Abstract
The co-inhibitory receptor programmed cell death (PD)-1, expressed by immune effector cells, is credited with a protective role for normal tissue during immune responses, by limiting the extent of effector activation. Its presently known ligands, programmed death ligands (PD-Ls) 1 and 2, are expressed by a variety of cells including cancer cells, suggesting a role for these molecules as an immune evasion mechanism. Blocking of the PD-1-PD-L signaling axis has recently been shown to be effective and was clinically approved in relapsed/refractory tumors such as malignant melanoma and lung cancer, but also classical Hodgkin’s lymphoma. A plethora of trials exploring PD-1 blockade in cancer are ongoing. Here, we review the role of PD-1 signaling in lymphoid malignancies, and the latest results of trials investigating PD-1 or PD-L1 blocking agents in this group of diseases. Early phase studies proved very promising, leading to the clinical approval of a PD-1 blocking agent in Hodgkin’s lymphoma, and Phase III clinical studies are either planned or ongoing in most lymphoid malignancies.
Background
Regulation of T-cell activation consists of two distinct signals. The primary signal is represented by a specific interaction between the T-cell receptor (TCR) and the antigen bound by the major histocompatibility complex molecule on the surface of the antigen presenting cells (APCs). The second signal is mediated through co-stimulation of lymphocyte receptor CD28 by B7 ligands (CD80, CD86) induced on the APC by pathogens, playing an important role in T-cell activation and tolerance. However, co-inhibitory signaling can limit activation and suppress effector T-cell actions and is as such credited with a protective role, by limiting immune damage to healthy tissue and inducing tolerance. Molecules such as cytotoxic T lymphocyte associated protein 4 (CTLA-4), programmed cell death 1 (PD-1) and its ligands, programmed death-ligand (PD-L) 1 and 2, are members of the B7/CD28 ligand–receptor family and represent the most investigated inhibitory immune checkpoints at present.Citation1
The PD-1 (CD279) receptor is a transmembrane protein of the immunoglobulin superfamily and was first identified and characterized in 1992 in mice.Citation2,Citation3 It is a co-inhibitory receptor found on the surface of T cells, B cells, monocytes, and activated natural killer cells.Citation4 The receptor interacts with its two ligands, PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273)Citation5,Citation6 expressed by APCs, PD-L1 being regarded as the main mediator of PD-1 dependent immunosuppression.Citation7 This ligand is constitutively expressed on T cells, B cells, macrophages, and dendritic cells, as well as nonimmune cells, such as endothelial cells, β pancreatic cells, glial cells, epithelial cells, and muscle fibers.Citation8–Citation10 In contrast to PD-L1, PD-L2 has a more narrow expression profile, restricted to APC and helper T cells, but an affinity approximately two to sixfold higher, hence the possibility of competition between ligands for the binding of the receptor.Citation5,Citation11,Citation12 Also, the mechanism of action of the two ligands differs: PD-L1 binds to both PD-1 and CD80, whereas PD-L2 interacts directly with PD-1.Citation6
The PD-1-PD-L pathway downregulates the immune response to maintain a balance between T cell activation and healthy tissue destruction, thus preserving peripheral tolerance ().Citation13–Citation15 T cell activation is followed by upregulation of PD-1 and production of cytokines, such as interferon (IFN)-γ and interleukin (IL)-4. These cytokines upregulate PD-L1 expression through a positive feedback mechanism, having a role in preventing autoimmunity and tissue destruction.Citation16,Citation17 In case of an inadequate immune response, prolonged antigen stimulation causes PD-1 upregulation and T cell exhaustion. The critical role of downregulation of the immune system through PD-1 stimulation has been demonstrated in a series of studies on chronic viral infections such as HIV, hepatitis B and hepatitis C, whereas CD8+ T cells have impaired proliferation responses and cytokine production, and are often described as exhausted T cells.Citation18–Citation20 In these cases there is a persistent T cell activation with PD-1 upregulation and, consecutively, PD-1-PD-L1 pathway stimulation, resulting in inactive T cells, infection persistence and a minimized immune aggressive effect on healthy tissues.Citation21
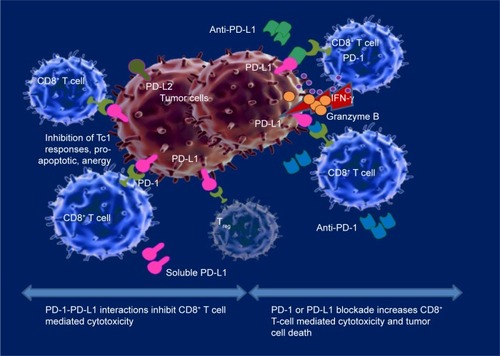
Figure 1 PD-1- PD-L1 axis blockade in cancer.
Abbreviations: PD, programmed cell death; PD-L1, programmed cell death receptor ligand-1.
Role of PD-1-PD-L pathway in cancer
Involvement of the PD-1-PD-L pathway in cancer has been demonstrated in a broad variety of solid malignancies, such as breast cancer, colon carcinoma, lung cancer, renal cell cancer, melanoma, ovarian cancer, bladder cancer, pancreatic cancer, and various hematologic malignancies.Citation7,Citation22–Citation26 PD-1 levels are considerably upregulated on tumor-infiltrating lymphocytes (TILs) in comparison to peripheral blood or healthy tissues infiltrating T cells, and consecutively TILs exert an impaired antitumor activity.Citation5,Citation27–Citation30 Compared to PD-1− lymphocytes, PD-1+ TILs exhibit an “exhausted” phenotype, through decreased TCR signaling, defective calcium flux and diminished cytokine production including IL-2, IFN-γ, and TNFα.Citation28,Citation29,Citation31–Citation34 PD-L1 expression is encountered in a large variety of tumors as well: lung, breast, colon, skin, ovarian, gastric, pancreatic cancers, and different types of hematologic malignancies.Citation17,Citation35–Citation38 The ligand is upregulated at the surface of cancer cells, intratumoral macrophages and APCs from the surrounding tumor microenvironment. PD-L1 appears to act as an antiapoptotic factor in cancer cells, as its expression is strongly associated with in vivo tumorigenesis and invasion, and in vitro resistance to T cell mediated lysis.Citation1,Citation39 The ligand upregulation is triggered by proinflammatory cytokines such as IFN-γ produced by lymphocytes present in the tumor microenvironment.Citation38 Therefore, activation of the PD-1-PD-L1 immune checkpoint pathway in cancer represents an adaptive mechanism of resistance used by cancer cells against TILs, suggesting the presence, yet exhaustion of an antitumor T-cell immune response.
In vitro studies have demonstrated that blockade of PD-1 or PD-L1 using monoclonal antibodies restored T cell cytotoxic capacity and IFN-γ production ().Citation1,Citation40 Subsequently, clinical studies have confirmed these findings, with PD-1 and PD-L1 blocking antibodies being successful at present and having been recently approved by the US Food and Drug Administration for the treatment of metastatic melanoma, nonsmall cell lung cancer, renal cell and urothelial carcinoma, head and neck cancer, and classical Hodgkin’s lymphoma (cHL) ().
Table 1 US Food and Drug Administration-approved PD-1 checkpoint blockers
Potential biomarkers for the efficacy of PD-1–PD-L blockade
PD-L1 and/or PD-1 expression were actively investigated as potential biomarkers to predict the efficacy of PD-1-PD-L1 axis blockade. Initial studies and preclinical data in solid tumors have found a correlation between PD-L1 expression and clinical benefits of PD-1 blockade, suggesting that the ligand might be a promising biomarker, with a better association to the treatment response in comparison with PD-1 expression.Citation41 A strong correlation between PD-L1+ expression in malignant cells and the response to PD-1 blockers has been demonstrated in lung cancer,Citation42 but also in melanoma,Citation43 breast cancer,Citation44 hepatocellular carcinoma,Citation45 and colorectal cancer,Citation46 whereas in renal cell carcinoma and urothelial carcinoma PD-L1+ infiltrating cells correlate best with response to anti-PD-L1 antibodies.Citation47–Citation49
Some of the difficulties encountered in PD-L1 evaluation were the limited tumoral tissue availability, the tissue heterogeneity, and the markers’ dynamic, the expression of which is influenced by infections, malignancies, and treatment. Although early phase studies in advanced solid cancers such as melanoma, lung cancer, colorectal cancer, renal-cell cancer, and prostate cancer demonstrated clinical benefits in PD-L1+ tumors and none in PD-L1− cohorts,Citation1,Citation7,Citation50 a recent Phase III randomized trial of nivolumab, an anti-PD-1 human IgG4 monoclonal antibody, in melanoma showed improved survival in all subgroups, regardless of the levels of PD-L1 expression, but objective response rates (ORRs) were significantly higher in the PD-L1+ subgroup (52.7%) than in the PD-L1− one (33.1%).Citation51 Nevertheless, even patients with tumors lacking PD-L1 expression can benefit from anti-PD-1 therapy, probably due to tumor microenvironment responsiveness.Citation1,Citation49,Citation52 Therefore, a lack of PD-L1 expression is not an appropriate biomarker for patient exclusion, with PD-L1 status being rather appropriate for stratification into groups that would benefit from anti-PD-1 monotherapy and groups that are in need of combination therapy in order to achieve a better response.Citation52 However, the recent approval by the US Food and Drug Administration (FDA) of anti PD-1 agent pembrolizumab for nonsmall cell lung cancer is conditional on the demonstration of tumor PD-L1 expression by an FDA-approved test.
The differences between data obtained in various clinical trials may be attributed to different cutoff values for PD-L1 expression varying in different trials from as low as 1%Citation53 to the more frequently used 5%,Citation54,Citation55 and even as high as 50% of tumor cells.Citation53 Uniformization and standardization of PD-L1 expression assessment, including the positive cutoff value, the selective staining of tumor cells or infiltrating immune cells and the types of antibody used, is of major significance for future trials. A sensitivity analysis of 20 trials of PD-1 axis blockers’ efficacy according to PD-L1 expression in solid tumors which used thresholds of 1% and 5% ligand expression by immunostaining, underscored the above shortcomings and concluded that a cutoff of 5% should be used for PD-L1 expression assessment.Citation56
The mechanism of anti-PD-1 therapy action differs between solid tumors and hematologic malignancies. PD-1 evaluation as a prognostic marker in lymphoid malignancies has yielded variable results. While in Hodgkin’s lymphoma, PD-1 expression correlated with overall survival (OS) being a stage-independent negative prognostic factor,Citation57,Citation58 the same receptor expressed by TILs represents a positive prognostic marker for progression-free survival (PFS) and OS in cases of follicular lymphomas.Citation59
Besides surface expression of PD-1 and its ligands, other biomarkers have been evaluated to predict efficacy of the PD-1 signaling blockade. These include the presence of soluble PD-L1 (sPD-L1) in patients’ sera,Citation60,Citation61 the ratio of immune cells subtypes in the tumor microenvironment,Citation62,Citation63 and immune gene expression signature.Citation64 Specific biomarkers of PD-1 axis blockade investigated in lymphoproliferative disease will be discussed in the respective sections below.
PD-1-PD-L1 pathway blockade in hematological malignancies
Hematological cancers have, too, developed diverse strategies of evading the immune system. Since the impressive effect of PD-1 blockade has been proven, PD-1 or PD-L1 targeted antibodies are being investigated for the treatment of various types of hematological malignancies. The use of immune checkpoint blockade in these pathologies is limited, but has shown clinical benefit in relapsed or refractory disease settings.Citation3 Markers of the PD-1 pathway, evaluated by immunohistochemistry or flow cytometry, have been confirmed in hematologic diseases such as multiple myeloma (MM), acute myeloid leukemia, and Hodgkin and non-Hodgkin lymphomas (NHL).Citation57,Citation65 Of the NHLs, PD-1 and ligands expression has been confirmed in chronic lymphocytic leukemia (CLL),Citation66 follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBL), anaplastic large-cell lymphoma, and angioimmunoblastic T-cell lymphoma (AITL).Citation57,Citation67–Citation69
The timing of PD-1 blockade initiation is crucial and there are several temporal aspects under consideration in the optimization of treatment outcome. First, initiation of anti-PD-1 antibody treatment prior to chemotherapy may enhance antitumor immune responses offering a better support for subsequent treatment. Second, use of immune checkpoint blockade concomitantly with classical chemotherapy could enhance the antitumor response by creating a tumor antigen-rich environment consecutive to cell lysis, which can further stimulate the immune system. Finally, a third option would be the administration of PD-1 blockers after the cytotoxic treatment, boosting the antitumor response during a period of immune reconstitution subsequent to chemotherapy-induced myelosuppression.Citation57 Also, chemotherapy induces PD-1 expression on immune cells, favoring the immune checkpoint blockade being administered postcytotoxic treatment.Citation21,Citation70,Citation71 Based on this reasoning, anti-PD-1 therapy could be applied either before autologous/allogeneic stem cells transplantation (ASCT), or to target residual disease after transplantation, when PD-1 pathways may serve as a tumor survival mechanism.Citation52,Citation72,Citation73
The response to PD-1 blockade varies significantly between different types of lymphoma, due to the diverse mechanisms responsible for the expression of co-inhibitory molecules, and no definitive correlations have been yet established. PD-L1 is an inducible molecule that can be stimulated by viral infections, TILs, or genetic changes within the tumor.Citation69,Citation74 Also, high levels of sPD-L1 may be secreted by tumor-infiltrating cells following proinflammatory cytokine stimulation.Citation36
While in solid tumors PD-L1 is highly expressed on cancer cells but minimally expressed in the surrounding normal tissue,Citation22 in up to 73% of T-cell NHL subtypes PD-L1 expression is more prevalent on tumor-infiltrating cells as compared to malignant cells, contributing to immune suppression.Citation75,Citation76
DLBCL cells employ multiple mechanisms for the upregulation of PD-1 and its ligands, occasionally present on the same tumor cell, in contrast to FL, where PD-1 is highly expressed in the microenvironment, highlighting different immune evasion strategies.Citation62,Citation68,Citation77,Citation78
Strong PD-L1 expression in DLBCL tumor cells is significantly associated with Epstein–Barr virus (EBV) infection, and is higher in activated B cell-like than in germinal center B cell-like phenotypes. The levels of PD-1+ TIL correlated positively with the level of PD-L1 expression in tumor cells or macrophages.Citation79
Similar to HL, where EBV latent membrane protein 1 (LMP-1) increases PD-L1 promoter activity, PD-L1 expression in DLBCL may be upregulated by EBV infection.Citation80,Citation81 Similar to genetic aberrations encountered in HL, several gene expression profiling studies demonstrated the presence of gains/amplifications of a region of chromosome 9p24 in ~70% of PMBL cases, leading to a high expression of PD-L1 and 2, which distinguishes PMBL from other types of DLBCL and could serve as a molecular diagnostic tool.Citation82–Citation84
Primary central nervous system lymphomas (PCNSLs) and primary testicular lymphomas have also been shown to exhibit 9p24.1 copy gain and chromosomal translocation of PD-L1/PD-L2, as well as EBV-mediated upregulation of the ligands in PCNSL.Citation85
An association between viral infections and PD-L1 expression was also observed in other lymphoid malignancies. Studies on EBV-positive natural killer/T-cell lymphoma found a high expression of PD-L1 in lymphoma cells, upregulated by LMP1 through the MAPK/NF-κB pathway. High PD-L1 expression (>38%) and serum sPD-L1 levels ≥3.4 ng/mL were interpreted as independent prognostic factors for lower complete remission (CR) rates, PFS, and OS.Citation86 Also, in extranodal NK/T-cell lymphoma (ENKTL) treated with asparaginase, high posttreatment sPD-L1 level (>1.12 ng/mL) was demonstrated to be a predictive biomarker for early relapse and poor prognosis and also a marker of minimal residual disease.Citation87 However, results from another study show that high PD-L1 expression in advanced stages of EBV+ ENKTL correlates with improves OS,Citation88 further studies being warranted to establish the prognostic value of PD-L1 expression in these cases. In adult T-cell leukemia/lymphoma (ATLL), HTLV-1 bZIP factor expressed by HTLV-1 infected cells upregulates PD-1 expression on both neoplastic and normal CD4+ T cells, but impedes its suppressive signals by inhibiting co-localization of PD-1 and tyrosine phosphatase (SHP-2), favoring the proliferation of infected cells and immune suppression.Citation89,Citation90 Both asymptomatic HTLV-1 carriers and ATLL patients express high PD-1 levels on HTLV-1-specific cytotoxic T cells, with elevated levels in patients with EBV and CMV co-infection. PD-L1 expression was only identified in ATLL cells of the patients and administration of an anti-PD-L1 or anti-PD-1 antibody stimulated HTLV+ CD8+ T cell immune response.Citation91,Citation92
In the next sections, we review results of PD-1 blockade studies in lymphoproliferative diseases, the most relevant clinical trial efficacy reports being summarized in .
Table 2 Selected clinical trial results of PD-1-PD-L checkpoint blockade efficacy in lymphoid malignancy
Hodgkin’s lymphoma
Hodgkin’s lymphoma (HL) is an ideal candidate for anti-PD-1 therapy, because of its particular histological structure, which involves a rather small number of primary tumor-associated CD-30+ Reed Sternberg or Hodgkin cells surrounded by a granuloma-like, immune cell-rich environment. A viral or genetic-induced PD-L1 overexpression by Reed Sternberg malignant cells was also described.Citation93 Thus, not surprisingly, of the several clinical studies evaluating PD-1 blockade efficiency in hematologic malignancies, the most promising results have been recorded for patients with cHL. Consecutively, cHL is the first hematologic malignancy in which an anti-PD-1 agent, nivolumab, has been approved, in early 2016, as salvage therapy after prior ASCT and brentuximab. The breakthrough therapy designation by the FDA is based on results of a Phase I (CheckMate-039)Citation93 and a Phase II (Checkmate-205)Citation94,Citation95 trial ().
The PD-L1 gene has been identified in HL and is located on the short arm of chromosome 9p24. Amplification of genetic material in the 9p24 region is associated with PD-1 ligand overexpression in nodular sclerosis HL and also PMBL: mostly PD-L1 in HL and PD-L2 in PMBL. Amplification of this region also results in amplification of JAK2, which through JAK2-STAT signaling further stimulates PD-1 ligand overexpression.Citation66,Citation83 Immune cells surrounding Reed Sternberg cells include PD-1+ T-cells, whose function and IFN-γ production can be stimulated by immune checkpoint blockade.Citation38,Citation65 Epstein–Barr infection, commonly associated with HL, is another mechanism involved in PD-L1 upregulation,Citation80 viral infections being known as able to exploit the PD-1-PD-L pathway in order to induce immune tolerance.Citation40,Citation52
Chronic lymphocytic leukemia
CLL is the most frequent B cell malignancy, characterized by an increased proliferation and accumulation of monoclonal CD5+ CD19+ B cells in the bone marrow, lymphoid organs, and peripheral blood, promoting a tumor microenvironment which dampens the immune response and favors malignant cell proliferation and treatment resistance.Citation96,Citation97 Recent studies described a functional impairment in the T-cell compartment (both CD4+ and CD8+) reflected by alterations of their number, function and memory, as a consequence of interaction with leukemic cells.Citation37,Citation98 T-cells are found in a state of chronic activation and have the tendency to accumulate, presenting an inversion of the normal CD4:CD8 ratio, higher levels of CD8+ T cells being associated with a more aggressive disease.Citation99
The PD-1–PD-L1 axis has been shown to be involved in CLL pathogenesis. Immunohistochemistry and immunofluorescence assays of PD-1 and PD-L1 expression in lymph nodes of CLL patients demonstrated the presence of PD1+ CD4+ T cells and PD-L1+ CD23+ B cells.Citation37 Both T cells and leukemic cells are present in lymph nodes proliferation centers, suggesting their probable interaction and involvement in CLL pathogenesis.
A study on the effects of early in vivo PD-L1 blockade in a mouse model of CLL showed that administration of anti-PD-L1 antibodies prevented disease progression. Decreased spleen sizes were observed, as well as changes in the tumor load and peripheral blood lymphocytosis. PD-L1 blockade prevented CD8:CD4 ratio inversion, T cell exhaustion, and determined increased CD8 cytotoxicity, growth fraction, in vivo proliferation, and CD8 synapse formation.Citation100–Citation102
PD-1 expression is found on CD4+ and CD8+ T cells, their proliferation being associated with a negative prognosis,Citation99 while PD-L1 expression on leukemic cells and mononuclear cells in peripheral blood and bone marrow is higher in CLL patients than in healthy controls.Citation66,Citation103 Apparently, ligand expression does not correlate with age, sex, LDH levels, white blood cell count or disease stage.Citation66 However, PD-L1 significantly correlates with PD-1, TIL number, and IFN-γ levels, PD-1–PD-L1 interaction suppressing intratumoral cytokine production, and ligand blockade stimulating IFN-γ secretion through a negative feedback mechanism.Citation66,Citation76
Rusak et al reported that patients with advanced stage CLL have a considerably increased number of PD1+ CD4+ T cells in the peripheral blood compared to patients with incipient stages of the disease.Citation104 Also, patients with lower levels of these lymphocytes achieved CR more frequently through PD-1 blockade, suggesting that the number of PD1+ CD4+ T cells correlates with the response obtained with this immunotherapy approach. These findings suggest that quantitative flow cytometric evaluation of PD1+ CD4+ T cells could be used for prognostic purposes in newly diagnosed patients.Citation104
Non-Hodgkin lymphoma
PD-1-PD-L blockade is under investigation in various types of NHL, based on evidence of frequent expression of PD-1, PD-L1, and PD-L2 in lymphoid malignancies. Cancer cells drive changes in the host immune response to generate unique microenvironments that promote cancer cell growth. The PD-1-PD-L1 interaction represents an important cause of lymphoma induced T-cell defects, causing changes in T-cell subsets, their effector function, expression of surface molecules, and gene expression profiles. Studies on FL and DLBCL found overexpression of 25 immune escape genes including the ones involved in PD-1–PD-L and CTLA-4 inhibitory axis, the LAG3 and TIM3/galectin T-cell exhaustion axis.Citation68 Expression of these genes and proteins is considerably higher in DLBCL than in FL. Detectable circulating soluble PD-L1 was reported in DLBCL, and was associated with a poor prognosis.Citation105 In a Phase I study of the anti-PD-1 nivolumab in patients with relapsed or refractory T and B-cell lymphomas ORR were 40% in patients with FL, 36% in DLBCL, 15% in mycosis fungoides (MF) and 40% in peripheral T-cell lymphoma (PTCL). In the same study it was discovered that malignant cells from MF expressed high levels of PD-L2 and fluorescence in situ hybridization assays revealed chromosomal rearrangements (disomy 9p, polysomy 9p, and PD-L2 translocation), responsible for the ligand overexpression.Citation25 Results of clinical trials of PD-1 axis blockade in NHL are summarized in .
Diffuse large B-cell lymphoma
PD-L1 is highly expressed by DLBCL (>60% of cases), as compared to other solid tumors, such as melanoma (30%) or nonsmall cell lung cancer (25%–36%).Citation68 PD-L1 expression by lymphoma cells is considered an independent factor for the OS, being associated with a poor prognosis. The number of PD-1+ TILs is associated with PD-L1+ lymphoma cells and PD-L1+ stromal cells, suggesting a role for the PD-1–PD-L1 pathway in the tumor microenvironment.Citation78
Pidilizumab, a humanized IgG1 monoclonal antibody, was initially developed as an anti-PD-1 antibody and was the first one to be evaluated in lymphoid malignancies. Phases I and II studies in DLBCL and FL showed promising results, prompting the development of further anti-PD-1 antibodies.Citation100 A multicenter Phase II clinical trial where pidilizumab was administered to patients with relapsed or refractory DLBCL after ASCT reported a 16 month PFS of 72%. The reported overall response rate among patients with measurable disease after ASCT was 51%, including 34% CRs and 17% partial remissions.Citation73 The PFS after posttransplant pidilizumab administration of 72% compares favorably to the PFS of 52% obtained after ASCT alone, in a cohort of autografted patients with chemosensitive DLBCL.Citation52,Citation73 Interestingly, by the end of 2015, the manufacturer of pidilizumab announced that the drug was no longer to be regarded as a PD-1 inhibitor. However, trials of pidilizumab in lymphoma yielded encouraging results and will go on, despite the mechanisms of the immune regulatory action of the drug not being precisely known.
Quan et al evaluated the efficacy of PD-1 blockade in Epstein–Barr virus (EBV)-associated DLBCL (EBV+ DLBCL), an aggressive lymphoma, highly resistant to current treatments and a potential target for immunotherapy. The number of effector/memory PD-1+ T cells is more elevated in the lymph nodes than in the peripheral blood, suggesting the immune suppressive effect of the tumor microenvironment in DLBCL. CD8+ PD-1+ and CD4+ PD-1+ T cells also expressed CTLA-4, marker of T cell exhaustion. Lymphoma cells upregulate PD-1 expression on T cells and inhibit their proliferation and secretion of IL-2, IFN-γ, tumor necrosis factor-α (TNF-α) and IL-10. PD-1 blockade reversed these effects, increasing T-cell proliferation and cytokine secretion. Also, it was shown in vitro that PD-1 blockade is more potent in EBV+ than in EBV− DLBCL.Citation81 A French multicenter randomized trial in DLBCL patients receiving standard chemoimmunotherapy versus high-dose therapy revealed that levels of sPD-L1, with a cutoff of 1.52 ng/mL, had negative predictive value for OS, elevated levels being significantly correlated with a poorer prognosis in the standard chemoimmunotherapy arm, and suggesting a potential benefit of PD-1 axis blocking therapy in these patients.Citation105
Follicular lymphoma
FL is a hematologic malignancy characterized by an indolent heterogeneous evolution, relapses alternating with remissions and a 7–10 years median survival. In 10%–15% of the cases it behaves aggressively or transforms to DLBCL, leading to poor treatment response and short survival.Citation59 Gene expression profile and immunophenotyping of nonmalignant cells from FL have highlighted the involvement of the microenvironment in the clinical evolution and treatment response.Citation59 Unlike CLL, where T cell defects also appear in the peripheral blood, in FL T cells are impaired only in the lymph nodes.
There is controversial evidence regarding PD-1 expression and its prognostic value in FL. Muenst et al showed that grade 1 FL has a higher number of PD-1+ TILs than secondary DLBCL derived from FL. Also, PD-1+ TILs may influence tumor behavior in FL and secondary DLBCL arising from FL, being associated with improved disease specific survival in these entities.Citation106
Carreras et al concluded that increased numbers of CD4+ and CD8+ lymphocytes are favorable prognostic markers. PD-1+ cells and Tregs are localized in the tumoral follicular compartment, playing a role in inhibition of T cell activation and immunomodulation of the microenvironment. PD-1+ TILs are an independent prognostic marker of survival in patients with FL and their number decreases with transformation to DLBCL.Citation59 In a study on 70 FL patients selected for either very good or very poor outcome, CD4+ follicular cells were associated with poor outcome, whereas PD-1+ follicular cells and CD8+ interfollicular cells were associated with a good outcome. As far as tumor-microenvironment cell ratios were concerned, high CD4/CD8 and CD4 follicular/interfollicular ratios appeared to be markers of poor outcome.Citation62 A study conducted by Myklebust et al showed that PD-L1 expression is present in histocytes, in T cell-rich areas between the follicles, playing an inhibitory role in T-cell activation.Citation77 High numbers of tissue-infiltrating macrophages were associated with unfavorable evolution and TILs from FL have an impaired activity, probably mediated by the malignant B cells of the lymphoma. Follicular localization of Tregs correlated with poor clinical outcome and increased risk of transformation.Citation77
FL microenvironment includes a variety of T-cell subsets that express PD-1: antitumor effector T cells (helper CD4+ T and cytotoxic CD8+ T cells), protumoral follicular helper T cells (TFH) and follicular regulatory T cells, with a role in suppressing lymphoma cells and TFH.Citation107 TFH are localized in intrafollicular regions and highly express PD-1, whereas exhausted T cells reside in interfollicular areas and express low levels of PD-1.Citation108 Therefore, inconsistency regarding the prognostic value of PD-1+ T cells in FL is probably caused by the multiple types of cells expressing PD-1 receptor and by the effects of the PD-1 blockade on every subset.Citation107 Patients with higher numbers of PD-1+ effector T cells may have a positive response at anti-PD-1 antibody administration, whereas patients with higher numbers of PD-1+ TFH may have no response or develop disease progression after PD-1 blockade.Citation109
Reported clinical trial results showed promising results for nivolumab in R/R FL, albeit not as good as in cHL ().
Cutaneous T-cell lymphoma (CTCL)/mycosis fungoides
PD-1 is frequently expressed in the early stages of CTCL, where >25% of atypical lymphocytes express the receptor. PD-1 expression diminishes in the tumor stage of the lymphoma and in cases of large cell transformation. PD-L1 is expressed by the majority of atypical lymphocytes during all stages of lymphoma evolution and increases with disease progression and large cell transformation.Citation110 Therefore, administration of anti-PD-1 antibodies may restore the immune function of the lymphocytes and could be used in the early stages of CTCL, when PD-1 expression is the most pronounced. In more advanced stages when PD-L1 is highly expressed and the lymphoma is more aggressive, administration of anti-PD-L1 antibodies should improve the antitumor immunity.Citation110
Peripheral T-cell lymphoma
PTCLs are malignancies derived from postthymic T-cells. The most common types include AITL and PTCL, not otherwise specified (NOS). Both AITL and PTCL are characterized by atypical lymphocytes in the paracortical zones of the lymph nodes. Results from a study on PD-1 expression in PTCL demonstrated that extrafollicular expansion of PD-1+ T cells was encountered in 93% of the AITL cases and 62% of PTCL.Citation111
In another study all cases of AITL showed reactivity for PD-1, which is expressed on the cell surface and in the cytoplasm of neoplastic CD4+ T cells. In reactive lymph nodes, PD-1 expression is mainly localized in the germinal centers, similar to another marker encountered in AITL, CXCL13, a chemokine that distinguishes AITL from PTCL.Citation67 However, studies showed that PD-1 can serve as a sensible, but not specific marker for the diagnosis of AITL and PTCL-NOS, because a similar abnormal PD-1 staining pattern is observed in nonmalignant diseases such as viral lymphadenitis, highlighting the importance of differential diagnosis in these situations.Citation111,Citation112
Multiple myeloma
MM, an incurable B cell malignancy, is a monoclonal gammopathy characterized by neoplastic proliferation of plasma cells and their accumulation in the bone marrow, causing bone marrow failure, anemia, and osteolytic bone lesions with secondary hypercalcemia. Excessive production of monoclonal protein leads to predisposition to infection and systemic amyloidosis with organ failure (renal, heart, liver, and nervous system).Citation113 The disease mainly affects the elderly and has a median survival of 4–5 years.Citation114
CD138+ malignant plasma cells and tumor microenvironment cells such as myeloid-derived suppressor cells have an increased expression of PD-L1 compared with normal plasma cells, in which expression of this ligand is insignificant.Citation115–Citation118 PD-L1+ plasma cells levels do not correlate with tumor burden, suggesting that the ligand expression is also influenced by factors from the microenvironment. Indeed, populations of dendritic cells accumulate in the bone marrow, express PD-L1 and affect T cell antitumor activity.Citation119 Stromal cells upregulate PD-L1 levels on myeloma cells, stimulate their proliferation, and dampen the response to chemotherapy, accelerated disease progression being observed in patients with high PD-L1 expression.Citation114,Citation120
Myeloma cells accumulate and exert their action in the bone marrow. Their local immunosuppressive effect leads to the presence of a PD-1+ T cell population with higher dysfunction compared to circulating T cells, as shown in preclinical studies.Citation23,Citation116,Citation121 PD-1 expression is also elevated on natural killer cells from myeloma patients compared to healthy controls.Citation122 Direct interaction between the ligand present on myeloma cells and the receptor expressed by T and natural killer cells inhibits the antitumor immune response and contributes to chemotherapy resistance, while administration of an anti-PD-1 antibody stimulates lymphocyte cytolytic activity against malignant cells and reduces the tumor growth induced by stromal cells.Citation122–Citation124
In myeloma patients’ serum, high concentrations of soluble PD-L1 were identified and this fraction might also interact with PD-1, contributing to the immune suppression. Correlations between soluble PD-L1, disease aggressiveness, and poorer responses to treatment have been established. A value over 2.78 ng/mL was proposed as an independent prognosis factor for a shorter PFS.Citation123
Administration of anti-PD-L1 antibodies after chemotherapy overthrows tumor-induced immunosuppression and restores lymphocyte production of IFN-γ consecutive to tumor antigen stimulation. When both CD4+ and CD8+ T cells were reactivated, PD-L1 blockade determined successful myeloma eradication in preclinical studies.Citation125,Citation126
Conclusion
Like with other cancers, immune checkpoint blockade inhibitors are a promising immunotherapeutic option in hematologic malignancies, and PD-1-PD-L1 axis blockers are the most investigated candidates to date. While there are over 600 ongoing clinical trials of PD-1-PD-L axis blockade in oncology, only a small proportion of these are investigating hematologic cancers. Nevertheless, results in hematologic malignancies are extremely promising, and the US FDA granted accelerated approval for nivolumab in cHL in 2016. Fuelled by these results, the number of clinical trials is increasing at a high rate, and many drugs in this class are currently under development. With more early trial results published at this high rate, we expect that immune checkpoint blockade will soon become an integral and well represented target, and feature as part of the management of hematologic malignancy.
Acknowledgments
This work was supported by a grant from the Romanian National Authority for Scientific Research and Innovation, CNCS – UEFISCDI, project number PN-II-RU-TE-2014-4-2074. We thank Dr Michael Edwards of Imperial College for his kind support in the English language polishing of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- McDermottDFAtkinsMBPD-1 as a potential target in cancer therapyCancer Med20132566267324403232
- IshidaYAgataYShibaharaKHonjoTInduced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell deathEMBO J19921111388738951396582
- BryanLJGordonLIBlocking tumor escape in hematologic malignancies: the anti-PD-1 strategyBlood Rev2015291253225260226
- AgataYKawasakiANishimuraHExpression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytesInt Immunol1996857657728671665
- ZhengPZhouZHuman cancer immunotherapy with PD-1/PD-L1 blockadeBiomark Cancer20157Suppl 2151826448693
- GhiottoMGauthierLSerriariNPD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1Int Immunol201022865166020587542
- BrahmerJRDrakeCGWollnerIPhase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlatesJ Clin Oncol201028193167317520516446
- LiangSCLatchmanYEBuhlmannJERegulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responsesEur J Immunol200333102706271614515254
- StanciuLABellettatoCMLaza-StancaVCoyleAJPapiAJohnstonSLExpression of programmed death-1 ligand (PD-L) 1, PD-L2, B7-H3, and inducible costimulator ligand on human respiratory tract epithelial cells and regulation by respiratory syncytial virus and type 1 and 2 cytokinesJ Infect Dis2006193340441216388488
- ZdrengheaMTJohnstonSLRole of PD-L1/PD-1 in the immune response to respiratory viral infectionsMicrobes Infect201214649549922285902
- LesterhuisWJSteerHLakeRAPD-L2 is predominantly expressed by Th2 cellsMol Immunol2011491–21322000002
- YoungnakPKozonoYKozonoHDifferential binding properties of B7-H1 and B7-DC to programmed death-1Biochem Biophys Res Commun2003307367267712893276
- FifeBTPaukenKEEagarTNInteractions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signalNat Immunol200910111185119219783989
- KeirMELiangSCGuleriaITissue expression of PD-L1 mediates peripheral T cell toleranceJ Exp Med2006203488389516606670
- LatchmanYELiangSCWuYPD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cellsProc Natl Acad Sci U S A200410129106911069615249675
- YamazakiTAkibaHIwaiHExpression of programmed death 1 ligands by murine T cells and APCJ Immunol2002169105538554512421930
- ShiLChenSYangLLiYThe role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignanciesJ Hematol Oncol2013617424283718
- BoniCFisicaroPValdattaCCharacterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infectionJ Virol20078184215422517287266
- LiZLiNLiFImmune checkpoint proteins PD-1 and TIM-3 are both highly expressed in liver tissues and correlate with their gene polymorphisms in patients with HBV-related hepatocellular carcinomaMedicine (Baltimore)20169552e574928033288
- DayCLKaufmannDEKiepielaPPD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progressionNature2006443710935035416921384
- SehgalAWhitesideTLBoyiadzisMProgrammed death-1 checkpoint blockade in acute myeloid leukemiaExpert Opin Biol Ther20151581191120326036819
- BrownJADorfmanDMMaFRBlockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine productionJ Immunol200317031257126612538684
- HamanishiJMandaiMIwasakiMProgrammed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancerProc Natl Acad Sci U S A200710493360336517360651
- NomiTShoMAkahoriTClinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancerClin Cancer Res20071372151215717404099
- LesokhinAMAnsellSMArmandPNivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib StudyJ Clin Oncol201634232698270427269947
- HamanishiJMandaiMMatsumuraNAbikoKBabaTKonishiIPD-1/PD-L1 blockade in cancer treatment: perspectives and issuesInt J Clin Oncol201621346247326899259
- BlankCBrownIPetersonACPD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cellsCancer Res20046431140114514871849
- AhmadzadehMJohnsonLAHeemskerkBTumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impairedBlood200911481537154419423728
- LiJJieHBLeiYPD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironmentCancer Res201575350851825480946
- BottaiGRaschioniCLosurdoAAn immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancersBreast Cancer Res201618112127912781
- ChaponMRandriamampitaCMaubecEProgressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytesJ Invest Dermatol201113161300130721346771
- MuenstSSoysalSDGaoFObermannECOertliDGillandersWEThe presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancerBreast Cancer Res Treat2013139366767623756627
- WeiFZhongSMaZStrength of PD-1 signaling differentially affects T-cell effector functionsProc Natl Acad Sci U S A201311027E2480E248923610399
- ZhangYHuangSGongDQinYShenQProgrammed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancerCell Mol Immunol20107538939520514052
- Cimino-MathewsAThompsonETaubeJMPD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomasHum Pathol2016471526326527522
- DongHStromeSESalomaoDRTumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasionNat Med20028879380012091876
- BrusaDSerraSCosciaMThe PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemiaHaematologica201398695396323300177
- YamamotoRNishikoriMKitawakiTPD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphomaBlood200811163220322418203952
- IwaiYIshidaMTanakaYOkazakiTHonjoTMinatoNInvolvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockadeProc Natl Acad Sci U S A20029919122931229712218188
- TelcianAGLaza-StancaVEdwardsMRRSV-induced bronchial epithelial cell PD-L1 expression inhibits CD8+ T cell nonspecific antiviral activityJ Infect Dis20112031859421148500
- TaubeJMKleinABrahmerJRAssociation of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapyClin Cancer Res201420195064507424714771
- ZhangYWangLLiYProtein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinomaOnco Targets Ther2014756757324748806
- GrossoJHCInzunzaDCardonaDMSimonJSGuptaAKSankarVAssociation of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumabJ Clin Oncol201331Suppl Abstract 3016
- MuenstSSchaerliARGaoFExpression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancerBreast Cancer Res Treat20141461152424842267
- ZengZShiFZhouLUpregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinomaPLoS One201169e2362121912640
- DroeserRAHirtCViehlCTClinical impact of programmed cell death ligand 1 expression in colorectal cancerEur J Cancer20134992233224223478000
- McDermottDFSosmanJASznolMAtezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and Immune Correlates From a Phase Ia StudyJ Clin Oncol201634883384226755520
- BellmuntJMullaneSAWernerLAssociation of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinomaAnn Oncology2015264812817
- HerbstRSSoriaJCKowanetzMPredictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patientsNature2014515752856356725428504
- TopalianSLHodiFSBrahmerJRSafety, activity, and immune correlates of anti-PD-1 antibody in cancerN Engl J Med2012366262443245422658127
- RobertCLongGVBradyBNivolumab in previously untreated melanoma without BRAF mutationN Engl J Med2015372432033025399552
- ArmandPImmune checkpoint blockade in hematologic malignanciesBlood2015125223393340025833961
- GaronEBRizviNAHuiRPembrolizumab for the treatment of non-small-cell lung cancerN Engl J Med2015372212018202825891174
- RizviNAMazieresJPlanchardDActivity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trialLancet Oncol201516325726525704439
- MotzerRJRiniBIMcDermottDFNivolumab for metastatic renal cell carcinoma: results of a randomized phase II trialJ Clin Oncol201533131430143725452452
- CarbogninLPilottoSMilellaMDifferential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancersPLoS One2015106e013014226086854
- BryanLJGordonLIReleasing the brake on the immune system: the PD-1 strategy for hematologic malignanciesOncology201529643143926091677
- MuenstSHoellerSDirnhoferSTzankovAIncreased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survivalHum Pathol200940121715172219695683
- CarrerasJLopez-GuillermoARoncadorGHigh numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphomaJ Clin Oncol20092791470147619224853
- TakahashiNIwasaSSasakiYSerum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancerJ Cancer Res Clin Oncol201614281727173827256004
- FinkelmeierFCanliOTalAHigh levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosisEur J Cancer20165915215927039170
- WahlinBEAggarwalMMontes-MorenoSA unifying microenvironment model in follicular lymphoma: outcome is predicted by programmed death-1 – positive, regulatory, cytotoxic, and helper T cells and macrophagesClin Cancer Res201016263765020068089
- HaHNamARBangJHSoluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapyOncotarget2016747766047661227780932
- BangYJChungHCShankaranVLBA-04Clinical outcomes and their correlation with gene expression in patients with advanced gastric cancer treated with pembrolizumab (MK-3475): KEYNOTE-012Ann Oncol201526Suppl 4iv118iv118
- TimmermanJHematologic cancers break down a ‘checkpoint’: targeting the PD-1/PD-L1 axisOncology (Williston Park)201529644044126091678
- GrzywnowiczMKarczmarczykASkorkaKExpression of programmed death 1 ligand in different compartments of chronic lymphocytic leukemiaActa Haematol2015134425526226159545
- YuHShahsafaeiADorfmanDMGerminal-center T-helper-cell markers PD-1 and CXCL13 are both expressed by neoplastic cells in angioimmunoblastic T-cell lymphomaAm J Clin Pathol20091311334119095563
- LaurentCCharmpiKGravellePSeveral immune escape patterns in non-Hodgkin’s lymphomasOncoimmunology201548e102653026405585
- ChenBJChapuyBOuyangJPD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignanciesClin Cancer Res201319133462347323674495
- YangHBueso-RamosCDiNardoCExpression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agentsLeukemia20142861280128824270737
- KronigHKremmlerLHallerBInterferon-induced programmed death-ligand 1 (PD-L1/B7-H1) expression increases on human acute myeloid leukemia blast cells during treatmentEur J Haematol201492319520324175978
- NordeWJMaasFHoboWPD-1/PD-L1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantationCancer Res201171155111512221659460
- ArmandPNaglerAWellerEADisabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trialJ Clin Oncol201331334199420624127452
- VranicSGhoshNKimbroughJPD-L1 Status in Refractory LymphomasPLoS One20161111e016626627861596
- WilcoxRAFeldmanALWadaDAB7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disordersBlood2009114102149215819597183
- AndorskyDJYamadaRESaidJPinkusGSBettingDJTimmermanJMProgrammed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cellsClin Cancer Res201117134232424421540239
- MyklebustJHIrishJMBrodyJHigh PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cellsBlood201312181367137623297127
- KiyasuJMiyoshiHHirataAExpression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphomaBlood2015126192193220126239088
- KwonDKimSKimPJClinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomasHistopathology20166871079108926426431
- GreenMRRodigSJuszczynskiPConstitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and post-transplant lymphoproliferative disorders: implications for targeted therapyClin Cancer Res20121861611161822271878
- QuanLChenXLiuAPD-1 blockade can restore functions of T-cells in epstein-barr virus-positive diffuse large B-cell lymphoma In vitroPLoS One2015109e013647626361042
- RosenwaldAWrightGLeroyKMolecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphomaJ Exp Med2003198685186212975453
- GreenMRMontiSRodigSJIntegrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphomaBlood2010116173268327720628145
- BentzMBarthTFBruderleinSGain of chromosome arm 9p is characteristic of primary mediastinal B-cell lymphoma (MBL): comprehensive molecular cytogenetic analysis and presentation of a novel MBL cell lineGenes Chromosomes Cancer200130439340111241792
- ChapuyBRoemerMGStewartCTargetable genetic features of primary testicular and primary central nervous system lymphomasBlood2016127786988126702065
- BiXWWangHZhangWWPD-L1 is upregulated by EBV-driven LMP1 through NF-kappaB pathway and correlates with poor prognosis in natural killer/T-cell lymphomaJ Hematol Oncol20169110927737703
- WangHWangLLiuWJHigh post-treatment serum levels of soluble programmed cell death ligand 1 predict early relapse and poor prognosis in extranodal NK/T cell lymphoma patientsOncotarget2016722330353304527105512
- KimWYJungHYNamSJExpression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosisVirchows Arch2016469558159027595782
- KinosadaHYasunagaJIShimuraKHTLV-1 bZIP factor enhances T-Cell proliferation by impeding the suppressive signaling of co-inhibitory receptorsPLoS Pathog2017131e100612028046066
- ShimauchiTKabashimaKNakashimaDAugmented expression of programmed death-1 in both neoplastic and non-neoplastic CD4+ T-cells in adult T-cell leukemia/lymphomaInter J Cancer20071211225852590
- KozakoTYoshimitsuMFujiwaraHPD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patientsLeukemia200923237538218830259
- YasumaKYasunagaJTakemotoKHTLV-1 bZIP factor impairs anti-viral immunity by inducing co-inhibitory molecule, T cell immunoglobulin and ITIM domain (TIGIT)PLoS Pathog2016121e100537226735971
- AnsellSMLesokhinAMBorrelloIPD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphomaN Engl J Med2015372431131925482239
- YounesAAndreas EngertASShippMargaretCheckmate 205: A Phase 2 Study of Nivolumab in Patients With Classical Hodgkin Lymphoma Following Autologous Stem Cell Transplantation and Brentuximab VedotinCopenhagen, DenmarkEHA212016
- TimmermanJMEngertAYounesACheckmate 205 update with minimum 12-Month Follow up: a phase 2 study of nivolumab in patients with relapsed/refractory classical Hodgkin lymphomaBlood20161282211101110
- HerishanuYKatzBZLipskyAWiestnerABiology of chronic lymphocytic leukemia in different microenvironments: clinical and therapeutic implicationsHematol Oncol Clin North Am201327217320623561469
- RamsayADRodriguez-JustoMChronic lymphocytic leukaemia – the role of the microenvironment pathogenesis and therapyBr J Haematol20131621152423617880
- RossmannEDJeddi-TehraniMOsterborgAMellstedtHT-cell signaling and costimulatory molecules in B-chronic lymphocytic leukemia (B-CLL): an increased abnormal expression by advancing stageLeukemia200317112252225414576733
- NunesCWongRMasonMFeganCManSPepperCExpansion of a CD8(+)PD-1(+) replicative senescence phenotype in early stage CLL patients is associated with inverted CD4:CD8 ratios and disease progressionClin Cancer Res201218367868722190592
- BatleviCLMatsukiEBrentjensRJYounesANovel immunotherapies in lymphoid malignanciesNat Rev Clin Oncol2016131254026525683
- GribbenJTumor microenvironment: a rational therapeutic target for lymphomas EHA Learning CenterPresented at the 15th symposium of the EHAJune 14; 2015
- BergerRRotem-YehudarRSlamaGPhase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignanciesClin Cancer Res200814103044305118483370
- GrzywnowiczMZaleskaJMertensDProgrammed death-1 and its ligand are novel immunotolerant molecules expressed on leukemic B cells in chronic lymphocytic leukemiaPLoS One201274e3517822532845
- RusakMEljaszewiczABolkunLPrognostic significance of PD-1 expression on peripheral blood CD4+ T cells in patients with newly diagnosed chronic lymphocytic leukemiaPol Arch Med Wewn20151257–855355926140546
- RossilleDGressierMDamotteDHigh level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trialLeukemia201428122367237524732592
- MuenstSHoellerSWilliNDirnhoferaSTzankovADiagnostic and prognostic utility of PD-1 in B cell lymphomasDis Markers2010291475320826917
- WestinJRChuFZhangMSafety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trialLancet Oncol2014151697724332512
- YangZZGroteDMZiesmerSCXiuBNovakAJAnsellSMPD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survivalBlood Cancer J20155e28125700246
- ChuFNeelapuSSAnti-PD-1 antibodies for the treatment of B-cell lymphoma: importance of PD-1+ T-cell subsetsOncoimmunology201431e2810124808975
- KantekureKYangYRaghunathPExpression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma/mycosis fungoidesAm J Dermatopathol201234112612822094231
- KrishnanCWarnkeRAArberDANatkunamYPD-1 expression in T-cell lymphomas and reactive lymphoid entities: potential overlap in staining patterns between lymphoma and viral lymphadenitisAm J Surg Pathol201034217818920087161
- XerriLChetailleBSerriariNProgrammed death 1 is a marker of angioimmunoblastic T-cell lymphoma and B-cell small lymphocytic lymphoma/chronic lymphocytic leukemiaHum Pathol20083971050105818479731
- BladeJCibeiraMTFernandez de LarreaCRosinolLMultiple myelomaAnn Oncol201021Suppl 7vii313vii31920943635
- AtanackovicDLuetkensTKrogerNCoinhibitory molecule PD-1 as a potential target for the immunotherapy of multiple myelomaLeukemia2014285993100024153012
- CastellaBFogliettaMSciancaleporePAnergic bone marrow Vgamma9Vdelta2 T cells as early and long-lasting markers of PD-1-targetable microenvironment-induced immune suppression in human myelomaOncoimmunology2015411e104758026451323
- HallettWHJingWDrobyskiWRJohnsonBDImmunosuppressive effects of multiple myeloma are overcome by PD-L1 blockadeBiol Blood Marrow Transplant20111781133114521536144
- LiuJHamrouniAWolowiecDPlasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathwayBlood2007110129630417363736
- YousefSMarvinJSteinbachMImmunomodulatory molecule PD-L1 is expressed on malignant plasma cells and myeloma- propagating pre-plasma cells in the bone marrow of multiple myeloma patientsBlood Cancer J20155e285
- SponaasAMMoharramiNNFeyziEPDL1 expression on plasma and dendritic cells in myeloma bone marrow suggests benefit of targeted anti PD1-PDL1 therapyPLoS One20151010e013986726444869
- TamuraHIshibashiMYamashitaTMarrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myelomaLeukemia201327246447222828443
- RosenblattJGlotzbeckerBMillsHPD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccineJ Immunother201134540941821577144
- BensonDMJrBakanCEMishraAThe PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibodyBlood2010116132286229420460501
- WangLWangHChenHSerum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myelomaOncotarget2015638412284123626515600
- GorgunGSamurMKCowensKBLenalidomide enhances immune checkpoint blockade-induced immune response in multiple myelomaClin Cancer Res201521204607461825979485
- JingWGershanJAWeberJCombined immune checkpoint protein blockade and low dose whole body irradiation as immunotherapy for myelomaJ Immunother Cancer201531225614821
- KearlTJJingWGershanJAJohnsonBDProgrammed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myelomaJ Immunol2013190115620562823616570
- AnsellSArmandPTimmermanJMNivolumab in patients (Pts) with relapsed or refractory classical Hodgkin lymphoma (R/R cHL): clinical outcomes from extended follow-up of a phase 1 study (CA209–039)Blood201512623583583
- ArmandPShippMARibragVPembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: long-term efficacy from the phase 1b keynote-013 studyBlood20161282211081108
- MoskowitzCHZinzaniPLFanaleMAPembrolizumab in relapsed/refractory classical Hodgkin lymphoma: primary end point analysis of the phase 2 keynote-087 studyBlood20161282211071107
- DingWLe-RademacherJCallTGPD-1 blockade with pembrolizumab in relapsed CLL including Richter’s transformation: an updated report from a phase 2 trial (MC1485)Blood20161282243924392
- JainNBasuSThompsonPANivolumab combined with ibrutinib for CLL and Richter transformation: a phase II trialBlood2016128225959
- ZinzaniPLRibragVMoskowitzCHPhase 1b Study of Pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma: results from the ongoing keynote- 013tTrialBlood201612822619619
- BadrosAZHyjekEMaNPembrolizumab in combination with pomalidomide and dexamethasone for Relapsed/Refractory Multiple Myeloma (RRMM)Blood201612822490490
- San MiguelJMateosM-VShahJJPembrolizumab in combination with lenalidomide and low-dose dexamethasone for Relapsed/Refractory Multiple Myeloma (RRMM): keynote-023Blood201512623505505
