Abstract
Purpose
Tongue cancer is an extremely aggressive disease and is characterized by a poor prognosis. It is a complex disease to treat and current therapies have produced mediocre results with many side effects. Some facts suggest that natural essences can support traditional cancer therapy by carrying out a synergistic function with chemotherapy. Therefore, we evaluated the antitumor effects of genistein on tongue carcinoma cells.
Methods
Genistein 20, 50 and 100 µM were used for 24, 48 and 72 hours on 3 tongue carcinoma cell lines. xCELLigence system was used to evaluate the effects on cell adhesion, proliferation and to calculate IC50 values. Both MTT assay and Trypan blue assay were used to evaluate alterations in cell viability, scratch assay for cell migration and Western blot analysis for expression of some proteins.
Results
Cell adhesion was inhibited especially between 20 and 50 µM of genistein treatment. Proliferation was reduced by 50% for treatments with 20 µM at 24 hours, with 20 or 50 µM at 48 and 50 µM at 72 hours (P<0.0001). Viability tests confirmed a proportional reduction in concentration of genistein and duration of treatments. Even cell migration was reduced significantly (P<0.001). Genistein down-regulates vitronectin, OCT4 and survivin.
Conclusion
This in vitro study clarifies the anti-tumor effect of genistein on tongue carcinoma. In vivo studies are needed to confirm these data and develop a suitable delivery system that is capable of acting directly on tumor.
Introduction
Tongue cancer is the most common tumor of oral squamous cell carcinoma (OSCC). Survival rates at 5 years since diagnosis appears stable, despite new treatments.Citation1–Citation3 In addition, the side effects have not been completely eliminated.
This has led to a growing interest in researching new molecules with selective effects against cancer cells without causing any damage to healthy cells and tissues.
Genistein (4′,5,7-trihydroxyisoflavone) is an isoflavone, found in soybeans and all its derivatives such as flour, sauces, oil, milk and cheese.Citation4 It is also found in other legumes such as lentils, beans, peas, chickpeas and whole grains such as wheat, rice, barley, rye and oats.
It has potent anti-cancer properties, including the inhibition of tyrosine kinase proteins and inhibition of cell cycle at G2/M phase; it is also able to promote apoptosis by activation of caspase-9 and -3.Citation5–Citation7
Recently, clinical trials have confirmed the chemopreventive effects of genistein on many types of cancers.Citation8–Citation17
Many studies have been conducted on breast cancer demonstrating that genistein modulates oxidative stress, acting on estrogen receptor (ER)α/ERβ ratio,Citation18 down-regulates the activity of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor (HER)-2/neu modulating the expression levels of nuclear factor (NF)-kBCitation19 and it inhibits protein phosphatase (PP2A), inducing growth reduction and apoptosis.Citation20
However, genistein has a poor bioavailability in nature. It can be found as β-glucoside, which is hydrolized, in the small intestine, in the form of aglycones,Citation21–Citation23 its active pharmacological part.
During digestion, due to the action of microflora, the genistein is transformed in various metabolites. In this phase, the absorption efficiency is often reduced because the intestinal flora can degrade aglycones and produce aromatic acids.
It is known, however, that the glycosidic form can also be transported intact into the cell through a carrier and then hydrolyzed by cytosolic β-glucosidases.Citation24–Citation26
Shehata et al have fabricated self-emulsifying phospholipid pre-concentrates using some bioactive surfactants and they have demonstrated that they can be good nanocarriers for genistein, thereby overcoming its poor oral bioavailability.Citation27
Meanwhile, Gavin et al studied a nanomocoadhesive system using lipid-based nanocarriers containing genistein, named nanoemulsions, and achieved excellent results in patients with oral cancer.Citation28
This study suggests that in situ application of genistein in tumors could help in overcoming obstacles associated with its poor bioavailability. This may be simpler for oral cancer since they are present in an anatomical site of easier access.
There are not many studies in literature on the effects of genistein on oral cancer, except for some that studied its capacity to inhibit tyrosine kinases,Citation29 in combination with other isoflavones, such as quercetinCitation30,Citation31 and biochanin A.Citation32 Moreover, there are no studies that specifically investigate the effects of genistein on tongue squamous cell carcinoma.
For this reason, we evaluated in vitro anti-tumor action of genistein using 3 cell lines of OSCC; PE/CA-PJ15, PE/CA-PJ49 and HSC-3 cell lines.
Three concentrations of genistein have been used to assess adhesion, proliferation, migration, cell viability at 3 time points, and we calculated the IC50 values.
In addition, we studied the expression of OCT4 and survivin, 2 important proteins responsible for promoting tumorigenesis, after treatment with 20, 50 and 100 µM of genistein at 24, 48 and 72 hours of treatment.
Materials and methods
Cell culture and treatment
PE/CA-PJ15, PE/CA-PJ49, HSC-3 are cell lines of tongue carcinoma (European Collection of Cell Cultures, ECACC) maintained at standard conditions of temperature and atmosphere (37°C and 5% CO2, respectively) for all tests used. We have used DMEM culture medium with 4,500 mg/L glucose for PE/CA-PJ15 and PE/CA-PJ49 cells and Roswell Park Memorial Institute 1640 medium (RPMI 1640) for HSC-3 cells (Life Technologies, Gibco, Grand Island, NY, USA). Both culture media were supplemented with 10% fetal bovine serum, L-glutamine (2 mM) and penicillin–streptomycin (100 U/mL) (Sigma Aldrich, Saint Louis, MO, USA). Genistein (Abcam, Cambridge, UK) was dissolved in dimethylsulfoxide (DMSO) at a stock concentration of 5 mM and serial dilutions of 20, 50 and 100 µM of genistein were prepared. The cell lines were treated for 24, 48 and 72 hours.
xCELLigence real-time cell analyzers
PE/CA-PJ15, PE/CA-PJ49, HSC-3 cells were resuspended in culture medium and then seeded, in triplicate, inside an E-plate of xCELLigence RTCA DP system (ACEA Biosciences Inc., San Diego, CA, USA). Genistein at concentrations of 20, 50 and 100 µM were added to cells in adhesion phase and the cells were monitored for 24, 48 and 72 hours. To evaluate its effects on cell adhesion, genistein was added to cells after their adhesion phase. Control cells were treated with equivalent volume of the vehicle of genistein; DMSO. The concentrations used were the same as indicated previously. Moreover, the same concentrations of genistein were added in the proliferation phase to evaluate the effects of this nutraceutical on cellular proliferation. xCELLigence system measured the impedances parameter called “Cell Index (CI)” and hence we calculated also IC50 values at 24, 48 and 72 hours. The measurement of the IC50 values was performed by the instrument as described in the literature, with a high reliability.Citation33,Citation34 They were expressed as the mean (M) ± standard error of the mean (SEM) (n=3).
MTT assay and Trypan blue exclusion test
Vybrant® MTT Cell Proliferation Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to evaluate cell viability of PE/CA-PJ15, PE/CA-PJ49, and HSC-3 cells after treatments with genistein. We seeded 5×104 cells in a total volume of 250 µL/well in a 96-well plate with 20, 50 and 100 µM of genistein for 24, 48 and 72 hours. Then, 100 µL of fresh culture medium and 10 µL of 12 mM MTT stock solution were added to each well after we had incubated the cells for 4 hours at 37°C. To each well, SDS-HCl solution (10 mL of 0.01 M HCl to 1 gm of SDS) was added for an incubation period of 12 hours in a humidified chamber at 37°C. The absorbance was read at 570 nm using the Multiskan™ GO Microplate Spectrophotometer (Thermo Fisher Scientific).
While PE/CA-PJ15, PE/CA-PJ49, HSC-3 cells were treated with genistein at the same concentrations and time points, Trypan blue exclusion method was used to validate the cell viability results obtained with MTT assay. A suspension of each cell line was mixed with 0.4% Trypan blue solution and, after 10 minutes, cells were counted automatically with JuLI™ FL (NanoEntek, Pleasanton, CA, USA).
Scratch assay
A monolayer of each cell line was scraped with a p200 pipette tip and then washed twice with Dulbecco’s phosphate buffered saline 1X (Life Technologies, Gibco) in order to remove debris. Then, cells were treated with the IC50 dose of genistein at 24 hours. Untreated cells were used as control. Initially, we acquired the first image (T0) and then the subsequent ones after 1 hour (T1), 2 hours (T2), 3 hours (T3), 5 hours (T4), 6 hours (T5) and 24 hours (T6) after treatment. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to calculate the size of wound and analyze all the acquired images. GraphPad Prism 7 (GraphPad Software, Inc., CA, USA) software was used for statistical evaluation.
Western blotting analysis
After treatments with genistein at the same concentrations and time points previously used, cells were lysated to obtain proteins. They were measured and, then, separated by 15% SDS polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Bovine serum albumin (BSA) 5% was used for 1 hour as blocking solution.
Membranes were incubated with vitronectin (1:150; BD Biosciences), OCT4 (1:700; Novus Biologicals, Littleton, CO, USA), survivin (1:1,000; Cell Signaling Technology Inc., Danvers, MA, USA), and β-actin (1:5,000; Sigma Aldrich), overnight at 4°C.
Then, peroxidase-conjugated secondary antibody was used (1:2,500; Santa Cruz Biotechnology, Dallas, TX, USA). Signals were acquired with enhanced chemiluminescence kit (ClarityTM Western ECL Substrate, Bio-Rad). UVP ChemiDoc-It®TS2 Imaging System (Analytik Jena AG, Jena, Germany) was used.
Statistical analysis
GraphPad Prism 7 software was used for Student’s t-test and one-way analysis of variance (ANOVA) and all data were expressed as the M ± SEM;Citation17 a P-values <0.05, P<0.01, P<0.001 were accepted as statistically significant.
Results
Effects of genistein on cell adhesion
PE/CA-PJ15, PE/CA-PJ49 and HSC-3 cell lines were treated with 20, 50 and 100 µM of genistein during cell adhesion phase. Through xCELLigence system, we monitored adhesion kinetics in real time of the treated cells, using untreated cells as control. After 24, 48 and 72 hours, the CI values were taken and adhesion curves were analyzed. Data obtained show clear effects of genistein on all cells used even at 24 hours after the treatment.
The CI values showed that the adhesion of HSC-3 treated with 20 µM of genistein appears to have reduced by 50% compared with the same untreated cells (). This reduction is evident at all time points considered, while, the values of CI of HSC-3 treated with 100 µM of genistein after 48 and 72 hours are negative ().
Figure 1 Genistein inhibited adhesion of tongue cancer cells.
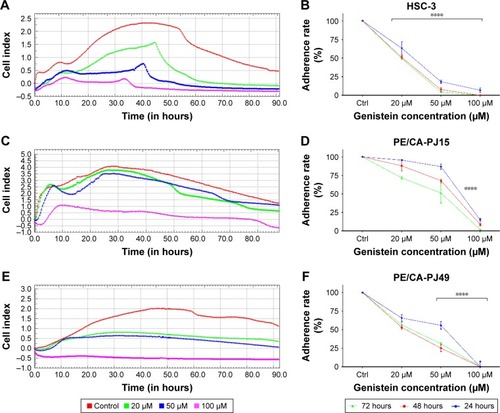
PE/CA-PJ15 cell adhesion was reduced by ~50%, following treatment with concentrations of genistein ranging between 50 and 100 µM, 24 and 48 hours after the treatment. However, for longer treatments, such as 72 hours, even 50 µM of genistein caused a halving of cell adhesion, compared with the untreated control ().
Cell adhesion of the PE/CA-PJ49 showed a reduction of about 50% when treated with an intermediate concentration between 20 and 50 µM of genistein, at all time points (). Furthermore, the values of CI of PE/CA-PJ49 treated with 100 µM of genistein were negative ().
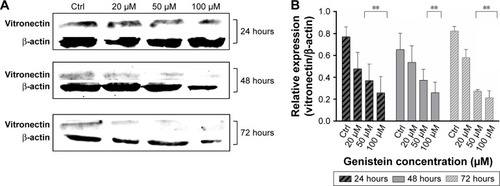
To evaluate the effects of genistein on cell adhesion of tongue carcinoma cells, we also studied the expression levels of the vitronectin protein. It is a glycoprotein found mainly in the extracellular matrix and promotes cell adhesion and spreading. From the Western blotting performed on protein lysates of the 3 cell types used, we noticed that the expression of vitronectin seems to have decreased as a result of treatments with increasing concentrations of genistein, especially those long-lasting. In fact, at 48 hours after treatment with 100 µM of genistein, vitronectin seems to be little expressed and almost unexpressed for treatments with 50 and 100 µM of genistein at 72 hours ().
Figure 2 Inhibition of vitronectin expression.
Abbreviation: Ctrl, control.
Variation on cell proliferation and IC50 determination
Genistein was added to all cancer cell lines in the cell proliferation phase. All cells were monitored in real time and all cell index values were taken at 24, 48 and 72 hours. In addition, they were converted into percentage values and we used the ANOVA as the statistical test to evaluate the cell proliferation percentage.
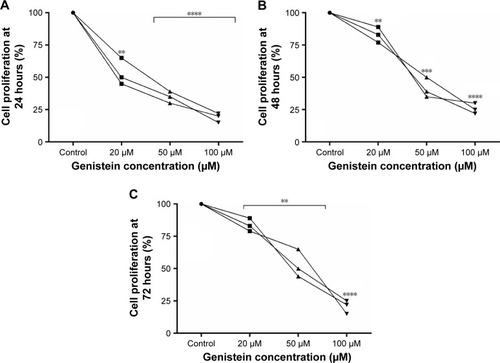
We found a 50% significant reduction of cell proliferation for all cells treated with 20 µM of genistein after 24 hours of treatment (); while, the same reduction was found at 48 hours in a concentration range between 20 and 50 µM of genistein (). Instead, cell proliferation seems to be reduced by 50% compared to the control at 72 hours in all cells treated with 50 µM of genistein (). In all cell lines, 100 µM of genistein resulted in a reduction of about 25% of proliferation at all time points considered. All the data were considered statistically significant for P<0.005, P<0.001, P<0.0001 ().
Figure 3 Variation in cell proliferation rate (%).
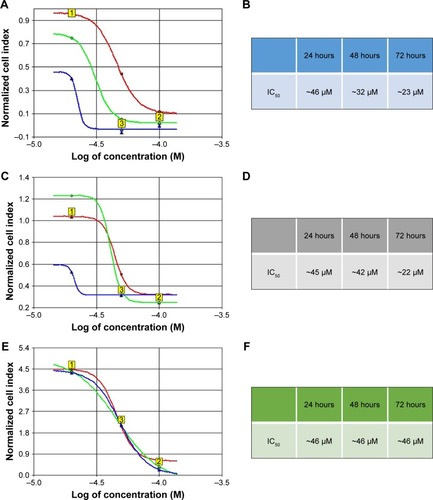
From the growth curves obtained by treating cells with 20, 50 and 100 µM of genistein, CI values were used to calculate the value of IC50 at 24, 48 and 72 hours. In particular, it was found that at 24 hours, the IC50 value was approximately 46 µM for all cell lines used (); while for treatments at 48 hours, the average IC50 value of the 3 cell lines used was approximately 40 µM (). However, there was a slight discrepancy between the lines for 72-hour treatments. In fact, for HSC-3 and PE/CA-PJ15 the IC50 value seemed to be around 22 µM (). Only for PE/CA-PJ49, this value was around 46 µM. This was probably because this line showed a higher resistance to treatment with genistein than the other ().
Figure 4 IC50 values.
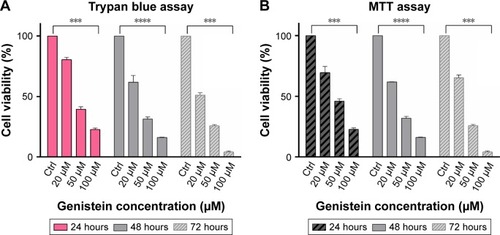
Effects of genistein on cell viability
For a better evaluation of genistein on cell viability, we used 2 simple tests: the MTT assay and the Trypan blue assay. In both assays, we noticed that genistein changed cell viability even 24 hours after treatment.
In fact, we noticed a reduction of about 50% of cell viability in each treated cell line with concentrations between 20 and 50 µM at 24, 48 and 72 hours (). Trypan blue assay confirmed almost all the data obtained with the MTT assay (). All the data were considered statistically significant for P<0.001 and P<0.0001.
Figure 5 Genistein reduces the viability of tongue cancer cells.
Abbreviation: Ctrl, control.
Determination of cell migration rate
To evaluate if genistein is able to reduce the migration of the tongue cancer cells, we used the Scratch assay. Tongue cells were treated with IC50 at 24 hours, previously calculated. The control had not undergone any treatment.
The treated cells migrated very little in the cut area (). The most significant effects were evident starting from T4 and, specifically, the wound of treated cells does not show any healing after 24 hours ().
Figure 6 Effects of genistein on cell migration.
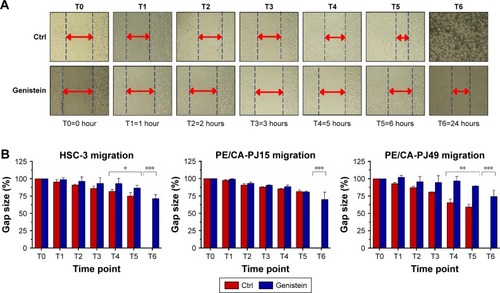
The untreated control showed an increasing level of healing of the wound during the considered times total healing after 24 hours ().
Evaluations of the timing at T4, T5 and T6 were most significant with P-value <0.05, P<0.01, P<0.001 ().
Effects of genistein on tumorigenesis
After treating cancer cells of the tongue with different concentrations of genistein, we evaluated the expression of OCT4 and survivin. OCT4 is a known protein because its overexpression promotes tumorigenesis in different types of cells. In tongue cancer cells, genistein appears to have an action on the expression of OCT4 only after high concentration treatments.
In fact, shows a reduction in those cells that were treated with 50 and 100 µM of genistein for each time point considered.
Figure 7 Genistein reduces tumorigenesis and promotes apoptosis.
Abbreviation: Ctrl, control.
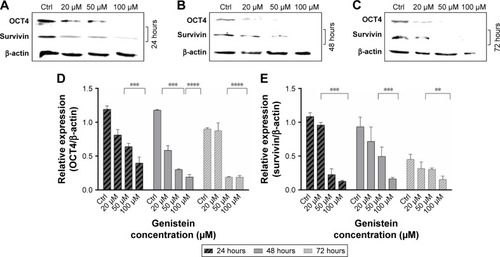
Moreover, expression of survivin appears to be extremely reduced as a result of treatments with concentrations of 50 and 100 µM at 24 hours (). This seems to be confirmed at all time points considered, and especially at a concentration of 100 µM, there is almost a complete inhibition ().
Discussion
Modern treatment protocols of OSCC did not lead to great results despite adoption of new surgical techniques and innovative chemotherapy formulations.
In recent years, the scientific community has been fascinated by the remarkable properties that natural substances possess, especially by many features that make them anti-cancer substances.
Genistein is an isoflavone isolated in 1899 from dyeing of genistra, characterized in 1926 and synthesized for the first time in 1928. It is well known to be present mainly in soybeans. It has a structure similar to estrogen and this characteristic seems to play an important role to help improve menopause symptoms.Citation35
Notable are also its anti-tumor effects, demonstrated especially in breast cancerCitation20,Citation36–Citation38 and prostate cancer.Citation39–Citation41
Even for OSCC, there are studies on genistein action, but they investigate the inhibitory activity of the protein tyrosine kinase,Citation29 its action on tumor angiogenesisCitation42 and its chemopreventive effects when used with quercetinCitation30,Citation31 and biochanin A,Citation32 which are other isoflavones.
Park et al have carried out an in vitro study on oral cancer cells, HSC-3 and KB, validating their results on nude mice. They demonstrated that genistein and cetuximab have a synergistic action in the inhibition of EGFR signaling pathway.Citation43
In literature, there are no papers that demonstrate the effects of genistein, as the only treatment against tongue carcinoma, which is considered the most common oral cancer and the most aggressive of all oral cancers.
Therefore, we studied the effects of this isoflavone on 3 lines of squamous cell carcinoma of the tongue; HSC 3, PE/CA-PJ15 and PE/CA-PJ49 cell lines.
We treated the cells with 3 concentrations of genistein; 20, 50 and 100 µM. The choice of concentrations was made by studying the literature data and, in particular, inspired by the work of Johnson et al. Even when the genistein was used with the biochanin A, the results on oral carcinoma cells were good. All in vitro assessments were made considering 3 time points; 24, 48 and 72 hours.
In this paper, our goal was to show how genistein inhibits adhesion, proliferation, viability and migration of cancer cells of the tongue.
It is known that alterations in cell adhesion affect the ability of cells to move. In particular, cancer cells need to be able to move and migrate in order to spread, and cell adhesion plays an important role in regulating cell movements and the spread of metastases.Citation44
Our data show that cell adhesion is decreased by almost 50% at concentrations between 20 and 50 µM, at 24 hours, in almost all cell types used compared with untreated cells. This decrease was confirmed at other time points within the range of the concentrations used.
This showed that the adhesion of the tongue cancer cells is greatly compromised by treatment with genistein.
Kingsley et al have shown that the adhesion of cells of OSCC (CAL 27 and SCC25 cell lines) was clearly decreased when cells were treated with soy protein extractCitation45 belonging to different isoflavones, including genistein.Citation46
In addition, to confirm the strong action of genistein on adhesion, we also evaluated the expression of vitronectin, a well known adhesion protein. Western blotting analysis demonstrates that the inhibition of the vitronectin after treatment with genistein is dose-dependent and is also down-regulated by treatment at 48 and 72 hours with high concentrations (50–100 µM).
Inhibitory effects of genistein are also confirmed by Skogseth et al. They demonstrate that genistein is able to reduce the adhesion of prostate cancer cells because it inhibits many adhesion proteins. This is probably because it is a strong inhibitor of tyrosine kinases.Citation47
Even for Haier et al, genistein has strong cell adhesion inhibition capacity in colon carcinoma cells. Indeed, it also seems to inhibit the expression of vitronectin in HT-29 cell lines.Citation48
In our study, we also evaluated the effects of genistein on cell proliferation. Also, in this case, we obtained a strong reduction of proliferation with high significance (P<0.0001) for all tongue cancer cells treated with concentrations between 20 and 50 µM at all time points.
Davis et al showed that genistein also has anti-proliferative action on prostate tumor cells.Citation49 Alhasan et al confirmed that genistein has a strong anti-proliferative effect in OSCC HN4 cell line treated with 50 µM of genistein, even favoring an arrest of the cell cycle and promotion of apoptosis.Citation50
In addition, we used the CI values obtained with the xCELLigence system to calculate the IC50 values of genistein at 24, 48 and 72 hours.
In all cell lines, on average, we found IC50 values of approximately 46 µM at 24 hours and approximately 40 µM at 48 hours. However, we obtained discordant IC50 values at 72 hours in each cell line used. This was probably because PE/CA-PJ49 cells are more aggressive than the other 2 cell lines used.
Johnson et al affirm that the IC50 value for the SCC15 and SCC25 cell lines, OSCC cells, is ~50 µM.Citation32 The same IC50 value was attributed to the breast cancer cells.Citation51
In addition, we showed that tongue carcinoma cells’ viability decreased by 50% when treated with concentrations between 20 and 50 µM of genistein, almost at all time points.
Alhasan et al have reported a significant reduction of the vitality and growth of OSCC cells after treatment with genistein.Citation50 Ye et al also argued that genistein inhibits the viability and proliferation of cells of head and neck cancer by also inducing apoptosis.Citation52
In our opinion, one of the most significant results, among those obtained, relates to cell migration. In fact, the treatment of all cells with the IC50 value of genistein at 24 hours showed a very significant reduction of cell migration after 24 hours; P<0.001. This shows that genistein can inhibit tongue carcinoma cells migration and so it can have a big effect on the inhibition of the metastasis processes.
This power of genistein seems to be confirmed for ovarian cancerCitation53 and OSCC.Citation42
Finally, we have shown that genistein is able to strongly inhibit, with treatment >50 µM, the expression of OCT4 and survivin in tongue cancer cells, thus reducing the tumorigenesis enough to be considered a good agent against this kind of tumor.
This inhibitory action of OCT4 expression seems to be also confirmed in embryonal carcinoma,Citation54 while Tian et al have shown that genistein down-regulates the expression of survivin in H446 small-cell lung cancer cells.Citation55
Conclusion
Genistein is a natural isoflavone whose effects have been studied fully in many neoplastic diseases.
However, its anti-tumor properties were evaluated, especially in combination with other isoflavones, against OSCC and there are no data in literature that make clear its effect, in particular on tongue carcinoma. There are several studies on the use of genistein for other cancers, very few on oral cancer and almost no specific study on tongue squamous cell carcinoma.
With this in vitro study, we can confirm that genistein acts on the tongue cancer cells by inhibiting efficaciously cell viability, proliferation, adhesion and migration.
This shows that genistein has excellent anti-cancer properties that can be explored for this type of tumor.
However, in vivo studies are needed to further validate these results and develop a suitable delivery system that acts directly in situ to overcome the problems concerning the bioavailability.
Author contributions
Dr FA designed the paper, collected data, drafted the manuscript, drew the figures and approved the final manuscript as submitted. Dr GT, Dr AC and Dr DP did statistical analysis with the data collected. Dr MRP drafted the initial manuscript with Dr FA and approved the final manuscript as submitted. Prof LLM coordinated and supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- LauALiKYYangWFSuYXInduction chemotherapy for squamous cell carcinomas of the oral cavity: a cumulative meta-analysisOral Oncol20166110411427688112
- AdkinsDLeyJMichelLnab-Paclitaxel, cisplatin, and 5-fluorouracil followed by concurrent cisplatin and radiation for head and neck squamous cell carcinomaOral Oncol2016611727688097
- SahuNGrandisJRNew advances in molecular approaches to head and neck squamous cell carcinomaAnticancer Drugs201122765666421178766
- RussoMRussoGLDagliaMUnderstanding genistein in cancer: the “good” and the “bad” effects: a reviewFood Chem201619658960026593532
- BanerjeeSLiYWangZSarkarFHMulti-targeted therapy of cancer by genisteinCancer Lett2008269222624218492603
- SpinozziFPagliacciMCMiglioratiGThe natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cellsLeuk Res19941864314398207961
- YamasakiMMineYNishimuraMGenistein induces apoptotic cell death associated with inhibition of the NF-kappaB pathway in adult T-cell leukemia cellsCell Biol Int201337774274723526666
- RohTKimSWMoonSHNamMJGenistein induces apoptosis by down-regulating thioredoxin-1 in human hepatocellular carcinoma SNU-449 cellsFood Chem Toxicol20169712713427597132
- YangYZangAJiaYGenistein inhibits A549 human lung cancer cell proliferation via miR-27a and MET signalingOncol Lett20161232189219327602162
- YangYMYangYDaiWWLiXMMaJQTangLPGenistein-induced apoptosis is mediated by endoplasmic reticulum stress in cervical cancer cellsEur Rev Med Pharmacol Sci201620153292329627467006
- AhnJCBiswasRChungPSCombination with genistein enhances the efficacy of photodynamic therapy against human anaplastic thyroid cancer cellsLasers Surg Med2012441084084923143780
- LazarevicBBoezelijnGDiepLMEfficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: a randomized, placebo-controlled, double-blind Phase 2 clinical trialNutr Cancer201163688989821714686
- AtefehZVahidCHasanNSaeedAMahnazHCombination treatment of glioblastoma by low-dose radiation and genisteinCurr Radiopharm20169325826327527071
- KhawAKYongJWKalthurGHandeMGenistein induces growth arrest and suppresses telomerase activity in brain tumor cellsGenes Chromosomes Cancer2012511096197422736505
- SuzukiRKangYLiXRoifeDZhangRFlemingJBGenistein potentiates the antitumor effect of 5-Fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cellsAnticancer Res20143494685469225202045
- KimSHKimCWJeonSYGoREHwangKAChoiKCChemopreventive and chemotherapeutic effects of genistein, a soy isoflavone, upon cancer development and progression in preclinical animal modelsLab Anim Res201430414315025628724
- Van CutsemEFindlayMOsterwalderBCapecitabine, an oral fluoropyrimidine carbamate with substantial activity in advanced colorectal cancer: results of a randomized phase II studyJ Clin Oncol20001861337134510715306
- Nadal-SerranoMPonsDGSastre-SerraJBlanquer-Rosselló MdelMRocaPOliverJGenistein modulates oxidative stress in breast cancer cell lines according to ERalpha/ERbeta ratio: effects on mitochondrial functionality, sirtuins, uncoupling protein 2 and antioxidant enzymesInt J Biochem Cell Biol20134592045205123871935
- ValachovicovaTSlivovaVBergmanHShuherkJSlivaDSoy isoflavones suppress invasiveness of breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent and -independent pathwaysInt J Oncol20042551389139515492830
- ZhaoQZhaoMParrisABXingYYangXGenistein targets the cancerous inhibitor of PP2A to induce growth inhibition and apoptosis in breast cancer cellsInt J Oncol20164931203121027574003
- MazurWPhytoestrogen content in foodsBaillieres Clin Endocrinol Metab199812472974210384822
- CassidyAPotential risks and benefits of phytoestrogen-rich dietsInt J Vitam Nutr Res200373212012612747219
- SetchellKDBrownNMDesaiPBioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplementsJ Nutr20011314 Suppl1362S1375S11285356
- ManachCScalbertAMorandCRémésyCJiménezLPolyphe-nols: food sources and bioavailabilityAm J Clin Nutr200479572774715113710
- WalleTAbsorption and metabolism of flavonoidsFree Radic Biol Med200436782983715019968
- ScalbertAWilliamsonGDietary intake and bioavailability of polyphenolsJ Nutr20001308S Suppl2073S2085S10917926
- ShehataEMElnaggarYSGalalSAbdallahOYSelf-emulsifying phospholipid pre-concentrates (SEPPs) for improved oral delivery of the anti-cancer genistein: development, appraisal and ex-vivo intestinal permeationInt J Pharm2016511274575627492016
- GavinAPhamJTWangDBrownlowBElbayoumiTALayered nanoemulsions as mucoadhesive buccal systems for controlled delivery of oral cancer therapeuticsInt J Nanomedicine2015101569158425759580
- SugiuraTShirasunaKHayashidoYSakaiTMatsuyaTEffects of human fibroblasts on invasiveness of oral cancer cells in vitro: isolation of a chemotactic factor from human fibroblastsInt J Cancer19966867747818980183
- BrowningAMWalleUKWalleTFlavonoid glycosides inhibit oral cancer cell proliferation – role of cellular uptake and hydrolysis to the aglyconesJ Pharm Pharmacol20055781037104216102260
- WalleTBrowningAMSteedLLReedSGWalleUKFlavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humansJ Nutr20051351485215623831
- JohnsonTLLaiMBLaiJCBhushanAInhibition of cell proliferation and MAP kinase and Akt pathways in oral squamous cell carcinoma by genistein and biochanin AEvid Based Complement Alternat Med20107335135818955325
- PanTKhareSAckahFIn vitro cytotoxicity assessment based on KC(50) with real-time cell analyzer (RTCA) assayComput Biol Chem20134711312024055763
- ZhangMAguileraDDasCMeasuring cytotoxicity: a new perspective on LC50Anticancer Res2007271A353817352213
- MariniHMinutoliLPolitoFEffects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trialAnn Intern Med20071461283984717577003
- AvciCBSusluerSYCaglarHOGenistein-induced mir-23b expression inhibits the growth of breast cancer cellsContemp Oncol (Pozn)2015191323526199568
- ChenJDuanYZhangXYeYGeBChenJGenistein induces apoptosis by the inactivation of the IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cellsFood Funct201563995100025675448
- OrlandoLSchiavonePCinieriSGenistein: the future of prevention and treatment of breast cancer?Cancer Biol Ther2011111091892021455031
- PaveseJMKrishnaSNBerganRCGenistein inhibits human prostate cancer cell detachment, invasion, and metastasisAm J Clin Nutr2014100Suppl 1431S436S24871471
- ChiyomaruTYamamuraSFukuharaSGenistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIRPLoS One201388e7037223936419
- ChiyomaruTYamamuraSZamanMSGenistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151PLoS One201278e4381222928040
- MyoungHHongSPYunPYLeeJHKimMJAnti-cancer effect of genistein in oral squamous cell carcinoma with respect to angiogenesis and in vitro invasionCancer Sci200394221522012708500
- ParkSJKimMJKimYKKimSMParkJYMyoungHCombined cetuximab and genistein treatment shows additive anti-cancer effect on oral squamous cell carcinomaCancer Lett20102921546319959278
- KhaliliAAAhmadMRA review of cell adhesion studies for biomedical and biological applicationsInt J Mol Sci2015168181491818426251901
- VainshteinJMSamuelsSTaoYImpact of xerostomia on dysphagia after chemotherapy-intensity-modulated radiotherapy for oropharyngeal cancer: prospective longitudinal studyHead Neck201638Suppl 1E1605E161226605872
- KingsleyKTruongKLowESoy protein extract (SPE) exhibits differential in vitro cell proliferation effects in oral cancer and normal cell linesJ Diet Suppl20118216918822432688
- SkogsethHHoltRULarssonEHalgunsetJTyrosine kinase inhibitors alter adhesivity of prostatic cancer cells to extracellular matrix componentsAPMIS2006114322523316643189
- HaierJNasrallaMNicolsonGLInfluence of phosphotyrosine kinase inhibitors on adhesive properties of highly and poorly metastatic HT-29 colon carcinoma cells to collagenInt J Colorectal Dis199914211912710367258
- DavisJNKucukOSarkarFHExpression of prostate-specific antigen is transcriptionally regulated by genistein in prostate cancer cellsMol Carcinog20023429110112112315
- AlhasanSAEnsleyJFSarkarFHGenistein induced molecular changes in a squamous cell carcinoma of the head and neck cell lineInt J Oncol200016233333810639578
- ScherbakovAMAndreevaOEApigenin inhibits growth of breast cancer cells: the role of ERalpha and HER2/neuActa Naturae20157313313926483970
- YeFWuJDunnTYiJTongXZhangDInhibition of cyclooxygenase-2 activity in head and neck cancer cells by genisteinCancer Lett20042111394615194215
- KimYSChoiKCHwangKAGenistein suppressed epithelial-mesenchymal transition and migration efficacies of BG-1 ovarian cancer cells activated by estrogenic chemicals via estrogen receptor pathway and downregulation of TGF-beta signaling pathwayPhytomedicine2015221199399926407941
- RegenbrechtCRJungMLehrachHAdjayeJThe molecular basis of genistein-induced mitotic arrest and exit of self-renewal in embryonal carcinoma and primary cancer cell linesBMC Med Genomics200814918847459
- TianTLiJLiBGenistein exhibits anti-cancer effects via down-regulating FoxM1 in H446 small-cell lung cancer cellsTumour Biol20143554137414524379139
