Abstract
Objective
Our objective was to examine whether adding induction chemotherapy to concurrent chemoradiotherapy improved survival in stage III nasopharyngeal carcinoma (NPC) patients, especially in low-risk patients at stage T3N0-1.
Materials and methods
We retrospectively analyzed 687 patients with stage T3N0-1 NPC treated with intensity-modulated radiation therapy (IMRT) plus concurrent chemotherapy (CC) with or without induction chemotherapy (IC). Propensity score matching (PSM) method was used to select 237 pairs of patients from two cohorts. Overall survival (OS), locoregional relapse-free survival (LRFS), distant metastasis-free survival (DMFS), and progression-free survival (PFS) were assessed by using the Kaplan–Meier method, log-rank test, and Cox regression analysis.
Results
No significant survival differences were observed between IC plus CC and CC cohorts with similar 4-year OS (91.7% vs 92.6%, P=0.794), LRFS, (92.7% vs 96.8%, P=0.138), DMFS (93.5% vs 94.3%, P=0.582), and PFS (87.5% vs 91.1%, P=0.223). In a univariate analysis, lower Epstein–Barr virus deoxyribonucleic acid (EBV DNA; <4,000 copies/mL) significantly improved 4-year DMFS (95.5% vs 91.6%, P=0.044) compared with higher EBV DNA (≥4,000 copies/mL). No factors were associated with 4-year OS, LRFS, DMFS, and PFS in a multivariate analysis. IC plus CC group experienced higher rates of grade 3–4 leucopenia (P<0.001) and neutropenia (P<0.001).
Conclusion
The addition of IC to CC in stage T3N0-1 NPC patients treated with IMRT did not significantly improve their survival. The IC group experienced higher rates of grade 3–4 hematological toxicities. Therefore, further investigation is required.
Introduction
Nasopharyngeal carcinoma (NPC) occurs at a high incidence rate in Southern China, especially in Hong Kong and Guangdong.Citation1 NPC exhibits high radiosensitivity, and radiotherapy (RT) is the primary and most effective treatment for pathologically confirmed NPC. Intensity-modulated radiation therapy (IMRT) has significantly improved local control and lowered radiation-induced toxicities compared with two-dimensional RT.Citation2,Citation3 A series of phase III clinical trials have established chemoradiotherapy as the standard treatment for locoregionally advanced NPC (LA-NPC) because it improves disease control and survival.Citation4–Citation10
However, whether the addition of induction chemotherapy (IC) would improve survival in LA-NPC patients remains controversial. Differing results have been found in several trials.Citation11–Citation14 Hui et alCitation14 showed that overall survival (OS) improved by adding IC (94.1% vs 67.7%, P=0.012), whereas 3-year progression-free survival (PFS) did not significantly improve (P=0.12). Fountzilas et alCitation12 and Tan et alCitation11 reported no significant survival benefit in LA-NPC patients treated with IC. Sun et alCitation13 recently published a phase III study showing that treatment with IC in LA-NPC patients (except T3-4N0) significantly improved 3-year failure-free survival, OS, and distant failure-free survival. Our recent retrospective studyCitation15 showed that the addition of IC significantly improved 5-year OS (P=0.022) and 5-year distant metastasis-free survival (DMFS; P=0.018) in stage IVa-b NPC patients treated with IMRT.
IC may be associated with high incidences of hematological acute toxicity and could affect chemoradiotherapy. A previous study showed that IC exhibited grade 3 and 4 toxicities, including leukopenia (P=0.046), neutropenia (P=0.029), and thrombocytopenia (P<0.001).Citation11 Therefore, IC may not improve survival benefits but increase hematological acute toxicity in low-risk stage III NPC patients. In order to assess the benefit of IC, we retrospectively analyzed stage T3N0-1 NPC patients treated with IMRT plus concurrent chemotherapy (CC) with or without IC. The propensity score matching (PSM) method was used to mimic randomized trials to reduce potential bias.Citation16,Citation17
Materials and methods
Patients
A total of 687 patients with stage T3N0-1 NPC treated with IMRT plus CC with or without IC were retrospectively examined in our institution between January 19, 2005, and December 27, 2012. Before treatment, a complete history of each patient was noted. Clinical examinations of the head and neck, hematological studies and biochemical profiles, pretreatment plasma Epstein–Barr virus deoxyribonucleic acid (pre-EBV DNA) level, fiberoptic nasopharyngoscopy with biopsy, magnetic resonance imaging (MRI) of the nasopharynx and neck, chest radiography, abdominal sonography, and a whole body bone scan using single photon emission computed tomography (CT) or positron emission CT were performed for each patient. Plasma EBV DNA level was measured by using real-time quantitative polymerase chain reaction as previously described.Citation18,Citation19 All the patients were restaged according to the seventh edition of the International Union against Cancer/American Joint Committee on Cancer staging system for NPC.Citation20 This study was approved by the Institutional Review Board of Sun Yat-Sen University Cancer Center. The requirement for written consent was waived as this is a retrospective study; however, oral consent was obtained via telephone.
Treatment
Radiation therapy
All the patients were treated using one daily fraction of IMRT for 5 days per week at our institution. Gross tumor volume (GTV) included the nasopharynx GTV (GTVnx) and the cervical lymph nodes GTV (GTVnd). High-risk clinical target volume (CTV-1) was defined as the GTVnx plus a 5–10 mm margin (2–3 mm posteriorly) to encompass the high-risk area and the whole nasopharynx, and low-risk clinical target volume (CTV-2) was defined as the CTV-1 plus a 5–10 mm margin (2–3 mm posteriorly) to encompass the low-risk area including parapharyngeal space, posterior parts of the nasal cavity, retropharyngeal nodal regions, clivus, pterygoid fossae, sphenoid sinus, pterygopalatine fossae, and the selective neck area. The prescribed doses were as follows: 66–72 Gy to the GTVnx, 60–68 Gy to the GTVnd, ≥60 Gy to the (CTV-1), and 54–56 Gy to the CTV-2 for >30–33 fractions.
Chemotherapy
Platinum-based chemotherapy was given to all the patients. IC consisted of docetaxel plus cisplatin (TP), paclitaxel plus carboplatin (TC), docetaxel plus cisplatin plus fluorouracil (TPF), or cisplatin/nedaplatin plus fluorouracil (PF) regimen for up to three cycles. Patients received a CC regimen of cisplatin/nedaplatin of 30–40 mg/m2 weekly or every 3 weeks during RT. The three weekly regimens consisted of 80–100 mg/m2 of cisplatin/nedaplatin, PF, or TP for up to seven cycles.
Follow-up
Patient follow-up was calculated from the first day of therapy to either the day of death or the last day of examination. The patients underwent physical examination, plasma EBV DNA level, endoscopy, MRI scans, chest radiography, abdominal sonography, and whole body bone scan every 3–6 months during the first 3 years and every 6–12 months thereafter until death. OS, locoregional relapse-free survival (LRFS), DMFS, and PFS were analyzed as end points, and these end points were measured from the date of the first therapy to the date of death, first locoregional relapse, distant metastasis, disease progression (locoregional relapse or distant metastasis), or the date of the last follow-up visit. Patients not having recent examination records were followed-up via telephone calls.
Statistical analysis
SPSS version 22.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analyses. We used the PSM method to match the patients between the two groups (IC plus CC and CC) at the ratio of 1:1 based on propensity scores. The scores were computed by using logistic regression based on the following covariates: gender, age, World Health Organization (WHO) pathological types (types I + II and III), smoking history, NPC family history, pre-EBV DNA, chemotherapy strategy (IC plus CC and CC), and N category (ie, N0 and N1). The balance of the covariates between the two groups was examined by using independent-samples t-test (continuous variable), χ2 test, or Fisher’s exact test (categorical variable). A cutoff of 4,000 copies/mL of pre-EBV DNA was used to define low versus high levels because this threshold has previously been shown to be a good prognostic factor.Citation21 The χ2 test or Fisher’s exact test was used to compare the patient characteristics. Survival rates were estimated by using the Kaplan–Meier method, and differences were compared by using the log-rank test. Multivariate analyses were performed by using the Cox proportional hazards model to calculate hazard ratios (HRs) and 95% CIs and to identify significant independent prognostic factors. In the multivariate analyses, the following parameters were included in the model as covariates for each analysis: gender, age (≤50 vs >50 years), WHO pathological types (types I + II and III), smoking history, NPC family history, N category (N0 and N1), pre-EBV DNA (≤4,000 vs >4,000 copies/mL), and chemotherapy strategy (IC plus CC and CC). Two-tailed P-values <0.05 were considered statistically significant.
Results
Patient characteristics
Before PSM, we compared the characteristics of all the 687 patients between two groups. The analysis showed that age, pre-EBV DNA, and N category were not balanced between the two groups (P=0.031, P<0.001, and P<0.001, respectively). In order to balance the characteristics and reduce potential bias, we selected 237 patient pairs from 687 T3N0-1 NPC patients by using PSM method. In the PSM cohort, the median age of patients was 44 years (range =21–70 years), including 330 men and 144 women with a ratio of 2.29:1. No significant differences were found in gender, age, pathology type, smoking, NPC family history, pre-EBV DNA, and N category between the IC plus CC group and the CC group ().
Table 1 Characteristics of stage T3N0-1 NPC treatment with IC + CC or CC before and after propensity score matching
Patterns of treatment failure
The median follow-up time was 49.4 months (range =1.4–117.7 months). Up to the last day of follow-up, 54 patients experienced disease progression. Furthermore, 33 of 54 (61.1%) patients showed disease progression in the IC plus CC group and 21 of 54 (38.9%) patients in the CC group. In total, 22 of 54 (40.7%) patients developed local/regional recurrence alone. A total of 5 of 22 (22.7%) patients in the IC plus CC group and 1 of 22 (4.5%) patients in the CC group developed both local and regional recurrence. Furthermore, 4 of 22 (18.2%) patients in the IC plus CC group and 5 of 22 (22.7%) patients in the CC group developed local recurrence, and 6 of 22 (27.3%) patients in the IC plus CC group and 1 of 22 (4.5%) patients in the CC group developed regional recurrence. In addition, 29 of 54 (53.7%) patients experienced distant metastases alone, which included 16 of 29 (55.2%) patients treated with IC plus CC and 13 of 29 (44.8%) patients treated with CC (P=0.565). Moreover, only two patients treated with IC plus CC and one patient treated with CC developed both distant metastases and recurrence. shows the summary of treatment failure.
Table 2 Patterns of treatment failure in 237 patient pairs with stage T3N0-1 NPC
Prognostic value of IC
The 4-year OS, LRFS, DMFS, and PFS for the patient population were 92.1%, 94.7%, 93.9%, and 89.2%, respectively. shows the summary of univariate analysis of prognostic factors, including gender, age, pathological type, smoking, NPC family history, pre-EBV DNA, chemotherapy strategy, and N category. Among these factors, gender, age, pathological type, smoking, NPC family history, and N category were not significantly associated with 4-year OS, LRFS, DMFS, and PFS. Patients with lower pre-EBV DNA (<4,000 copies/mL) demonstrated a significant improvement in 4-year DMFS (95.5% vs 91.6%, P=0.044) compared with those with higher EBV DNA (≥4,000 copies/mL). However, we did not detect a significant difference in pre-EBV DNA in 4-year OS, LRFS, and PFS rates. Patients treated with IC plus CC and CC alone resulted in similar 4-year OS (91.7% vs 92.6%, P=0.794; ), 4-year LRFS (92.7% vs 96.8%, P=0.138; ), 4-year DMFS (93.5% vs 94.3%, P=0.582; ), and 4-year PFS (87.5% vs 91.1%, P=0.223; ). Although there were no significant differences in survival improvement between the two groups, CC group was numerically superior to the IC plus CC group.
Figure 1 Kaplan–Meier survival curves for IC plus CC and CC alone in 237 patient pairs.
Abbreviations: CC, concurrent chemotherapy; IC, induction chemotherapy.
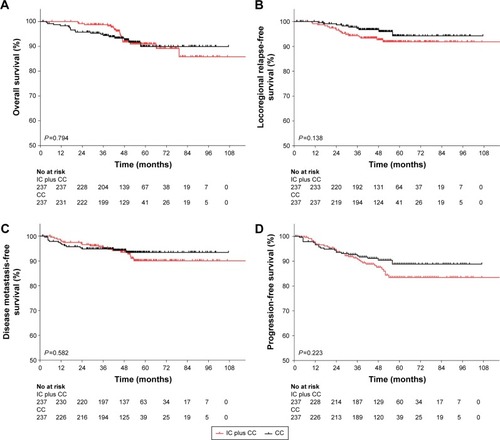
Table 3 Summary of prognostic factors in 237 patient pairs with stage T3N0-1 NPC by univariate analysis
Multivariate analysis was performed to adjust for various prognostic factors. The factors were included as covariates in accordance with univariate analysis (). Consistent with the univariate analysis, gender, age, pathological type, smoking, NPC family history, chemotherapy strategy, and N category were not associated with 4-year OS, LRFS, DMFS, and PFS. Only pre-EBV DNA showed an approximately significant difference in 4-year DMFS (HR =1.05; 95% CI =0.38–0.83; P=0.05).
Table 4 Summary of prognostic factors in 237 patient pairs with stage T3N0-1 NPC by multivariate analysis
Grade 3–4 hematological toxicities
In the IC plus CC treatment group, 82 patients experienced grade 3–4 leucopenia, and 97 patients experienced grade 3–4 neutropenia. IC plus CC treatment significantly increased the incidence of grade 3–4 leucopenia (P<0.001) and neutropenia (P<0.001) compared with CC treatment. The rates of grade 3–4 anemia and thrombocytopenia exhibited no significant difference in either treatment group ().
Table 5 Grade 3–4 hematological toxicities in 237 patient pairs with stage T3N0-1 NPC
Discussion
Previous phase II/III clinical trials showed conflicting outcomes when IC was added to concurrent chemoradiotherapy (CCRT) in LA-NPC patients.Citation11–Citation14 Tan et al’sCitation11 and Hui et al’sCitation14 studies included stage III–IVB NPC patients and Fountzilas et al’s studyCitation12 included stage IIB–IVB patients, but only Hui et al’s study significantly increased 3-year OS (94.1% vs 67.7%, P=0.012).Citation14 When stage T3-4N0 NPC patients were excluded to enhance the power for survival, the trial showed a significant improvement in 3-year PFS (P=0.034), OS (P=0.029), and DMFS (P=0.031) in LA-NPC patients.Citation13 Therefore, no published study exists which assesses the effect of IC in stage T3N0-1 patients, which could be defined as low-risk LA-NPC because of low T and N categories. Therefore, our retrospective study is the first to analyze IC plus CC treatment and CC treatment alone in stage T3N0-1 NPC patients treated with IMRT.
As it is a propensity-matched study, the groups were shown to be well matched for prognostic factors. Other than exploring conventional prognostic factors (ie, gender, age, WHO pathological type, chemotherapy strategy, and N category), we also considered some carcinogenic factors, including smoking history and family history of NPC. Our previous studyCitation22 revealed that pretreatment of cigarette smoking was a negative prognostic factor of death, locoregional recurrence, distant metastasis, and disease progression. Guo et alCitation23 reported that the ever-smokers suffered a higher risk of locoregional disease recurrence compared with never-smokers in LA-NPC patients. Ouyang et alCitation24 published that patients who had a first-degree family history of NPC had higher rates of OS and DMFS than those without a family history. However, smoking history and family history of NPC were not associated with survival in both univariate analysis and adjusted multivariate analysis. Plasma EBV DNA has been proven a prognostic biomarker in several studies.Citation25–Citation27 Leung et alCitation28 demonstrated that high levels of EBV DNA pretreatment were associated with the incidence of distant metastasis. Moreover, pre-EBV DNA has been reported a prognostic factor using a cutoff of 4,000 copies/mL to define low versus high levels.Citation21 Our results showed an increased risk of distant metastasis in patients with high EBV DNA (≥4,000 copies/mL) compared with those with low EBV DNA (<4,000 copies/mL), whereas there was no significant difference in 4-year OS, LRFS, and PFS.
Chemoradiotherapy is the standard treatment for LA-NPC. Chemoradiotherapy has been shown to significantly improve patient survival in several phase III randomized clinical trials.Citation4–Citation10 For patients with bulky and/or extensive nodal disease (N2-3), there is higher potential for metastasis; CC is not adequate.Citation29,Citation30 Moreover, T4-classified tumors are considered to result in a poor prognosis.Citation31 Consequently, N2-3 and/or T4 patients can be defined as a high-risk group of LA-NPC; therefore, IC is needed to reduce metastasis and improve survival. Xu et alCitation32 demonstrated that CCRT achieved higher 3-year DMFS rates (94.9% vs 80.1%, P=0.03) in N0-1 LA-NPC patients, but not in N2-3 tumors. In the present study, we chose stage T3N0-1 NPC patients treated with IMRT as a low-risk group to assess the value of IC. Results showed that IC plus CC did not improve survival compared with CC. However, the results showed that CC group was numerically superior to the IC plus CC group in 4-year OS (92.6% vs 91.7%), 4-year LRFS (96.8% vs 92.7%), DMFS (94.3% vs 93.5%), and PFS (91.1% vs 87.5%). We considered that IC increased hematological acute toxicities and reduced the tolerance of CC, which resulted in poor treatment intensity.
A previous trial showed that IC caused higher rates of grade 3 and 4 hematological toxicities, including leukopenia (52% vs 37%, P=0.046), neutropenia (24% vs 12%, P=0.029), and thrombocytopenia (14% vs 0%, P<0.01) compared with the chemoradiotherapy group in LA-NPC patients.Citation11 Our study showed that IC did not improve survival benefits and increased hematological acute toxicities. The IC group experienced a significantly higher incidence of grade 3–4 leucopenia (34.6% vs 16%, P<0.001) and neutropenia (40.9% vs 5.9%, P<0.001). These hematological toxicities may compromise the delivery of subsequent CC with dose reduction.
The present study had several limitations. First, our study is a single institutional retrospective study in an endemic area; therefore, a selection bias existed. Second, although all the patients in the cohort received platinum-based chemotherapy, the regimen and cycles of IC and CC were in disunity. IC included TP, TC, TPF, or PF regimens, whereas CC regimens consisted of cisplatin/nedaplatin, PF, or TP. Furthermore, there was no IMRT dose-fractionation consensus in NPC treatment. Third, only acute hematological toxicities were evaluated. Nonhematological mucositis, vomiting, and late toxicities were not acquired because of the long time interval of the cohort, and some information was missing. Fourth, besides plasma pre-EBV DNA,Citation33 pretreatment serum lactate dehydrogenase was reported to be associated with distant metastasis,Citation34,Citation35 but we did not include this factor into our analysis. Finally, we assessed only 4-year survival; however, the investigation should be 5 years or longer to evaluate the survival.
Conclusion
IC did not significantly improve survival in stage T3N0-1 NPC patients treated with CC. Furthermore, IC increased the incidence of grade 3–4 hematological toxicities, and it is not recommended for low-risk LA-NPC patients. Further confirmation is warranted in prospective studies.
Acknowledgments
We thank the Sun Yat-Sen University Cancer Center for their assistance with trial monitoring, data management, and statistical analysis. We appreciate the anonymous editors and reviewers who reviewed this manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
- WeeJTHaTCLoongSLQianCNIs nasopharyngeal cancer really a “Cantonese cancer”?Chin J Cancer201029551752620426903
- PengGWangTYangKYA prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinomaRadiother Oncol2012104328629322995588
- ZhangMXLiJShenGPIntensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-upEur J Cancer201551172587259526318726
- KwongDLShamJSAuGKConcurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial studyJ Clin Oncol200422132643265315226332
- WuXHuangPYPengPJLong-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinomaAnn Oncol20132482131213623661293
- ChanATLeungSFNganRKOverall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinomaJ Natl Cancer Inst200597753653915812080
- LinJCJanJSHsuCYLiangWMJiangRSWangWYPhase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survivalJ Clin Oncol200321463163712586799
- ChenYSunYLiangSBProgress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of ChinaCancer2013119122230223823576020
- LeeAWTungSYChuaDTRandomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinomaJ Natl Cancer Inst2010102151188119820634482
- WeeJTanEHTaiBCRandomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic varietyJ Clin Oncol200523276730673816170180
- TanTLimWTFongKWConcurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinomaInt J Radiat Oncol Biol Phys201591595296025832687
- FountzilasGCiuleanuEBobosMInduction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluationAnn Oncol201223242743521525406
- SunYLiWFChenNYInduction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trialLancet Oncol201617111509152027686945
- HuiEPMaBBLeungSFRandomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinomaJ Clin Oncol200927224224919064973
- LanXWZouXBXiaoYRetrospective analysis of the survival benefit of induction chemotherapy in stage IVa-b nasopharyngeal carcinomaPLoS One2016118e160758
- D’AgostinoRJPropensity score methods for bias reduction in the comparison of a treatment to a non-randomized control groupStat Med19981719226522819802183
- AustinPCThe use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experimentsStat Med20143371242125824122911
- ShaoJYZhangYLiYHComparison of Epstein-Barr virus DNA level in plasma, peripheral blood cell and tumor tissue in nasopharyngeal carcinomaAnticancer Res20042464059406615736452
- LoYMChanLYLoKWQuantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinomaCancer Res19995961188119110096545
- EdgeSBComptonCCThe American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNMAnn Surg Oncol20101761471147420180029
- LeungSFZeeBMaBBPlasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinomaJ Clin Oncol200624345414541817135642
- OuyangPYSuZMaoYPPrognostic impact of cigarette smoking on the survival of patients with established nasopharyngeal carcinomaCancer Epidemiol Biomarkers Prev201322122285229424252872
- GuoSSHuangPYChenQYThe impact of smoking on the clinical outcome of locoregionally advanced nasopharyngeal carcinoma after chemoradiotherapyRadiat Oncol2014924625424191
- OuyangPYSuZMaoYPLiangXXLiuQXieFYPrognostic impact of family history in southern Chinese patients with undifferentiated nasopharyngeal carcinomaBr J Cancer2013109378879423807164
- AnXWangFHDingPRPlasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapyCancer2011117163750375721319149
- PengHGuoRChenLPrognostic impact of plasma Epstein-Barr virus DNA in patients with nasopharyngeal carcinoma treated using intensity-modulated radiation therapySci Rep201662200026924234
- LinJCWangWYChenKYQuantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinomaN Engl J Med2004350242461247015190138
- LeungSFChanATZeeBPretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated typeCancer200398228829112872347
- LinJCLiangWMJanJSJiangRSLinACAnother way to estimate outcome of advanced nasopharyngeal carcinoma–is concurrent chemoradiotherapy adequate?Int J Radiat Oncol Biol Phys200460115616415337551
- LeeAWLauWHTungSYPreliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study GroupJ Clin Oncol200523286966697516192584
- ChenLLiuLZChenMPrognostic value of subclassification using MRI in the t4 classification nasopharyngeal carcinoma intensity-modulated radiotherapy treatmentInt J Radiat Oncol Biol Phys201284119620222300569
- XuTZhuGHeXYingHHuCA phase III randomized study comparing neoadjuvant chemotherapy with concurrent chemotherapy combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma: updated long-term survival outcomesOral Oncol2014502717624315404
- PengHChenLZhangYSurvival analysis of patients with advanced-stage nasopharyngeal carcinoma according to the Epstein-Barr virus statusOncotarget2016717242082421627008701
- WanXBWeiLLiHHigh pretreatment serum lactate dehydrogenase level correlates with disease relapse and predicts an inferior outcome in locally advanced nasopharyngeal carcinomaEur J Cancer201349102356236423541571
- ZhouGQRenXYMaoYPPrognostic implications of dynamic serum lactate dehydrogenase assessments in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapySci Rep201662232626928265
