitivity, 60.47% accuracy) (). Multivariate logistic regression analysis did not show any significant result (data not shown).
NMI LG vs NMI HG BC
When patients with NMI LG versus NMI HG tumor were analyzed by means of logistic regression, only miR-17-5p gave an acceptable model (70% sensitivity, 63.89% accuracy) (). In the multivariate logistic regression analysis, the combination of miR-106a-5p, miR-17-5p, miR-19a-3p and miR-146a-5p gave the best model in terms of sensitivity and accuracy (76.92% and 80.77%, respectively) ().
We also analyzed the association between miRNAs and disease-free survival of patients through Kaplan–Meier analysis. The optimal cut-off value for each miRNA was calculated by ROC analysis and used to dichotomize expression levels (high and low groups). No significant association between miRNA expression and disease-free survival was recorded (data not shown).
Discussion
Genome-wide miRNA expression signatures have been used to precisely identify aberrant miRNAs expression signatures in BC.Citation23 Moreover, emerging evidence demonstrated that miRNAs play a pivotal role in the regulation of immunological functions and the prevention of autoimmunity. Several NLRs were validated as specific targets of selected miRNAs, while others were only predicted through algorithms (). In the present study, we evaluated inflammation-related miRNAs targeting NLRs known to be altered in BC. In our previous report, we observed an enhanced expression of NLRP3, NLRP4, NLRP9 and NAIP messenger RNAs (mRNAs) in urine sediments from patients with BC with respect to healthy controls. Furthermore, we highlighted the up-regulation of NLRP3, NLRP4 and NLRP9 in both Ta and LG tumors after stratifying by T stage, grade and risk of recurrence and progression of BC.Citation11 In contrast, NLRP4 and NLRP9 were over-expressed in NMI LG tumors; these results suggested the potential role of inflammasomes in the early development of BC, when an innate immunity reaction occurs in the early stage of tumorigenesis. In the same study, the authors reported that NAIP mRNA level was significantly higher in patients with HG tumors than healthy controls and patients with bladder inflammation. Same results were obtained when HR patients were analyzed, suggesting an essential role of NAIP in BC progression.
Our study showed that miR-146a-5p, miR-141-3p and miR-19a-3p were differentially expressed in urine sediments from patients harboring BC, subjects with bladder inflammation and age-matched healthy controls. Down-regulation of miR-19 in CTR1 group compared with both BC and CTR0 subjects is a relevant finding since pro-inflammatory responses through miR-19a-dependent mechanisms have been recently clarified.Citation24 MiR-19a-3p is predicted to target NLRP9 and its increased expression, together with NLRP4, has been reported in less malignant tumors in our previous work.Citation11 Moreover, miR-19a-3p significantly decreases the SOCS3 mRNA level with consequent enhanced IFN-α and IL-6 signal transduction.Citation24 The regulation of SOCS molecules by miR-19a and its involvement in inflammatory processes has been also reported in multiple myeloma cells.Citation25 Down-regulation of miR-19a in post-digital rectal examination urine sediments from patients harboring prostate cancer has also been shown in our previous study.Citation26 MiR-146a-5p is aberrantly expressed in thyroid,Citation27 prostateCitation28 and gastric cancers.Citation29 Increased miR-146a-5p expression seems to inhibit cell proliferation, migration and invasion.Citation29 Targeting of STAT by miR-146a has been reported by Hou et al, who analyzed its effects on the IFN-α signaling pathway in hepatocytes infected by hepatitis B virus.Citation30 In patients with BC, a genetic variant of miR-146a-5p is associated with high risk of recurrence.Citation31 According to the recent literature, our results showed increased expression of miR-146a-5p in BC compared with CTR0, while no significant modifications in CTR1 subjects compared with both BC and CTR0 groups were recorded. These results seem to be in contrast with the up-regulation of NLRs in BC patients reported in our previous work.Citation11 Nonetheless, miR-146a-3p could exert a prominent role in carcinogenesis beyond controlling inflammation. This could be especially true since a validated interaction between miR-146a-5p and its NLR target mRNA has not yet been validated. In fact, miR-146-a-5p levels in BC patients did not differ significantly from CTR1 group, which instead occurs for miR-19a-3p and miR-141-3p. Studies on miR-141 have shown its involvement in different autoimmune diseases, such as systemic lupus erythematosus,Citation32 inflammatory bowel disease,Citation33 psoriasisCitation34 and other immune-related diseases. Although its role in different cancers is well known as well,Citation14 no significant modifications in miR-141 expression between BC patients and controls has been reported in our study. Regarding T stage stratification, miR-223-3p, miR-17-5p and miR-185-5p up-regulation in both NMI and muscle invasive BC has been described.Citation35 In our study, only an increased expression of miR-185–5p in T2 tumors () was found compared with both Ta and T1 ones, while neither miR-223-3p nor miR-17-5p showed significant alterations between muscle invasive versus NMI tumors. Besides NLRP3, other genes have demonstrated to be specifically targeted by miR-223-3p. In particular, ZEB1 was proven to be suppressed by miR-223–3p in BC cell lines treated with Ginkgolide B.Citation36 Furthermore, SEPT6 was reported to be specifically targeted by miR-223-3p in prostate cancer cell lines.Citation37 Among miRNAs involved in inflammation, miR-17-5p deserves particular attention as member of miR-17/92 cluster involved in tumorigenesis, immune diseases, cardiovascular diseases, neurodegenerative diseases and aging.Citation14 MiR-17 has been recently discovered to destabilize the thioredoxin-interacting protein mRNA, which is responsible for the activation of the NLRP3 inflammasome, causing Caspase-1 cleavage and IL-1β secretion.Citation38 Tazi et al identified the Mirc1/Mir17–92 cluster as a potential negative regulator of autophagy-related genes in cystic fibrosis macrophages.Citation39 In our analysis, miR-17-5p was significantly up-regulated in HG (), HR () and NMI HG tumors (). Similar pattern of expression was reported for miR-106a-3p and miR-19-a-3p (–). Large-scale miRNA microarray studies established the oncogenic nature of miR-106a for colon, pancreas and prostate carcinomas.Citation40 High levels of circulating miR-106a have been associated with risk of aggressive lung cancerCitation41 and gastric cancer together with miR-17–5p.Citation42 Moreover, chemoresistance and poor survival of patients with ovarian cancer were associated with the up-regulation of miR-106a.Citation43 Similarly, a recent study outlined the oncogenic properties of miR-19a demonstrating its overexpression in BC tissue. A correlation between miR-19a levels and cancer aggressiveness was also outlined.Citation44 In the present study, increased expression of miR-106a-5p, miR-17-5p and miR-19a-3p was associated with HG and HR BC. Therefore, the prognostic value of selected miRNAs was assessed; miR-17-5p seemed to discriminate HG from LG BC with 76.92% sensitivity and 57.14% accuracy. Furthermore, miR-170-5p was able to predict HR patients compared with LR counterparts. Similar results were observed for miR-19a-3p (82.24% sensitivity, 60.47% accuracy). Finally, the accuracy of selected miRNAs in discriminating NMI LG from NMI HG patients was investigated: again, in the univariate model, miR-17-5p performed the best (56.25% specificity, 70% sensitivity, 63.89% diagnostic accuracy). The combination of miR-106a-5p, miR-17-5p, miR-19a-3p and miR 146a-5p outperformed miR-17-5p (+6.92% sensitivity, +16.88% accuracy). Our results highlight a correlation between miRNA expression levels and disease severity. The molecular recognition of patients harboring HG tumors exerts a crucial importance in clinical practice. Inflammasome components and their regulatory pathways could allow the recognition of those BC subtypes with unfavorable prognosis needing both early diagnosis and surgical therapy. Moreover, as future perspective, they may become novel targets to develop a targeted immunotherapy because of their role in cancer immunity.
Conclusion
Our study showed that miR-146a-5p, miR-141-3p and miR-19a-3p are differentially expressed in urine sediments from patients harboring BC, subjects with bladder inflammation and age-matched healthy controls. Furthermore, increased expression of miR-106a-5p, miR-17-5p and miR-19a-3p was associated with HG and HR BC. According to T stage stratification, only miR-185-5p was significantly higher in muscle invasive tumors with respect to NMI ones.
The increased expression of miRNAs targeting NLRs in HG and HR BC is in accordance with the decrease in NLR mRNAs involved in tumorigenesis and progression of BC. Our data highlight the pivotal role of NLRs in BC and confirm the hypothesis that their regulation is mediated by miRNA. Then, these inflammasome-related molecules may be used as potential candidates for non-invasive diagnostic tool of BC. However, the potential ability of urine miRNAs for the prediction of tumor stage and grade needs further investigations because the accuracy obtained in the present study does not allow their clinical use as diagnostic tools compared with more achievable urine cytology.
Acknowledgments
This work was supported by Fondazione Cassa di Risparmio Terni e Narni. The research was performed in the Department of Surgical and Biomedical Sciences, Via Mazzieri 3, 05100 Terni, University of Perugia, Italy. Ettore Mearini and Giulia Poli share first authorship.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- KirkaliZChanTManoharanMBladder cancer: epidemiology, staging and grading, and diagnosisUrology200566Suppl 6A43416399414
- Van RhijnBWBurgerMLotanYRecurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategyEur Urol200956343044219576682
- LosaAHurleRLemboALow dose bacillus Calmette-Guerin for carcinoma in situ of the bladder: long-term resultsJ Urol20001631687110604316
- VroomanOPJWitjesJAUrinary markers in bladder cancerEur Urol200853590991618162285
- ZitvogelLKeppOGalluzziLKroemerGInflammasomes in carcinogenesis and anticancer immune responsesNat Immunol201213434335122430787
- MottaVSoaresFSunTPhilpottDJNOD-like receptors: versatile cytosolic sentinelsPhysiol Rev201595114917825540141
- Henao-MejiaJElinavEStrowigTFlavellRAInflammasomes: far beyond inflammationNat Immunol201213432132422430784
- GrivennikovSIGretenFRKarinMImmunity, inflammation, and cancerCell2010140688389920303878
- AggarwalBBShishodiaSSandurSKPandeyMKSethiGInflammation and cancer: how hot is the link?Biochem Pharmacol200672111605162116889756
- PoliGBrancorsiniSCochettiGBarillaroFEgidiMGMeariniEExpression of inflammasome-related genes in bladder cancer and their association with cytokeratin 20 messenger RNAUrol Oncol20153312505.e1e7
- Di LevaGGarofaloMCroceCMMicroRNAs in cancerAnn Rev Pathol2014928731424079833
- GuancialEABellmuntJYehSRosenbergJEBermanDMThe evolving understanding of microRNA in bladder cancerUrol Oncol201432141
- WangGChanESKwanBCExpression of microRNAs in the urine of patients with bladder cancerClin Genitourin Cancer201210210611322386240
- SnowdonJBoagSFeilotterHIzardJSiemensDRA pilot study of urinary microRNA as a biomarker for urothelial cancerCan Urol Assoc J201371–2283222630336
- NeelyLARieger-ChristKMNetoBSA microRNA expression ratio defining the invasive phenotype in bladder tumorsUrol Oncol2010281394818799331
- YoshinoHSekiNItesakoTChiyomaruTNakagawaMEnokidaHAberrant expression of microRNAs in bladder cancerNat Rev Urol201310739640423712207
- PignotGCizeron-ClairacGVacherSmicroRNA expression profile in a large series of bladder tumors: identification of a 3-miRNA signature associated with aggressiveness of muscle-invasive bladder cancerCancer20131321124792491
- XuZYuYQGeYZMicroRNA expression profiles in muscle-invasive bladder cancer: identification of a four-microRNA signature associated with patient survivalTumor Biol2015361081598166
- SutterwalaFSHaaskenSCasselSLMechanism of NLRP3 inflammasome activationAnn N Y Acad Sci20141319829524840700
- MacLennanGTKirkaliZChengLHistologic grading of non-invasive papillary urothelial neoplasmsEur Urol200751488989817095142
- BabjukMBöhleABurgerMEAU guidelines on non-muscle-invasive bladder cancerEur Urol20115958459421269756
- YonghuaHJiahaoCXiaokunZMicroRNA expression signatures of bladder cancer revealed by deep sequencingPLoS One201163e1828621464941
- CollinsAS1McCoyCELloydATStevensonNJmiR-19a: an effective regulator of SOCS3 and enhancer of JAK-STAT signallingPLoS One201387e6909023894411
- PichiorriFSuhSSLadettoMMicroRNAs regulate critical genes associated with multiple myeloma pathogenesisProc Natl Acad Sci U S A200810535128851289018728182
- EgidiMGGuelfiGCochettiGCharacterization of kallireins and microRNAs in urine sediment for the discrimination of prostate cancer from benign prostatic hyperplasiaJ Cancer Sci Ther201574
- GrahamMEHartRDDouglasSSerum microRNA profiling to distinguish papillary thyroid cancer from benign thyroid massesJ Otolaryngol Head Neck Surg2015443326341226
- XuBHuangYNiuXHsa-miR-146a-5p modulates androgen-independent prostate cancer cells apoptosis by targeting ROCK1Prostate201575161896190326306811
- LiHXieSLiuMThe clinical significance of downregulation of mir-124–3p, mir-146a-5p, mir-155–5p and mir-335–5p in gastric cancer tumorigenesisInt J Oncol20144519720824805774
- HouZHHanQJZhangCTianZGZhangJmiR146a impairs the IFN-induced anti-HBV immune response by downregulating STAT1 in hepatocytesLiver Int2014341586823890093
- WangMChuHLiPGenetic variants in miRNAs predict bladder cancer risk and recurrenceCancer Res201272236173618222846912
- WangGTamLSLiEKSerum and urinary free microRNA level in patients with systemic lupus erythematosusLupus201120549350021372198
- PekowJRKwonJHMicroRNAs in inflammatory bowel diseaseInflamm Bowel Dis201218118719321425211
- ZibertJRLøvendorfMBLitmanTMicroRNAs and potential target interactions in psoriasisJ Dermatol Sci201058317718520417062
- HaneklausMGerlicMO’NeillLAMastersSLmiR-223: infection, inflammation and cancerJ Intern Med2013274321522623772809
- ZhiYPanJShenWGinkgolide B inhibits human bladder cancer cell migration and invasion through microRNA-223-3pCell Physiol Biochem20163951787179427744452
- WeiYYangJYiLMiR-223–3p targeting SEPT6 promotes the biological behavior of prostate cancerSci Rep20144754625519054
- LernerAGUptonJPPraveenPVIRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stressCell Metab201216225026422883233
- TaziMFDakhlallahDACautionKElevated Mirc1/Mir17–92 cluster expression negatively regulates autophagy and CFTR (cystic fibrosis transmembrane conductance regulator) function in CF macrophagesAutophagy201612112026203727541364
- VoliniaSCalinGALiuCGA microRNA expression signature of human solid tumors defines cancer gene targetsProc Natl Acad Sci U S A200610372257226116461460
- BoeriMVerriCConteDMicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancerProc Natl Acad Sci U S A201110893713371821300873
- TsujiuraMIchikawaDKomatsuSCirculating microRNAs in plasma of patients with gastric cancersBr J Cancer201010271174117920234369
- HuhJHKimTHKimKDysregulation of miR-106a and miR-591 confers paclitaxel resistance to ovarian cancerBr J Cancer2013109245246123807165
- FengYLiuJKangYmiR-19a acts as an oncogenic microRNA and is up-regulated in bladder cancerJ Exp Clin Cancer Res2014336725107371
- XuXWuXJiangQDownregulation of microRNA-1 and microRNA-145 contributes synergistically to the development of colon cancerInt J Mol Med20153661630163826459459