Abstract
Recombinant immunotoxins (RITs) are proteins that contain a toxin fused to an antibody or small molecules and are constructed by the genetic engineering technique. RITs can bind to and be internalized by cells and kill cancerous or non-cancerous cells by inhibiting protein synthesis. A wide variety of RITs have been tested against different cancers in cell culture, xenograft models, and human patients during the past several decades. RITs have shown activity in therapy of several kinds of cancers, but different levels of side effects, mainly related to vascular leak syndrome, were also observed in the treated patients. High immunogenicity of RITs limited their long-term or repeat applications in clinical cases. Recent advances in the design of immunotoxins, such as humanization of antibody fragment, PEGylation, and modification of human B- and T-cell epitopes, are overcoming the above mentioned problems, which predict the use of these immunotoxins as a potential therapeutic method to treat cancer patients.
Introduction
Recombinant immunotoxins (RITs) are chimeric proteins for cancer therapy that contain a toxin fused to a targeting moiety. After the initial success of antibody therapy for cancer, monoclonal antibodies (Mabs) were used to link with the toxin molecules, which have higher specificity in targeting and higher potency in killing cancer cells. Thirty-five years ago, RITs were created by chemically conjugating a whole protein toxin to an Mab or a protein toxin devoid of its natural binding domain.Citation1,Citation2 Other immune proteins such as cytokines and growth factors have also been conjugated and genetically fused to toxins.Citation3 The traditional immunotoxins coupled the toxin and the targeting moiety by chemical method, but the cumbersome steps and the high cost encouraged the development of novel RITs. Nowadays, these RITs are generally synthesized by recombinant DNA techniques through constructing conditional expression plasmid and expressing interest protein in Escherichia coli rapidly and efficiently. RITs combine the cell-killing power of toxin and the specificity of antibody therapies. The careful design of both target moiety and toxin is the key of a successful therapy, because each type of cancer cell expresses different kinds of surface antigens. Compared with other commonly used immunotherapies, RITs’ cytocidal action is not dependent on antibody or complement-dependent cytotoxicity, but it is dependent on the specificity of the targeting moiety and the high activity of the toxin moiety, which will induce development of resistance. RITs directly target the surface of cancer cells, and 1 single internalized toxin molecule could kill the cell. Based on this, patients would not develop myelosuppression dose-limiting toxicity (DLT). Some RITs have been approved by the US Food and Drug Administration (FDA) for the treatment of cancer, such as denileukin diftitox (ONTAK®). This review considers RITs with different target moieties and toxins directed to cancer cells and focuses on those that have been tested clinically in the recent decades.
Mechanism of action of bacterial toxins
The target moiety facilitates the transfer and internalization of RITs into cancer cells. For this purpose, various bacterial toxins have been used in RITs targeting cancer cells, and among these toxins, Pseudomonas exotoxin (PE) and Diphtheria toxin (DT) are the most commonly used bacterial toxins. Nearly all toxins kill cancer cells by enzymatically inhibiting protein synthesis. Similarly, both PE and DT also inhibit adenosine diphosphate (ADP)-ribosylate elongation factor 2 (EF-2) in the cytosol,Citation4 which is a critical component of the protein synthesis machinery. Each toxin catalyzes the ADP-ribosylate of his-699 of EF-2, which is posttranslationally modified to a diphthamide residue.Citation5 Despite their similar action, PE and DT differ greatly in amino acid sequence, and PE’s binding domain is located near to its amino terminal, but DT is located on the opposite terminal.
PE is a single-chain protein, consisting of 613 amino acids in length, which is further composed of 3 functional domains.Citation6,Citation7 Domain Ia (amino acids 1–252) is the binding domain, domain II (amino acids 253–364) mediates translocating the toxin to the cytosol, and domain III (amino acids 400–613) contains the ADP-ribosylating enzyme that inactivates EF-2 in the cytosol (). The function of domain Ib (amino acids 365–399) is unclear. PE kills the cells following these steps (): 1) Lys613 is removed by a carboxypeptidase in the plasma.Citation8 2) Domain Ia binds to the α2 macroglobulin receptor on animal cells and internalizes via endosomes to the Golgi.Citation9 3) The protease furin cleaves domain II between amino acids 279 and 280.Citation10 4) The disulfide bond that joins the 2 fragments generated by proteolysis is reduced.Citation11 5) Amino acids 609–612 (arginine–alutamic acid–aspartic acid–leucine, REDL) bind to an intracellular sorting receptor that transports the carboxy terminal fragment from the Golgi to the endoplasmic reticulum (ER).Citation12,Citation13 6) Amino acids 280–313 mediate translocation of the toxin to the cytosol.Citation14,Citation15 7) The ADP-ribosylating enzyme in amino acids 400–602 inactivates EF-2.Citation4 8) Though inhibition of protein synthesis is sufficient to induce cell death eventually, cell death from toxins is facilitated by apoptosis.Citation16,Citation17
Figure 1 Structure of widely used toxins and immunotoxins based on them.
Abbreviations: PE, Pseudomonas exotoxin; DT, Diphtheria toxin; IL-2, interleukin-2; Rgel, recombinant gelonin; dgA, deglycosylated ricin A chain; SMPT, N-succimidyl-oxycarbonyl-α-methyl-α-(2-pyridyldithio)-toluene.
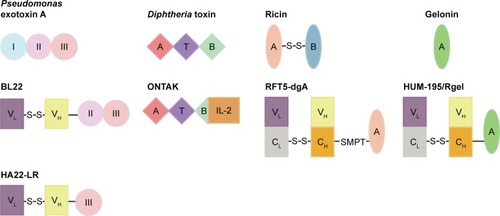
Figure 2 Mechanism of ADCs and immunotoxins based on PE A and DT.
Abbreviations: ADC, antibody–drug conjugate; ER, endoplasmic reticulum; PE, Pseudomonas exotoxin A; DT, Diphtheria toxin; ADP-ribose, adenosine diphosphate ribose; eEF2, eukaryotic elongation factor 2; RIT, recombinant immunotoxins.
Full-length 535-amino acid DT is a single-chain protein containing an enzymatic A domain (amino acids 1–193) and a binding B domain (amino acids 482–535).Citation18 A translocation or transmembrane (T) domain is located in the center of the molecule ().Citation19 DT undergoes the following steps to kill cells (): 1) DT is proteolytically cleaved outside the cell between Arg193 and Ser194,Citation20 which is within a disulfide loop formed by Cys186 and Cys201. 2) DT binds on the cell surface via carboxyl residues 482–535 to a complex of heparin-binding epidermal growth factor (EGF)-like growth factor precursor and CD9.Citation18 3) DT internalizes in an endosome and unfolds at low pH,Citation21 and the disulfide bond between amino acids 186 and 201 is reduced. 4) The TH8 (amino acids 326–347) and TH9 (amino acids 358–376) domains form a hairpin that inserts in the membrane of the endosome and forms a channel through which the enzymatic fragment translocates to the cytosol.Citation22 5) In the cytosol, nicotinamide adenine dinucleotide (NAD) binds to the active-site cleft of DT (amino acids 34–52), and the ADP ribose of NAD is transferred to EF-2.Citation23,Citation24 6) Similar to PE, DT also induces cell death by apoptosis.Citation17
It has been shown that one or only a few of those toxin molecules delivered to the cytosol are sufficient to kill a target cell.Citation25 However, the inhibition of protein synthesis by toxins was thought to be lethal recently.Citation26,Citation27 Because some toxin-treated cancer cells appear to survive from toxin treatment which suggests the existence of resistance to RIT-mediated apoptosis. Therefore, assays that focus more precisely on the mechanisms of cell death have been developed by these toxins later. The activities of bacterial toxin and RITs have been connected to apoptosis in some cell systems, but the mechanisms have not been extensively stated.Citation17,Citation28,Citation29 Keppler et al indicated that a PE-based RIT, B3(Fv)-PE38, kills MCF-7 breast cancer cell line by 2 mechanisms: one is due to the inhibition of protein synthesis caused by inactivation of EF-2 and the other requires caspase activation.Citation30 There was also evidence that PE toxin-induced apoptosis of human mast cells involves downregulation of anti-apoptotic proteins and activation of caspase-8 and -3 pathways.Citation31 When treated with BL22, B cells of chronic lymphocytic leukemia (CLL) underwent programmed cell death that was characterized by caspase-9 and caspase-3 activation, poly(adenosine diphosphate[ADP]-ribose)polymerase (PARP) cleavage, DNA fragmentation, and membrane flipping.Citation32 From the above, cell death mediated by bacterial toxins, such as PE and DT, is often facilitated by caspase-dependent programmed cell death, in addition to the inhibition of protein synthesis.
Mechanism of action of plant toxins
Plant toxins are classified into 2 classes, holotoxins and hemitoxins. Holotoxins, or class I ribosome-inactivating proteins, include ricin, abrin, modeccin, and mistletoe lectin. Hemitoxins, which are also referred to as class II ribosome-inactivating proteins, include gelonin, saporin, bouganin, and bryodin.Citation33 Plant toxins such as ricin and gelonin also arrest protein synthesis but by inactivating the ribosome instead of EF-2.Citation34,Citation35 As shown in , holotoxins such as ricin contain both binding and catalytic domains, whereas hemitoxins contain only catalytic domains. Only the catalytic domains of both holotoxins and hemotoxins translocate to the cytosol, and hence the binding domains of holotoxins would be removed by reduction of the disulfide bond prior to translocation. Plant toxins have been reported to prevent the association of EF-1 and EF-2 with the ribosomal subunit by removing the base of A4324 in 28s ribosomal RNA (rRNA).Citation36 Ricin also removes the neighboring base G4323. These toxin-mediated processes stimulate the apoptotic pathway, as well as the bacterial toxins, leading to cell death in the end. How plant toxins translocate to the cytosol from the cell surface is still unknown. The intracellular transport of ricin is dependent on sorting receptors that cycle between the ER and the Golgi.Citation37
RITs targeting hematologic malignancies
Generally, receptors of cytokines and growth factors are overexpressed in all types of cancer cells. In hematologic malignancies, several receptors are overexpressed compared with normal blood cells and have been targeted successfully in several studies (). Here, we discuss the recently published RITs targeting hematologic malignancies in clinical application with different surface markers.
Table 1 RITs targeting hematologic malignancies
Interleukin-2 receptor (IL-2R)
Interleukin-2 (IL-2), one of the first lymphokines to be identified, plays a central role in the clonal expansion of activated T cells by interacting with its specific cell surface receptor (IL-2R). IL-2R, which binds the IL-2 with high affinity, is composed of 3 subunits (alpha [CD25], beta [CD122], and gamma [CD132]). IL-2R is overexpressed in hematologic malignancies such as adult T-cell leukemia (ATL), cutaneous T-cell lymphoma (CTCL), Hodgkin’s disease (HD), and other B- and T-cell leukemias and lymphomas. But only a small percentage of normal T cells are IL-2R positive. Thus, IL-2R has been broadly used to target leukemias and lymphomas.
Denileukin diftitox (ONTAK, DAB389IL-2)
Denileukin diftitox was granted initial approval in 2001 for the treatment of CTCL. Targeting domain of this RIT does not contain an antibody but a recombinant human IL-2 fused to the C-terminus of the DT toxin. The ligand targets those cells that express the IL-2R, which is transiently expressed on activated T cells but constitutively present in a number of hematologic malignancies. It does not cause generalized DT-related toxicity because the binding parts of the DT are replaced by the IL-2 segment targeting only those cells expressing IL-2R.Citation38
In a single-arm Phase III trial, patients with IL-2R- positive CTCL received denileukin diftitox (9 or 18 μg/kg/d) for 5 consecutive days every 3 weeks. This study demonstrated 20% partial remission (PR), 10% complete regressions (CRs), and 30% overall response rate (ORR) in 71 patients. The duration of response ranged from 2.7 to >46.1 months with a median of 6.9 months. The side effects included flu-like symptoms, acute infusion-related events, and a vascular leak syndrome (VLS). Through measuring the neutralizing antibody of denileukin diftitox, there was no difference of tolerability at the 2 dose levels.Citation39
A later Phase III trial confirmed and improved the response rate of denileukin diftitox with placebo.Citation40 A total number of 144 patients with CTCL were assigned to 9 μg/kg/d denileukin diftitox, 18 μg/kg/d denileukin diftitox, or placebo infusion. The agents were administered for 5 consecutive days every 3 weeks. Compared with 15.9% ORR for placebo-treated patients (2% CR and 13.6% PR), 44% of patients (n=100) treated with denileukin diftitox achieved response (10% CR and 34% PR). Higher ORR was observed in the 18 μg/kg/d group than 9 μg/kg/d group (49.1% vs 37.8%, respectively). In addition, progression-free survival was significantly longer for patients treated with denileukin diftitox. This study demonstrated that denileukin diftitox had a significant and durable effect with an acceptable safety profile in patients with CTCL. Based on this study, Prince et alCitation41 examined the efficacy and safety of denileukin diftitox in 36 patients with IL-2R low expression skin biopsies. The result demonstrated that the safety profile of denileukin diftitox in IL-2R low expression disease was similar to that in IL-2R-positive disease, which suggests that IL-2R low expression would not preclude clinical response to denileukin diftitox in patients with CTCL.
Denileukin diftitox has also been reported to reduce the percentage of regulatory T cells (Tregs) in the peripheral blood of patients with renal carcinoma, ovarian cancer, and melanoma.Citation42–Citation46 It is used infrequently because of poor tolerability and some kinds of side effects, but previously reported adverse effects can be managed effectively by supportive measures without dose reduction.Citation47 Hence, denileukin diftitox can be used for the treatment of CTCL currently.
IL-2Rα (CD25)
The human IL-2Rα, also described as the Tac antigen or CD25, is a 55-kDa membrane glycoprotein (p55). The deduced amino acid sequence of IL-2Rα predicts a mature protein of 251 amino acids with a signal peptide of 21 amino acids in length. The amino-terminal 219 residues constitute the extracellular region. The next 19 residues constitute the membrane spanning region, and the carboxy-terminal 13 residues, the cytoplasmic region. Mutational analysis showed that the N-terminal 83 residues of the IL-2Rα, especially residues 1–6 and 35–43, were essential for its binding function.Citation48
LMB-2 [anti-Tac(Fv)-PE38]
LMB-2, also named anti-Tac(Fv)-PE38, consists of a modified PE toxin and an antibody fragment. The antibody fragment, which contains the VH of anti-Tac fused to the VL via a 15-amino acid linker, selectively binds the α-subunits of IL-2R.Citation49 LMB-2 has been shown to be cytotoxic to CD25+ malignant cells that were either established cell lines or directly obtained from patients with hematologic malignancies.Citation50–Citation53 LMB-2 produced CR in murine xenografts in vivo.Citation49 Blood levels of LMB-2 causing tumor regression in mouse are well tolerated by monkeys.Citation54
The Phase I trial of LMB-2 in patients with hematologic malignancies began in 1996.Citation55 In this test, 4 patients with CD25+ hairy cell leukemia (HCL) received LMB-2 at 3 dose levels (30, 40, and 63 μg/kg every other day [QOD] ×3). All patients reacted to LMB-2 after a single cycle; 1 patient who received 2 cycles had a CR, marked by regression of HCL cells from blood and marrow, and did not relapse after 11 months. Three additional patients had 98%–99.8% reductions in malignant circulating cells. This study represents evidence that LMB-2 may be an effective new therapy for patients with CD25+ HCL.
To evaluate the pharmacokinetics, toxicity, immunogenicity, and antitumor activity of LMB-2, more patients with hematologic malignancies received dose levels that ranged from 2 to 63 μg/kg for a total of 59 cycles.Citation56 Seven PRs were observed in patients with CTCL, HCL, HD, ATL, and CLL. One HCL patient achieved a durable CR for >20 months. All 4 patients with HCL responded to LMB-2. At the maximum tolerated dose (MTD; 40 μg/kg QOD ×3), toxicity was transient and included transaminase elevation and fever. Only 6 of 35 patients developed neutralizing antibodies after the first cycle. This study demonstrated that LMB-2 has clinical antitumor activity in CD25+ hematologic malignancies, especially HCL.
Although LMB-2 showed antitumor activity in Phase I trial, its application was limited by immunogenicity and rapid tumor growth between cycles. Currently, LMB-2 is being investigated in combination with fludarabine and cyclophosphamide for patients with ATL.Citation57 In the previous report, treatment with fludarabine was associated with lower immunogenicity to murine antibodies, and the fludarabine–cyclophosphamide combination was associated with reductions in normal T and B cells. Patients received fludarabine (25 mg/m2) and cyclophosphamide (250 mg/m2) for 3 consecutive days before cycles began. Two weeks later, patients were treated cyclically every 3 weeks. Fludarabine and cyclophosphamide were administered on days 1, 2, and 3, followed by 30–40 μg/kg LMB-2 on days 3, 5, and 7. An ORR of 50% with 2 CRs and 2 PRs was achieved in 8 evaluable patients, 1 of 2 CRs recurred after 6 months only in a sanctuary site. The toxicity of LMB-2 was not increased by fludarabine and cyclophosphamide, while doses of fludarabine and cyclophosphamide used were also without DLT. With fludarabine and cyclophosphamide, normal T and B cells were reduced to 70% and 96% on average, respectively, which allowed more cycles to result in long-term remission. However, additional patients will be needed to determine if chemotherapy can delay immunogenicity of LMB-2 significantly.
RFT5-dgA
RFT5-dgA is an anti-CD25-ricin A chain RIT that is formed by a murine anti-CD25 monoclonal antibody (immunoglobulin G [IgG]) and a deglycosylated ricin A chain (dgA). RFT5-dgA was prepared by using the hindered heterobifunctional cross-linker to dgA.Citation58 Bell et al evaluated the effect of depleting activated T cells with RFT5-dgA through an in vitro model of acute HIV infection, and the results showed that RFT5-dgA inhibited viral production by activated CD25+ HIV-infected cells and suppressed the breed of infection to uninfected T cells.Citation59 The antitumor activity of this RIT was evaluated in severe combined immunodeficiency (SCID) mice with disseminated human Hodgkin’s lymphoma (HL)Citation60 and in nude mice with subcutaneous solid HL.Citation61 In these mice, 40 μg of RFT5-dgA decreased the diameter of >60% of solid Hodgkin and Reed–Sternberg (H-RS) tumors and inhibited the growth of HL in the majority of the treated mice. And RFT5-dgA was at least 7 times more effective than other ricin A chain-based RITs.Citation62
RFT5-dgA was tested in a Phase I trial that involved 15 patients with refractory HL.Citation63 In this dose escalation trial, all patients received RFT5-dgA intravenously on days 1, 3, 5, and 7 for doses per cycle of 5, 10, 15, or 20 mg/m2. After the treatment of 1–4 cycles, 7 of 15 patients made anti-ricin antibodies and 6 of 15 patients developed anti-mouse antibodies. Only 2 PRs were achieved, and 9 of 15 patients got progressive disease. Side effects were mainly related to VLS, which was characterized by decreases in serum albumin, hypotension, edema, myalgia, and tachycardia. In conclusion, RFT5-dgA showed encouraging efficacy at a dose level, and multiple cycles of treatment could be given without cumulative toxicities. In an extension of this Phase I trial, 5 additional patients were treated at the MTD (15 mg/m2).Citation64 Tumor evaluations were performed after the end of completion of treatment. Overall, 2 patients at the MTD achieved PRs lasting 2 and 21 months, respectively. Of 20 patients, 10 showed progressive disease. Half of the patients developed anti-ricin antibodies or anti-mouse antibodies.
According to the preceding studies, more patients were enrolled in the subsequent trial at the MTD.Citation65,Citation66 A total of 27 patients were treated at this level, and all patients had signs of progressive disease before treatment with RFT5-dgA. Of 17 evaluable patients, 2 patients achieved PRs and 1 minor response (MR) and 5 stable diseases. Eleven of 16 patients receiving 2 or more cycles produced anti-ricin antibodies or anti-mouse antibodies, and the side effect in this study remained moderate and related to VLS.
CD19
CD19 is the hallmark differentiation antigen of the B lineage and has been proposed to serve as an important co-receptor molecule in conjunction with CD21 and CD81 for modifying signals generated through the B-cell antigen receptor complex.Citation67,Citation68 CD19 is a lineage-specific glycoprotein expressed on follicular dendritic cells and B cells. It is present on B cells from the earliest recognizable B-lineage cells during development to B-cell blasts but is lost on mature plasma cells. CD19 has been used to diagnose cancers that raised from cells – notably B-cell lymphomas.Citation69 Treatments targeting CD19 have begun to enter trials from 2012.Citation70,Citation71 Most experimental anti-CD19 drugs work by exploiting the presence of CD19 to treat B-cell cancers specifically. But another study indicated that CD19 plays an active role in driving the growth of these cancers, which suggests that CD19 and its downstream signaling may be a better therapeutic target.Citation72
Anti-B4-bR
Anti-B4-bR is an anti-CD19-blocked ricin (bR) RIT that contained an anti-B4 Mab chemically linked to intact ricin (A + B chain).Citation73 The reactivity of anti-B4-bR with tissues lacking the B4 epitope was diminished by chemically blocking the natural binding site of the B chain.Citation74 But the B chain of bR RITs can facilitate the efficient internalization of the A chain because both the ricin A and B chains have carbohydrate residues that are recognized by liver cells.Citation75,Citation76 Anti-B4-bR has been shown to be highly cytotoxic for B4-positive cells, and the cytotoxicity is restricted to these cells.Citation73
Anti-B4-bR induced clinical responses when administered either as continuous infusionsCitation77 or as a daily bolus.Citation73 Thirty-four patients with relapsed or refractory B-cell neoplasms (twenty-six NHL, four CLL, four ALL) received 7-day continuous infusion of anti-B4-bR at dose levels ranging from 10 to 70 mg/kg/d. Potentially, therapeutic serum levels of anti-B4-bR could be maintained for 4 days in patients treated at the MTD. And there were 2 CRs, 3 PRs, and 11 transient responses (TRs).Citation77 Another Phase I trial that enrolled 25 patients with refractory B-cell malignancies was conducted to detail the toxicity and clinical response data when anti-B4-bR was administered as daily bolus infusions for 5 consecutive days.Citation73 One CR, 2 PRs, and 8 mixed responses/TRs were observed after the treatment of anti-B4-bR. Human anti-mouse antibody and anti-ricin antibody were developed in 9 patients. These studies indicated that anti-B4-bR can be administered safely both by continuous infusion and as a daily bolus infusion. In a Phase I trial using anti-B4-bR immunotoxin, 11 of 12 patients with B-cell non-Hodgkin’s lymphoma (B-NHL) remained in CR to the treatment.
HD37-dgA
HD37-dgA was constructed by linking the murine HD37 Mab via a sterically hindered disulfide cross-linker, N-succinimidyl-oxycarbonyl-α-methyl-α-(2-pyridyldithio)-toluene (SMPT), to dgA. Stone et alCitation78 used 2 regimens, intermittent bolus infusion and continuous infusion, for the administration of HD37-dgA to patients with NHL in 2 concomitant Phase I trials. In the intermittent bolus regimen, 2, 4, 8, 16, and 24 mg/m2 of HD37-dgA were divided into 4 equal doses administered every other day. Of 23 evaluable patients, 1 patient achieved CR that persisted >40 months and 1 patient achieved PR (overall 9%). In the continuous infusion regimen, HD37-dgA was administered continuously at 2 dose levels (9.6 and 19.2 mg/m2) for 8 days. One of 9 (11%) evaluable patients developed PR on the continuous infusion regimen. The MTD of each regimen was 16 and 19.2 mg/m2/8 d, respectively. The DLT mainly consisted of VLS, aphasia, and acrocyanosis on the 2 regimens. Almost 25% of patients on the bolus infusion regimen and 30% on the continuous infusion regimen developed antibody against mouse Ig and/or ricin A chain antibody. This test showed that HD37-dgA can be administered safely and can be used in the later clinical trial. In the following Phase II trial, HD37-dgA was used as adjuvant treatment for patients with relapsed B-NHL combined with anti-B4-bR; 26 of 49 patients remained in CR.
CD22
CD22 is a molecule belonging to the sialic acid-recognizing immunoglobulin lectin (SIGLEC) superfamily of lectins.Citation79 It is a B-lineage-restricted surface molecule that modulates B-cell receptor signaling and mediates cellular adhesion. Mature B cells express CD22 on their surface, while it is found to a lesser extent on some immature B cells. It prevents the overactivation of the immune system and the development of autoimmune diseases.Citation80 It is also expressed on B cells in most B-cell leukemias and lymphomas; therefore, it is thought to be a potential targeting therapy for B-cell leukemias and lymphomas.
BL22 [RFB4(dsFv)–PE38/CAT3888]
BL22, which is also named RFB4(dsFv)–PE38 or CAT3888, was the first RIT designed to kill CD22 overexpressed cells. It contained a single-chain Fv of the anti-CD22 BFR4 antibodyCitation81 fused to a 38 kDa portion of PE toxin. In preclinical studies, BL22 was cytotoxic to a wide variety of CD22+ cell linesCitation82,Citation83 and fresh malignant cells from patients.Citation84 And it induced CRs in murine xenograft models at doses tolerated by cynomolgus monkeys.Citation85
To assess the clinical activity of BL22, a dose escalation trial was approved.Citation86 Of the 31 patients with B-cell cancers, 16 had HCL and were resistant to cladribine. Between 0.2 and 4.0 mg BL22 diluted in 50 mL of 0.2% albumin in 0.9% sodium chloride was administered intravenously every other day for a total of 3 doses. Of the 16 patients, 11 had CRs and 2 had PRs, while 6 of them had a CR after only one cycle of BL22. Surprisingly, only 4 of the 16 patients generated neutralizing antibodies against the BL22 after cycles 4, 1, 2, and 4, respectively.
In the following study, 46 patients with B-cell hemato-logic malignancies were enrolled, of whom 11 patients had CLL, 4 patients had NHL, and 31 patients had HCL.Citation87 Of the 31 HCL patients, 16 had a high response rate to BL22 in an interim report.Citation86 BL22 was administered QOD ×3 doses per cycle, dose levels were 3–50 μg/kg. There were no drug-related deaths, and the MTD was determined to be 40 μg/kg. Of the 31 patients with HCL, 19 (61%) CRs and 6 (19%) PRs were achieved, and the ORR was 81%. Only one CLL patient had a PR. The most common DLT was hemolytic uremic syndrome (HUS). HUS was observed in 4 HCL patients and 1 NHL patient during cycles 2/3 and cycle 1, respectively. In these Phase I trials, 65% of CRs were achieved after only one cycle of BL22, so retreatment may not be essential for all patients.
In the Phase II trial, BL22 was limited to 1 cycle and only retreated those patients who did not achieve the cytopenia level for CR. Thirty-six patients suffering from refractory/relapsed HCL were enrolled in this phase II trial.Citation88 A dose rate of 40 μg/kg BL22 was given QOD ×3 on cycle 1. Patients without hematologic remission (HR) were retreated at 30 μg/kg QOD ×3 every 4 weeks for at least 8 weeks after cycle 1, while 50% of the patients (n=18) had responses after just 1 cycle (CR, 25%; PR, 25%), and 56% were retreated 2–13 cycles selectively. After retreatment, 17 CRs (47%), 5 HRs (14%), and 4 PRs (11%) for an ORR of 72% were observed. Compared with patients with spleens either absent or >200 mm (14 of 36), patients with lower baseline spleen height than 200 mm had higher CR (64% vs 21%) and ORR (95% vs 36%). In addition, the median time to relapse of cytopenias has not been reached after nearly 7 years.Citation89
To evaluate the activity of BL22 in hematologic malignancies of children, Wayne et alCitation90 conducted a pre-clinical and Phase I clinical study (ClinicalTrials.gov number: NCT00077493). The results showed that BL22 was cytotoxic (median IC50 =9.8 ng/mL) to CD22+ fresh bone marrow or blasts from children with acute lymphoblastic leukemia (ALL) and also prolonged leukemia-free survival of xenografts. Although no obvious responses were achieved in adults, however, transient clinical activity was seen in most of the subjects.
BL22 has significant activity in HCL with a safety profile, but its activity in CLL, ALL, and NHL was limited.Citation86,Citation87 All cases in the Phase II trial generated neutralizing antibodies that neutralized the toxin portion of BL22, and it cannot achieve equal effect in children as well as in adults. So it is necessary to construct an improved RIT.
Moxetumomab pasudotox (CAT-8015/HA22)
BL22 achieved CR rates of 47%–61% in patients with HCL in Phase I and II trials.Citation86–Citation88 But it was less effective in patients with CLLCitation87 who had lower CD22 expression. Consequently, hotspot mutagenesis was used to increase the affinity of BL22, and the resulting protein was called moxetumomab pasudotox, CAT-8015, or HA22. Moxetumomab pasudotox contains Thr-His-Trp instead of Ser-Ser-Tyr at positions 100, 100A, and 100B in the antigen-binding site of VH. It was determined to have higher cytotoxicity and a 14-fold improved binding affinity, as a result of lower off-rate.Citation91 Moxetumomab pasudotox has antitumor activity in animal xenograft models and a safety profile in cynomolgus monkeys in preclinical studies.Citation92
To determine its safety and efficacy in the treatment of HCL, 28 patients were enrolled in a Phase I trial.Citation93 Moxetumomab pasudotox was given at 5–50 μg/kg QOD ×3 for 1–16 cycles (median, 4 cycles), including 3 patients each at 5, 10, 20, and 30 μg/kg, 4 patients at 40 μg/kg, and 12 patients at 50 μg/kg. After all 114 cycles of moxetumomab pasudotox were administered, major responses were observed at all dose levels. The ORR ranges from 67% to 100% at each dose level without apparent correlation with dose. The ORR of 86% was achieved in 28 patients, whereas only one patient (5%) made neutralizing antibodies after cycle 1. Neutralizing antibodies were detected in 5 of 20, 1 of 13, and 1 of 9 patients after cycles 2, 3, and 4, respectively, but not in patients receiving 5–16 cycles. Thus, it permitted us to retreat most of the HCL patients to increase the chance and degree of response. Remarkable difference had been noted between moxetumomab pasudotox and BL22 in the results of clinical trials. In this HCL clinical trial, the highest dose for treatment was 50 μg/kg, a level higher than the MTD for BL22, and DLT was not observed. The ORR of moxetumomab pasudotox, 86%, was higher than the 72% ORR of the Phase II trial of BL22,Citation88 and response rates at all dose levels were high.
Arons et al analyzed the plasma levels of 49 patients who achieved moxetumomab pasudotox to determine the relationship between response and high CD22 density on HCL cells.Citation94 Those analyzed included the original 28 enrolled prior,Citation93 an additional 20 at the highest dose level (50 μg/kg), and 1 additional patient enrolled prior. Moxetumomab pasudotox was administered to 49 patients at different dose levels every other day for 3 doses. There were 28 (57%) CRs and 88% ORR of 49 patients, while CRs at the highest dose level and lower doses were 64% and 44%, respectively. The difference in micro-residual disease (MRD)-free CR rate was significant (39% vs 6%, P=0.02). Correlations between dose level and both peak level and area under the curve were varied in each dose level. At the highest dose level, CR was more likely with lower volume of distribution and clearance. The pharmacokinetics analysis showed that moxetumomab pasudotox can achieve durable CR in relapsed and refractory HCL patients without MRD. This study also indicates that lower HCL tumor burden minimizes the CD22 “sink” effect allowing higher plasma levels and suggests that patients with lower tumor burden may improve the chance of the durable CR. Furthermore, no DLT or HUS cases were observed in any of the cycles administered to 49 patients.Citation95 Moxetumomab pasudotox is the only known agent that can eliminate MRD in HCL in a high percentage of patients without causing myelosuppressive toxicities.
RFB4-dgA
CD22 molecules expressing the RFB4 epitope are present in 60%–70% of NHL cells. RFB4-dgA was prepared with the anti-CD22 Mab, RFB4, coupled to chemically dgA via the heterobifunctional cross-linker (SMPT). The antibodies used to construct RITs targeting RFB4 contain a mouse IgG1-k and a Fab′ of RFB4. RITs prepared by these antibodies have had notable potential for patients with NHL. Phase I studies of IgG-RFB4-dgA and Fab′-RFB4-dgA have been completed using an intermittent bolus regimen.Citation96,Citation97 Patients with relapsing NHL were treated with Fab′-RFB4-dgA, via 4-h bolus infusion regimen every second day, 5 of 14 (36%) evaluable patients achieved PRs.Citation96 IgG-RFB4-dgA was tested in a similar NHL patient group treated by the same regimen; 1 CR and 5 PRs (ORR, 25%) were observed in 24 patients,Citation97 and a continuous infusion Phase I study of IgG-RFB4-dgA has also been completed.Citation98 In this subsequent Phase I trial, IgG-RFB4-dgA was administered over 8 days with comparable clinical responses (4 of 18 PRs, 22%).Citation98 In all these trials, 20%–40% of the patients achieved responses and the DLT mainly consisted of VLS.Citation99
Combotox (RFB4-dgA + HD37-dgA)
Combotox is a 1:1 mixture of HD37-dgA and RFB4-dgA, which are RITs that target the CD19 and CD22 antigens, respectively. A previous Phase I trial showed that RFB4-dgA induced 24% PRs and 1 long-lasting CR by continuous infusion regimen.Citation98 A previous Phase I trial using continuous infusion of HD37-dgA also showed evidence of anti-tumor activity,Citation78 concordant with the less potent action of it in vitro,Citation100 and in SCID mice with Daudi lymphoma.Citation101 Preclinical data showed that combotox is effective in killing cells in the Daudi disseminated lymphoma in SCID mice.Citation101
To determine the MTD, clinical pharmacology, toxicity, and clinical responses of combotox, a Phase I trial was conducted involving 22 patients with refractory B-cell lymphoma.Citation102 All patients expressed CD19 and CD22 on at least 30% of their tumor cells. Patients received a continuous infusion of combotox at 3 dose levels ranging from 10 to 30 mg/m2/192 h. After the treatment, only patients with circulating tumor cells (CTCs) in peripheral blood tolerated all doses without major toxicity, and prior therapies of these patients appeared to have little impact on toxicity. Analysis of the results indicated the fact that both lots of HD37-dgA tested in the trial had a tendency to aggregate after thawing, and the multimerization of HD37-dgA may have contributed to patient toxicity. Toxicities induced by combotox in this trial, including VLS and HUS, were similar to other types of RITs reported in previous trials. However, combotox appeared to be safe in patients with even minimal numbers of CTCs.Citation102
Combotox, the mixture of HD37-dgA and RFB4-dgA, can bind to and kill human precursor-B-ALL blasts in vitro, and the combination was more effective than either RIT alone.Citation103 Encouraging clinical data were observed in pediatric patients with pre-B-ALL.Citation104 Hence, combotox is a new candidate for the treatment of patients with relapsed B-ALL. In the subsequent Phase I trial, combotox was administered to adult patients with refractory or relapsed B-ALL. Seventeen patients received combotox at 5 different dose levels (3, 5, 6, 7, and 8 mg/m2) by intravenous infusion. A cycle of treatment of combotox included 3 doses administered every other day. All patients experienced decreased peripheral blasts following the treatment of combotox, and 1 PR was observed.Citation105 The DLT still related to VLS. Thus, combotox can be safely administered to adult patients with refractory B-ALL.
CD30
CD30 shows sequence homology to members of the tumor necrosis factor (TNF) receptor superfamily and is expressed only in activated but not resting T and B cells. It was initially described as a surface marker on neoplastic cells of HD.Citation106 Screening for CD30 expression in normal and neoplastic cells led to recognition of CD30 as an activation-induced antigen mostly expressed on lymphoid cells and to the identification of a new category of NHL: CD30/Ki-1-positive anaplastic large-cell lymphoma (ALCL) by using monoclonal antibodies.Citation107–Citation109 The CD30/CD30 ligand system triggers cytolytic cell death in malignant lymphoma cell line and induces proliferation and cytokine production in T cells or neutrophils.Citation110
Ki-4.dgA
Ki-4.dgA was constructed by linking the anti-CD30 Mab (ki-4) via a sterically hindered linker to dgA. Normal human organs revealed no major cross-reactivity of the anti-CD30 Mabs. Ki-4.dgA was 5 times more potent in vitro than other anti-CD30 dgA-RITs tested previously and showed high efficacy in the treatment of human HL in SCID mice.Citation111 Therefore, ki-4.dgA was selected for a Phase I trial in patients with refractory CD30+ HL and NHL.
In the first Phase I trial that aimed to determine the MTD, DLT, antitumor activity, and pharmacokinetics of ki-4. dgA, 17 patients with relapsed CD30+ lymphoma received escalating doses (5, 7.5, or 10 mg/m2/cycle) QOD ×4 of the RIT for 1–3 cycles.Citation66,Citation112 One PR and 1 MR were achieved in 15 evaluable patients. Side effects and DLT were also associated with VLS, which is similar to RIT RFT5-dgA.Citation65,Citation66 Seven of 17 (40%) patients developed anti-ricin antibodies, and only one patient made anti-mouse antibodies.
RITs targeting other molecules
CD33 and HUM-195/Rgel
CD33 is a surface protein that is found on hematopoietic colony-forming cells but not in their more primitive precursors.Citation113,Citation114 CD33 has structural and binding characteristics that identify it as a member of the sialoadhesin family.Citation115 Studies using samples from patients indicate that in 90% of patients with AML, leukemic cells express CD33.Citation116 HUM-195/Rgel was prepared by the humanized anti-CD33 Mab, M195, conjugated to gelonin.Citation117 HUM-195/Rgel was used in CD33+ cell lines and xenografts in nude mice.Citation118 In a Phase I trial of refractory myeloid leukemias, HUM-195/Rgel induced a 38%–50% decrease in peripheral blood blasts or bone marrow blasts in 7 of 28 patients,Citation117,Citation119 and there was no CR or PR.
CD3 and UCHT1 (A-dmDT390-bisFv)
The CD3 antigen was first identified as a glycoprotein that is present on the surface of all human T lymphocytes.Citation120 As more diverse labeling and immunoprecipitation methods were used, the structure of the CD3 antigen appeared to be more complex. The CD3 was expressed as a complex composed of 4 glycoproteins (CD3-γ, δ, ε, and ζ).Citation121,Citation122 CD3 is overexpressed in T-cell malignancies and is a potential target for the treatment of these diseases. UCHT1 (A-dmDT390-bisFv) is a kind of RIT composed of 2 single-chain Fv fragments of an anti-CD3ε Mab fused to DT390. UCHT1 was administered to 5 patients with CTCL by intravenous infusions.Citation123 Two of 5 patients had PRs lasting 1 and >6 months. Anti-DT antibodies developed in all patients after 2 weeks, and the side effects of UCHT1 were fever, nausea, chills, hypoalbuminemia, and transaminasemia.
IL-3R and DT388-IL3
IL-3 is a cytokine that supports the proliferation and differentiation of myeloid progenitors, but it is absent from mature myeloid cells.Citation124 It is overexpressed in myeloid leukemic progenitors and used as a therapeutic target. DT388-IL3, an RIT fused with a Met-His linker, showed antitumor activities in an SCID model of acute myeloid leukemia (AML).Citation99 A Phase I trial of DT388-IL3 was constructed in patients with chemorefractory AML and myelodysplasia (MDS).Citation125 One CR and 1 PR were observed out of 40 evaluable patients with AML and 1 PR out of 5 patients with MDS.
GM-CSFR and DT388-GM-CSF
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine responsible for the growth and differentiation of granulocytes and macrophages. GM-CSF receptor (GM-CSFR) is overexpressed in leukemic cells and targeted in leukemia.Citation99 The RITs GM-CSF-PE38KDEL and DT388-GM-CSF exhibited specific cytotoxicity in leukemic cell lines and patients, and DT388-GM-CSF was more cytotoxic than GM-CSF-PE38KDEL.Citation126 Thirty-one patients with refractory AML were treated with DT388-GM-CSF in a Phase I trial, all of those patients were resistant to chemotherapy.Citation127 One CR and 2 PRs were observed among these patients, and the major DLT was cytokine release syndrome. Neutralizing antibodies against DT were observed in 28 of 31 patients.
RITs targeting solid tumors
RITs have produced many durable CRs in refractory HCL, where patients rarely develop neutralizing antidrug antibodies (ADAs) to the toxin component of the RIT. Targeting solid tumors with RITs is much more difficult than targeting hematologic malignancies. Not only because the cellular junctions between cells are tighter and the tumor was more tightly packed, but also the patients are less immunosuppressed and more likely to develop neutralizing antibodies to the toxin. Later, we discuss the recently published RITs targeting solid tumors ().
Table 2 RITs targeting solid tumors
Mesothelin (MSLN)
MSLN is a cell-surface glycoprotein that is normally expressed only in mesothelial cells, and it is also overexpressed in many solid tumors including mesothelioma,Citation128 pancreatic adenocarcinoma,Citation129,Citation130 cholangiocarcinoma,Citation131 nonmucinous ovarian cancer,Citation132 triple-negative-type breast cancer,Citation133 gastric cancer,Citation134,Citation135 cervical cancer,Citation136 and lung adenocarcinoma.Citation137 Otherwise, MSLN is not critical because MSLN knockout mice grow normally and have no discernible phenotype.Citation138 All the above make MSLN one of the few targeting antigens with sufficient differential expression to allow safe treatment of solid tumor.
SS1P [SS1(dsFv)-PE38]
Phage display technology was used to generate new Fvs binding to MSLN. SS1P is an RIT obtained that underwent affinity improvement, which consists of the PE38 fragment fused to a murine anti-MSLN variable antibody fragment. SS1P was developed for systemic therapy of patients with MSLN-positive solid tumors. One Phase I, dose escalation study of single-agent SS1P was performed in 34 patients with MSLN-expressing advanced mesothelioma (n=20), ovarian (n=12), and pancreatic cancers (n=2).Citation139 Dose level escalated from 8 to 60 μg/kg, and there were 3 patients enrolled at each dose level. The initial cohort of 17 patients received SS1P QOD ×6 doses, and the MTD was 18 μg/kg. DLT included urticaria (1 patient) and grade 3 VLS (2 patients). To allow further dose escalation, 17 patients were treated QOD ×3 doses and MTD was 45 μg/kg. But SS1P was well tolerated at the highest dose level with pleuritis as the DLT. Following this study, another Phase I trial was performed in patients with chemoresistant solid tumor expression MSLN.Citation140 SSIP was administered by continuous infusion for 10 days and repeated cycles at 4-week intervals until the observation of neutralizing antibodies or progressive disease. Twenty-four patients received 4, 8, 12, 18, and 25 μg/kg/d ×10. One of 6 patients, who received the maximum dose level of SS1P, had DLT because of the reversible VLS. Neutralizing antibodies were observed in 18 (75%) of 24 patients, and 5 (21%) patients received a second cycle. Only one patient had a PR. These 2 Phase I trials showed similar efficacy and toxicity of SS1P by different administration schedule. The majority of patients developed ADAs after their first cycle. The main DLT included on-target pleura. These studies demonstrated the need for an effective means to suppress the host immune reaction to SS1P.
So SS1P was tested in patients with newly diagnosed malignant mesothelioma in combination with standard cisplatin and pemetrexed.Citation141 Pemetrexed (500 mg/m2 on day 1) and cisplatin (75 mg/m2 on day 1) were administered every 3 weeks for up to 6 cycles with escalating doses of SS1P. SS1P was administered i.v. on days 1, 3, and 5 during only first 2 cycles at 4 dose levels from 25 to 55 μg/kg. In this study, the toxicity of the combination was similar to that observed with the single agent. Grade 3 toxicities associated with SS1P included fatigue, hypoalbuminemia, back pain, and hypotension. The grade 3 fatigue was dose limiting in only 1 patient at 55 μg/kg. Of 20 evaluable patients, 12 (60%) had a PR. Of 13 patients who received the MTD (45 μg/kg), 10 (77%) had a PR. This compares favorably to the response rate (41.3%) reported for pemetrexed and cisplatin alone.Citation142 Overall, SS1P given with pemetrexed and cisplatin is safe and exhibits significant antitumor activity in patients with pleural mesothelioma, but the hematologic suppression caused by the chemotherapy failed to delay development of neutralizing ADAs.
In a pilot study, SS1P was tested in combination with the pentostatin plus cyclophosphamide immunosuppressive regimen.Citation143 After depleting T and B cells through immunosuppressive regimen, antibody formation was largely delayed, thereby allowing multiple cycles of therapy with SS1P that was previously limited to 1 therapeutic cycle. The immune modulation regimen allowed 2 patients to receive 4 or 6 cycles of SS1P. In this study, of 10 patients with advanced, refractory mesothelioma, 3 patients experienced durable response that persisted for >18 months and 2 patients responded to chemotherapy after immunotoxin therapy. As a result, it is essential to reduce the immunogenicity of SS1P to develop better therapeutic effect.
Ovarian antigen and OVB3-PE
OVB3-PE is the first PE-based RIT that was tested in a clinical trial. It consisted of a murine antibody that targets an unknown antigen on ovarian cancer cells fused to the entire PE toxin,Citation144 but it failed to be used in more patients after its first clinical trial. OVB3-PE was administered to 23 patients with refractory ovarian cancer intraperitoneally,Citation144 but it showed a high level of non-specific toxicity, including central nervous system (CNS) toxicity, transient elevation of liver enzymes, and gastrointestinal (GI) toxicity. Moreover, 100% of the patients made antibodies against the PE toxin 14 days after the first therapy. Human anti-mouse antibodies were also detected in 75% of patients 28 days after therapy.
CD25 and LMB-2
LMB-2 is a CD25-directed PE-based RIT that was usually used to treat CD25+ hematologic malignancies. Considering CD25+ CD4+ Treg cells regulate peripheral self-tolerance and possess the ability to suppress antitumor responses,Citation145 LMB-2 was administered to 8 patients with metastatic melanoma followed by Melanoma Antigen Recognized By T-cells 1 and gp100-specific peptide vaccination.Citation145 LMB-2 administration resulted in a reduction in Treg numbers and did not augment the immune response to cancer vaccination. This study indicated that LMB-2 can selectively mediate a transient partial reduction in circulating and tumor-infiltrating Treg cells in vivo.
ErbB2
The ErbB receptors constitute a group of related transmem-brane proteins that belong to the subclass I of the receptor tyrosine kinase superfamily. Four members of this family have been identified: ErbB/epidermal growth factor receptor (EGFR), ErbB2 (HER2/Neu), ErbB3 (HER3), and ErbB4 (HER4).Citation146,Citation147 Overexpression of ErbB glycoprotein and its tyrosine kinase activity induces loss of growth control and plays an important role in the development of several human cancers.Citation148–Citation150 ErbB2 has been reported to be minimally expressed in normal tissues.Citation151 Thus, ErbB2 is an attractive target for immunotherapy.
ERB-38
ERB-38 is an RIT composed of the Fv portion of Mab e23 that reacts with ErbB2, fused to PE38, a truncated form of PE.Citation152 The IC50 of ERB-38 is 0.2–4 ng/mL on the various ErbB2-positive tumor cell lines. ERB-38 is capable of causing CR in nude mice bearing epidermoid carcinoma and breast cancer. Then ERB-38 was administered in a Phase I trial in patients with advanced carcinoma.Citation152 In this trial, 5 breast cancer patients and 1 esophageal cancer patient were treated with 3 doses of ERB-38. But hepatotoxicity was observed in all patients. Immunohistochemistry showed the presence of ErbB2 on hepatocytes.
ScFv(FRP5)-ETA
ScFv(FRP5)-ETA is a recombinant single-chain antibody toxin that contained a scFv portion of murine Mab FRP5, which recognizes the extracellular domain of ErbB2, linked to a truncated ETA.Citation153 ScFv(FRP5)-ETA displayed potent antitumoral activity against a wide range of tumor cells including breast and ovarian carcinomas,Citation153–Citation155 prostate carcinomas,Citation156 and squamous cell carcinomas.Citation157,Citation158 ScFv(FRP5)-ETA effectively inhibited growth of established murine tumor xenografts.Citation153,Citation155,Citation157,Citation158 ScFv(FRP5)-ETA was applied first in 11 patients with metastatic breast and colorectal cancers and with malignant melanoma from 4 clinical centers.Citation159 ScFv(FRP5)-ETA was administered by intratumoral injection into cutaneous lesions for 7–10 days. Of 10 evaluated patients, treatment-induced shrinkage of tumors was observed in 6 patients and 4 CRs and 2 PRs among these 6 patients. Systemic liver toxicity was observed only in 1 patient treated at the highest daily dose levels.
Lewis Y
Lewis Y (LeY) is overexpressed as a surface membrane component of many solid tumors, and it is also expressed on gastrointestinal epithelium and in the pancreas.Citation160
LMB-1
LMB-1 was the first RIT reported to have anti-tumor activity targeting an epithelial tumor.Citation161 It is composed of an Mab that recognizes LeY, B3, chemically linked to PE38. The clinical test of LMB-1 was conducted in 38 patients with LeY-positive solid tumors who had failed conventional therapy. Objective antitumor activity was observed in only 5 patients, and 1 CR was observed in a patient with metastatic breast cancer. A tumor reduction >75% and resolution of clinical symptoms lasting for >6 months were observed in a colon cancer patient. The major toxicity of LMB-1 was VLS, but 33/38 of patients made antibodies against LMB-1 3 weeks after the first cycle of treatment.Citation161
SGN-10 (BR96 sFv-PE40)
SGN-10 is an RIT consisting of sFv regions of the murine Mab, BR96, fused to a binding-defective portion of PE toxin (PE40).Citation162 BR96 binds to the LeY carbohydrate antigen that is overexpressed on the surface of many human solid tumors.Citation160 SGN-10 showed significant antitumor activity in murine xenografts of human breast and lung tumors and made CR in these tumor xenografts.Citation163,Citation164 On the basis of these favorable data, SGN-10 was developed for clinical trial in 46 patients with LeY-positive advanced carcinomas.Citation165 In this test, cohorts of 3 patients were treated at each dose level on days 1, 4, 8, and 11, followed by a 14-day break. A treatment cycle was 28 days, and patient received 2 or more cycles until there was disease progression or unacceptable toxicity. No CR or PR was observed after 8-week treatment, although 31% patients had stable disease. In this study, DLT was due to GI toxicity rather than to VLS. The immunogenicity of the toxin portion limits the ability of SGN-10 by day 11 of therapy.Citation165
IL-4R and NBI-3001 [IL-4(38-37)-PE38KDEL]
IL-4R is overexpressed on many different tumor surfaces. Human malignant glioma cell lines have been shown to express IL-4R,Citation166,Citation167 and primary cell lines from glioma also express IL-4R,Citation168 but not normal brain cells. To treat these tumors that overexpress IL-4R on their surface, NBI-3001 was composed of circularly permuted IL-4 fused to a truncated portion of PE. NBI-3001 was highly cytotoxic to glioblastoma cell linesCitation169 and made CRs in murine xenografts of human glioma in all the animals.Citation170 NBI-3001 was first used in 9 patients with malignant high-grade gliomas.Citation171 Six of 9 patients showed glioma necrosis, and one of them also showed extensive necrosis of tumor leading to CR. Additional patients were treated to determine the appropriate dose level for patients with malignant glioma.Citation172 Thirty-one patients with astrocytoma were enrolled in this following trial, and 25 of them were diagnosed with glioblastoma multiforme (GBM), while the other 6 patients were diagnosed with anaplastic astrocytoma. The results showed decreased signal intensity in the tumor consistent with tumor necrosis after the treatment in most patients.
EpCAM and VB4-845
Epithelial cell adhesion molecule (EpCAM) is overexpressed in many carcinomas relative to normal tissue, as in the case of transitional cell carcinoma (TCC).Citation173,Citation174 TCC refers to those bladder tumors derived from urothelial tissue. And increase of EpCAM expression was regarded as these cancers progress from lower to higher grades.Citation173,Citation175,Citation176 Thus, EpCAM is a potent clinically relevant antigen for targeted treatment of bladder cancer. VB4-845 is an RIT that targets EpCAM+ cancer cells. It contains an anti-EpCAM humanized scFv fused to a truncated form of PE that lacks the cell-binding domain.Citation177 VB4-845 was tested in a clinical Phase I trial to determine its safety, tolerability, immunogenicity, and efficacy.Citation178 Sixty bacillus Calmette-Guerin (BCG)-intolerant patients with TCC or in situ carcinoma were enrolled in this test. CRs were observed in 39% of patients after the treatment. Although the majority of patients developed antibodies against the toxin portion of VB4-845, VB4-845 therapy was safe and without the most adverse events.
Discussion
The introduction of the “magic bullet” concept by Paul Ehlrich led to the search for agents that can selectively target cancer cells. After the initial success of antibody therapy for cancer, Mabs reacting with cancer cells became widely available. The first RITs that consist of a protein toxin fused to a Mab targeting moiety were constructed in the early 1980s. From then on, toxins from a variety of plants and bacteria were investigated, as well as the continuous optimization of the targeting moiety. To make the RIT more suitable for clinical development, portions of the toxin that were not essential for processing or cytotoxic activity were deleted from the sequence, and point mutations were created in the native toxins to improve activity, limit immunogenicity, or reduce off-target toxicity. Nowadays, the generation of an RIT involves the genetic fusion of a modified form of the toxin and a cell-selective ligand. The ligand can be a recombinant antibody or an antibody fragment, carbohydrate antigen, growth factor, or tumor-associated antigen. To be superior to conventional treatments, the ligand must be directed toward antigens that are exclusively or at least preferentially expressed on tumor cells compared to normal tissues. Meanwhile, one or only a few of toxin molecules delivered to the cytosol is sufficient to kill a target cell. So RITs perfectly combine the high specificity of targeting ligand and the excellent cytotoxic activity of toxins.
As one of the most commonly used antibody drugs, antibody–drug conjugates (ADCs) also have the higher specificity and lower toxicity compared with standard chemotherapy. ADCs consist of an Mab chemically attached to a highly toxic chemotherapy agent for use in traditional systemic therapy for cancers.Citation179 The antibody portion localizes the drug to the tumor but limits its deposition elsewhere, increases antitumor activity, and decreases systemic toxicity of the drug.Citation180,Citation181 Although ADCs have so many advantages, RITs have several favorable properties not shared by ADCs. First, the RITs induced the cell death by disrupting the process of protein synthesis (). And RITs could bring cell death by activation of caspases, which means that RITs can also be used to treat apoptosis-resisted cancers. Second, the mechanism action of novel RIT allows easy combination with standard agents.Citation179 Third, unlike chemotherapy agent used in ADCs, RITs can effectively kill nondividing cells, and RITs have little cross-resistance with other chemotherapy agents. And last, ADCs can cause off-target toxicity due to inappropriate payload, but RITs do not have this problem. In addition, RITs and ADCs share the same principles of selecting targets for therapy. But the requirement of differential expression is more stringent for RITs, so many targets suitable for ADCs are not suitable for RITs.
Because of higher specificity of RITs, selecting differential expressed surface markers is more essential than the category of toxin. Otherwise, RITs will induce some side effects and toxicities. Variety of toxicities has been observed with RITs that have limited the long-term treatment and efficacy in clinical practice. The most common toxicity for these agents is VLS characterized by weight gain, generalized edema, hypoalbuminemia, and orthostatic hypotension. RITs-mediated damage to endothelial cells appears to be responsible for VLS, because the cytotoxic protein must traverse endothelial cells to exit the blood vessels. Studies have shown that PE binds directly to endothelial cells, while truncated PE requires a ligand that reacts with the endothelium and a mutant form of PE showed less VLS.Citation182,Citation183 In addition, mutant toxins that lack enzymatic activity do not cause VLS, suggesting that VLS is an off-target effect of RITs.Citation183 Ricin toxin contains short amino acid motifs that bind endothelial cells.Citation184 Modification or deletion of these motif sequences reduced toxin-induced VLS.Citation185,Citation186 Most recently, Weldon et al deleted nearly entire PE domain II to prevent VLS, while preserving 2 of 3 putative endothelial binding motifs.Citation187 Clinical factors may also induce the VLS. Clinical factors, such as administration of RITs, vary among patients that may affect the severity of VLS. Ricin-based RITs have reported more severe VLS than PE-based RITs. A retrospective study of HL patients showed that patients with a history of prior radiation therapy will have more frequent and more severe VLS.Citation188 In addition to mutations of short amino acid motifs of toxin part, patients receiving premedication with dexamethasone have also been shown to have less severe VLS.Citation189
As a novel class of immune therapeutics, RITs have been used in the treatment of many kinds of tumors. The clinical responses present a promising approach to fighting cancers. RITs produced limited responses in relapsed and refractory hematologic malignancies mainly because of their side effects and high immunogenicity. Nevertheless, their half-lives were too limited for diffusion to occur in solid tumors. Immunogenicity of protein drugs and antibody drugs suddenly attracts broad attention around the world. For regulatory agencies, immunogenicity assessments are required for the licensure of all biologics to ensure safety and efficacy of the proteins. The causes mainly include 2 aspects: 1) immunogenicity will affect safety and efficacy of the drugs, even life-threatening interaction with endogenous protein and 2) lacking of efficient predictive tools means ADAs can be detected only in late Phase III trials after significant expenses have accrued. Based on a wide range of clinical trials, the incidence of immunogenicity after 1 cycle of RIT ranges from 50% to 100% for solid tumors and from 0% to 40% for hematologic tumors.Citation99 Patients with solid tumors are more likely to develop neutralizing antibodies to the toxin because of their less immunosuppression. The presence of neutralizing antibodies lowers the level of active RITs and their efficacy. There is a multitude of factors responsible for ADA formation against RITs. Studies on immunogenicity to RITs indicate that ADA had a strong correlation with a decrease in drug serum concentrations and resultant reduced efficacy.Citation190 ADAs impact the PK of RITs in diverse ways. They can enhance clearance besides sustaining the circulation of RITs. ADA–RIT complexes circulating in the bloodstream trigger regular endogenous elimination processes that are mediated by the reticuloendothelial system, predominantly phagocytic cells in the liver and spleen. The complexes are internalized and undergo subsequent lysosomal degradation. At the same time, ADA–RIT complexes are often clearly slower than the free activated RIT, and compensatory upregulation of shed target may result in concentration increases in total, while “free” concentrations are actually decreasing.Citation191 Moreover, the mechanism of action of an ADA-induced impact on the PK of RITs still needs further and extensive investigation.
At present, several approaches have been used to prevent the development of neutralizing antibodies of RITs in patients. 1) To diminish the immunogenicity of RITs, the new generation of RITs consists of a humanized Fab or Fv fragment of antibody.Citation192,Citation193 It was reported that humanized RIT lost some of its epitopes.Citation194 But the majority of the antibodies that have been found were against the toxin portion of RITs.Citation195 Genetic engineered single-chain variable fragments (scFVs) also are fused to toxins instead of full-size antibodies.Citation196 Compared with full-size Mab therapeutics, low immunogenicity scFV toxin therapeutics has several pros. Antibody fragments, such as scFVs, penetrate tissues and tumors more rapidly and deeply than full-size Mab. In addition, the scFVs have been suggested to permit binding to cryptic epitopes not accessible to full-size Mabs.Citation197 2) The most useful method for some biologic agents, such as interferonCitation198 and L-asparaginase,Citation199 is PEGylation. Covalent attachment of polyethylene glycol (PEG) to RIT has been found to be useful in “masking” the immunogenic epitopes in the protein.Citation200 PEGylation also prolongs the circulation of RIT by reducing renal clearance.Citation201 3) Domain II of PE appeared to be the most immunogenic portion of the PE molecule.Citation144 Mazor et al found that domain II of PE was sensitive to protease digestion and that almost all of domain II except the furin cleavage site (amino acids 274–284) could be removed without loss of activity.Citation200 4) Liu et alCitation202 identified the human B-cell epitopes of PE toxin by M13 phage display. Then they constructed a variant RIT with point mutations of the residues that make up the B-cell epitopes. The variant RIT, which with a deletion of domain II and 7-point mutations that modified human B-cell epitopes, had significantly reduced reactivity with human antisera and retained cytotoxic and antitumor activity.Citation202 Liu et al used this approach to develop a new RIT, RG7787.Citation202 The cytotoxic activity of RG7787 was significantly improved, but the immunogenicity results are not clear yet.Citation193,Citation203 5) Mazor et alCitation204 used the similar approach to develop 2 variant RITs that have their T-cell epitopes removed or suppressed.Citation205 The immunogenicity results suggested that removal of T-cell epitopes is more effective than the removal of B-cell epitopes.Citation200 All these approaches were widely used in the production of new RITs, especially the deletion of domain II of PE toxin and modification of human B- or T-cell epitopes (). The smaller molecular weight, the lower immunogenicity of RITs. So we constructed an RIT with small molecules that consisted of a PE38 toxin and 17 amino acids of amidated gastrin. This RIT, named rG17PE38KDEL, targets the overexpression of cholecystok II receptor (CCK2R) on gastric cancer cells, and it has lower immunogenicity in xenograft model, which means it can be used in further development and application.
Figure 3 Development of the PE-based immunotoxins to increase the toxicity and to decrease the immunogenicity.
Abbreviations: PE, Pseudomonas exotoxin A; RIT, recombinant immunotoxins; MSLN, mesothelin.
Recent advances in the design and administration of RITs are overcoming partial challenges. Serial modifications have been used to reduce nonspecific toxicities, to increase stability and to improve targeted cellular killing. To overcome another major challenge, immunogenicity, several approaches were developed in the past years. Onda et alCitation206 developed the less immunogenic PE by engineering variant of the toxin. Based on this strategy, a bispecific ligand-directed toxin EGF4KDEL-7mut was developed, in which human EGF and IL-4 are linked to low immunogenic variant of PE38. It showed the dual benefit by increasing targeting specificity and reducing immunogenicity. Fused micromolecule or ligand also allowed long-term treatment of tumors. An RIT aiming at the treatment of gastric cancer, rG17PE38, was developed by our laboratory, in which amidated gastrin 17 (rG17) was linked to truncated modificatory PE38. It can suppress the growth of tumor and prolong the survival time in murine xenograft models, and the tumor-bearing mouse did not develop the neutralizing antibody against rG17PE38 after continuous infusion.
Several RITs have shown remarkable success against hematologic malignancies. It had been reported that RITs targeting MSLN produced major tumor regression in some patients with advanced mesothelioma.Citation179 Although there is a further step to mitigate nonspecific toxicities and to enhance the activity of the toxin, we still can anticipate exciting successes in the future application of RITs based on appropriate combinations of cancers and selective target.
Conclusion
The success rate of immunotoxin therapy in clinical trials of leukemia has attracted more effects toward the newer and enhanced immunotoxins, but the immunotoxins targeting solid tumors did not prove effective as expected. Toxicity and immunogenicity remain major concerns, but recently they have been overcome partly by different strategies. As potential antineoplastic agents, immunotoxins are receiving more attention once again and they could be ideal molecules for combination therapy.
Acknowledgments
We thank Song Zhang, Jiang Chang, Chang Li, Ke Zhao, Yu-Ting Guan, Si-Yu Chen, Wen-Qiang He, and Yuan-Yuan Zhang for assistance with data collection and discussion. Furthermore, we express our gratitude to Dr Waqas Ahmad (Section of Epidemiology & Public Health, College of Veterinary and Animal Science, Jhang, Pakistan) for revising the paper for English language. This work was supported by the Key Project in Jilin Province (No 20120966), Specialized Research Fund for Doctoral Program of Colleges and Universities (No 20120061110078), the Fundamental Research Funds for the Platform Base Construction Project in Jilin University (No 2014ZKF01), National Natural Science Foundation Young Investigator Grant Program (No 81401953), the Youth Research Fund of Science and Technology Development Plan of Jilin Province (No 20160520162JH), and the Class General Financial and the special Financial Grant from the China Postdoctoral Science Foundation (No 2014M561303 and No 2015T80312).
Disclosure
The authors report no conflicts of interest in this work.
References
- MooltenFLCooperbandSRSelective destruction of target cells by diphtheria toxin conjugated to antibody directed against antigens on the cellsScience1970169394068704986716
- KrolickKAVillemezCIsaksonPUhrJWVitettaESSelective killing of normal or neoplastic B cells by antibodies coupled to the A chain of ricinProc Natl Acad Sci U S A198077954196968912
- CawleyDBHerschmanHRGillilandDGCollierRJEpidermal growth factor-toxin A chain conjugates: EGF-ricin A is a potent toxin while EGF-diphtheria fragment A is nontoxicCell19802225635706256086
- CarrollSFCollierRJActive site of Pseudomonas aeruginosa exotoxin A. Glutamic acid 553 is photolabeled by NAD and shows functional homology with glutamic acid 148 of diphtheria toxinJ Biol Chem198726218870787112885323
- PhanLDPerentesisJPBodleyJWSaccharomyces cerevisiae elongation factor 2. Mutagenesis of the histidine precursor of diphthamide yields a functional protein that is resistant to diphtheria toxinJ Biol Chem199326812866586688473309
- AlluredVSCollierRJCarrollSFMcKayDBStructure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolutionProc Natl Acad Sci U S A1986835132013243006045
- HwangJFitzgeraldDJAdhyaSPastanIFunctional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coliCell19874811291363098436
- HesslerJLKreitmanRJAn early step in Pseudomonas exotoxin action is removal of the terminal lysine residue, which allows binding to the KDEL receptorBiochemistry (Mosc)199736471457714582
- KounnasMZMorrisREThompsonMRFitzGeraldDJStricklandDKSaelingerCBThe alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin AJ Biol Chem19922671812420124231618748
- ChironMFFrylingCMFitzGeraldDJCleavage of Pseudomonas exotoxin and diphtheria toxin by a furin-like enzyme prepared from beef liverJ Biol Chem19942692718167181768027078
- McKeeMLFitzGeraldDJReduction of furin-nicked Pseudomonas exotoxin A: an unfolding storyBiochemistry (Mosc)199938501650716513
- KreitmanRJPastanIImportance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptorBiochem J1995307129377717988
- ChaudharyVKJinnoYFitzGeraldDPastanIPseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicityProc Natl Acad Sci U S A19908713083122104981
- TheuerCKasturiSPastanIDomain II of Pseudomonas exotoxin A arrests the transfer of translocating nascent chains into mammalian microsomesBiochemistry (Mosc)1994331958945900
- TheuerCPBuchnerJFitzGeraldDPastanIThe N-terminal region of the 37-kDa translocated fragment of Pseudomonas exotoxin A aborts translocation by promoting its own export after microsomal membrane insertionProc Natl Acad Sci U S A19939016777477788356083
- Keppler-HafkemeyerAKreitmanRJPastanIApoptosis induced by immunotoxins used in the treatment of hematologic malignanciesInt J Cancer2000871869410861457
- BrinkmannUBrinkmannEGalloMPastanICloning and characterization of a cellular apoptosis susceptibility gene, the human homologue to the yeast chromosome segregation gene CSE1Proc Natl Acad Sci U S A1995922210427104317479798
- RolfJMGaudinHMEidelsLLocalization of the diphtheria toxin receptor-binding domain to the carboxyl-terminal Mr approximately 6000 region of the toxinJ Biol Chem199026513733173372332431
- ChoeSBennettMJFujiiGThe crystal structure of diphtheria toxinNature199235763752162221589020
- WilliamsDPWenZWatsonRSBoydJStromTBMurphyJRCellular processing of the interleukin-2 fusion toxin DAB486-IL-2 and efficient delivery of diphtheria fragment A to the cytosol of target cells requires Arg194J Biol Chem19902653320673206772243114
- D’SilvaPRLalaAKUnfolding of diphtheria toxin identification of hydrophobic sites exposed on lowering of pH by photolabelingJ Biol Chem19982732616216162229632679
- KaulPSilvermanJShenWHRoles of Glu 349 and Asp 352 in membrane insertion and translocation by diphtheria toxinProtein Sci1996546876928845758
- WilsonBABlankeSRReichKACollierRJActive-site mutations of diphtheria toxin. Tryptophan 50 is a major determinant of NAD affinityJ Biol Chem19942693723296233018083236
- BennettMJEisenbergDRefined structure of monomelic diphtheria toxin at 2.3 A resolutionProtein Sci199439146414757833808
- YamaizumiMMekadaEUchidaTOkadaYOne molecule of diphtheria toxin fragment a introduced into a cell can kill the cellCell1978151245250699044
- MattooARFitzgeraldDJCombination treatments with ABT-263 and an immunotoxin produce synergistic killing of ABT-263-resistant small cell lung cancer cell linesInt J Cancer2013132497898722821746
- TrainiRBen-JosefGPastranaDVABT-737 overcomes resistance to immunotoxin-mediated apoptosis and enhances the delivery of Pseudomonas exotoxin-based proteins to the cell cytosolMol Cancer Ther2010972007201520587662
- MorimotoHBonavidaBDiphtheria toxin- and Pseudomonas A toxin-mediated apoptosis. ADP ribosylation of elongation factor-2 is required for DNA fragmentation and cell lysis and synergy with tumor necrosis factor-alphaJ Immunol19921496208920941517572
- KochiSKCollierRJDNA fragmentation and cytolysis in U937 cells treated with diphtheria toxin or other inhibitors of protein synthesisExp Cell Res199320812963028359223
- Keppler-HafkemeyerABrinkmannUPastanIRole of caspases in immunotoxin-induced apoptosis of cancer cellsBiochemistry1998374816934169429836586
- JenkinsCESwiatoniowskiAIssekutzACLinT-JPseudomonas aeruginosa exotoxin A induces human mast cell apoptosis by a caspase-8 and -3-dependent mechanismJ Biol Chem200427935372013720715205454
- DeckerTOelsnerMKreitmanRJInduction of caspase-dependent programmed cell death in B-cell chronic lymphocytic leukemia by anti-CD22 immunotoxinsBlood200410372718272614525789
- BolognesiAPolitoLTazzariPLIn vitro anti-tumour activity of anti-CD80 and anti-CD86 immunotoxins containing type 1 ribosome-inactivating proteinsBr J Haematol2000110235136110971392
- WalshMJDoddJEHautbergueGMRibosome-inactivating proteinsVirulence20134877478424071927
- ConcanavalinAGelonin, a new inhibitor of protein synthesis, nontoxic to intact cellsJ Biol Chem198025514694769537391060
- EndoYMitsuiKMotizukiMTsurugiKThe mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxinsJ Biol Chem198726212590859123571242
- WescheJRapakAOlsnesSDependence of ricin toxicity on translocation of the toxin A-chain from the endoplasmic reticulum to the cytosolJ Biol Chem199927448344433444910567425
- FossFMDAB 389 IL-2 (ONTAK): a novel fusion toxin therapy for lymphomaClin Lymphoma20001211011611707818
- OlsenEDuvicMFrankelAPivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphomaJ Clin Oncol200119237638811208829
- PrinceHMDuvicMMartinAPhase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphomaJ Clin Oncol201028111870187720212249
- PrinceHMMartinAGOlsenEAFivensonDPDuvicMDenileukin diftitox for the treatment of CD25 low-expression mycosis fungoides and Sézary syndromeLeuk Lymphoma2013541697522738414
- DannullJSuZRizzieriDEnhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cellsJ Clin Invest2005115123623363316308572
- BarnettBKryczekIChengPZouWCurielTJRegulatory T cells in ovarian cancer: biology and therapeutic potentialAm J Reprod Immunol200554636937716305662
- MahnkeKSchönfeldKFondelSDepletion of CD4+ CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitroInt J Cancer2007120122723273317315189
- MorseMAHobeikaACOsadaTDepletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccinesBlood2008112361061818519811
- RaskuMAClemALTelangSTransient T cell depletion causes regression of melanoma metastasesJ Transl Med200861118171482
- Sue McCannMSNAkilovOEGeskinLAdverse effects of denileukin diftitox and their management in patients with cutaneous T-cell lymphomaClin J Oncol Nurs2012165E16423022942
- RobbRJRuskCMNeeperMPStructure-function relationships for the interleukin 2 receptor: location of ligand and antibody binding sites on the Tac receptor chain by mutational analysisProc Natl Acad Sci U S A19888515565456583135551
- KreitmanRJBailonPChaudiharyVKFitzGeraldDJPPastanIRecombinant immunotoxins containing anti-Tac(Fv) and derivatives of Pseudomonas exotoxin produce complete regression in mice of an interleukin-2 receptor-expressing human carcinomaBlood19948324264348286741
- KrietmanRJChaudharyVKWaldmannTWillinghamMCFitzGeraldDJPastanIThe recombinant immunotoxin anti-Tac (Fv)-Pseudomonas exotoxin 40 is cytotoxic toward peripheral blood malignant cells from patients with adult T-cell leukemiaProc Natl Acad Sci U S A19908721829182952236041
- KreitmanRJChaudharyVKWaldmannTACytotoxic activities of recombinant immunotoxins composed of Pseudomonas toxin or diphtheria toxin toward lymphocytes from patients with adult T-cell leukemiaLeukemia1993745535628464234
- SaitoTKreitmanRJHanadaSCytotoxicity of recombinant Fab and Fv immunotoxins on adult T-cell leukemia lymph node and blood cells in the presence of soluble interleukin-2 receptorCancer Res1994544105910648313362
- KreitmanRJBatraJKSeetharamSChaudharyVKFitzGeraldDJPastanISingle-chain immunotoxin fusions between anti-Tac and Pseudomonas exotoxin: relative importance of the two toxin disulfide bondsBioconjug Chem1993421121207873642
- KreitmanRJPastanITargeting Pseudomonas exotoxin to hematologic malignanciesSemin Cancer Biol199565297306 Elsevier8562907
- KreitmanRJWilsonWHRobbinsDResponses in refractory hairy cell leukemia to a recombinant immunotoxinBlood199994103340334810552943
- KreitmanRJWilsonWHWhiteJDPhase I trial of recombinant immunotoxin anti-Tac (Fv)-PE38 (LMB-2) in patients with hematologic malignanciesJ Clin Oncol20001881622163610764422
- KreitmanRJSinghRStetler-StevensonMWaldmannTAPastanIRegression of adult T-cell leukemia with anti-CD25 recombinant immunotoxin LMB-2 preceded by chemotherapyBlood2011118212575
- ThorpePEWallacePMKnowlesPPNew coupling agents for the synthesis of immunotoxins containing a hindered disulfide bond with improved stability in vivoCancer Res19874722592459313499221
- BellKDRamiloOVitettaESCombined use of an immunotoxin and cyclosporine to prevent both activated and quiescent peripheral blood T cells from producing type 1 human immunodeficiency virusProc Natl Acad Sci U S A1993904141114158434001
- WinklerUGottsteinCSchonGSuccessful treatment of disseminated human Hodgkin’s disease in SCID mice with deglycosylated ricin A-chain immunotoxinsBlood19948324664758286745
- EngertAMartinGAmlotPWijdenesJDiehlVThorpePImmunotoxins constructed with anti-CD25 monoclonal antibodies and deglycosylated ricin a-chain have potent anti-tumour effects against human Hodgkin cells in vitro and solid Hodgkin tumours in miceInt J Cancer19914934504561917143
- EngertAGottsteinCWinklerUExperimental treatment of human Hodgkin’s disease with ricin A-chain immunotoxinsLeuk Lymphoma1994135–64414488069189
- EngertADiehlVSchnellRA phase-I study of an anti-CD25 ricin A-chain immunotoxin (RFT5-SMPT-dgA) in patients with refractory Hodgkin’s lymphomaBlood19978924034109002941
- SchnellRVitettaESchindlerJClinical trials with an anti-CD25 ricin A-chain experimental and immunotoxin (RFT5-SMPT-dgA) in Hodgkin’s lymphomaLeuk Lymphoma1998305–65255389711915
- SchnellRVitettaESchindlerJTreatment of refractory Hodgkin’s lymphoma patients with an anti-CD25 ricin A-chain immunotoxinLeukemia200014112913510637488
- SchnellRBorchmannPStaakJOClinical evaluation of ricin A-chain immunotoxins in patients with Hodgkin’s lymphomaAnn Oncol200314572973612702527
- WeissALittmanDRSignal transduction by lymphocyte antigen receptorsCell19947622632748293463
- CambierJCPleimanCMClarkMRSignal transduction by the B cell antigen receptor and its coreceptorsAnnu Rev Immunol19941214574868011288
- ScheuermannRHRacilaECD19 antigen in leukemia and lymphoma diagnosis and immunotherapyLeuk Lymphoma1995185–63853978528044
- JuneCHCAR T cells for leukemia and moreCancer Res2012728 supplL1L3
- CoghlanANovel gene therapy cures leukaemia in eight daysNew Sci2013217291010
- ChungEYPsathasJNYuDLiYWeissMJThomas-TikhonenkoACD19 is a major B cell receptor–independent activator of MYC-driven B-lymphomagenesisJ Clin Invest201212262257226622546857
- GrossbardMLFreedmanASRitzJSerotherapy of B-cell neoplasms with anti-B4-blocked ricin: a phase I trial of daily bolus infusionBlood19927935765851370636
- GoldmacherVSScottCFLambertJMCytotoxicity of gelonin and its conjugates with antibodies is determined by the extent of their endocytosisJ Cell Physiol198914112222342528553
- BlakeyDCSkilleterDNPriceRJThorpePEUptake of native and deglycosylated ricin A-chain immunotoxins by mouse liver parenchymal and non-parenchymal cells in vitro and in vivoBiochim Biophys Acta198896821721783257705
- BlakeyDCWatsonGJKnowlesPPThorpePEEffect of chemical deglycosylation of ricin A chain on the in vivo fate and cytotoxic activity of an immunotoxin composed of ricin A chain and anti-Thy 1.1 antibodyCancer Res19874749479523492271
- GrossbardMLLambertJMGoldmacherVSAnti-B4-blocked ricin: a phase I trial of 7-day continuous infusion in patients with B-cell neoplasmsJ Clin Oncol19931147267377683045
- StoneMJSausvilleEAFayJWA phase I study of bolus versus continuous infusion of the anti-CD19 immunotoxin, IgG-HD37-dgA, in patients with B-cell lymphomaBlood1996884118811978695836
- CrockerPRClarkEAFilbinMSiglecs: a family of sialic-acid binding lectinsGlycobiology199882vvi9498912
- HattaYTsuchiyaNMatsushitaMShiotaMHagiwaraKTokunagaKIdentification of the gene variations in human CD22Immunogenetics199949428028610079291
- LiJ-LShenG-LGhetieM-AThe epitope specificity and tissue reactivity of four murine monoclonal anti-CD22 antibodiesCell Immunol1989118185992463099
- MansfieldEChironMFAmlotPPastanIFitzGeraldDJRecombinant RFB4 single-chain immunotoxin that is cytotoxic towards CD22-positive cellsBiochem Soc Trans19972527099191188
- MansfieldEAmlotPPastanIFitzGeraldDJRecombinant RFB4 immunotoxins exhibit potent cytotoxic activity for CD22-bearing cells and tumorsBlood1997905202020269292538
- KreitmanRJMarguliesIStetler-StevensonMWangQ-CFitzGeraldDJPastanICytotoxic activity of disulfide-stabilized recombinant immunotoxin RFB4 (dsFv)-PE38 (BL22) toward fresh malignant cells from patients with B-cell leukemiasClin Cancer Res2000641476148710778980
- KreitmanRJWangQ-CFitzGeraldDJPastanIComplete regression of human B-cell lymphoma xenografts in mice treated with recombinant anti-CD22 immunotoxin RFB4 (dsFv)-PE38 at doses tolerated by cynomolgus monkeysInt J Cancer199981114815510077166
- KreitmanRJWilsonWHBergeronKEfficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemiaN Engl J Med2001345424124711474661
- KreitmanRJSquiresDRStetler-StevensonMPhase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignanciesJ Clin Oncol200523276719672916061911
- KreitmanRJStetler-StevensonMMarguliesIPhase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemiaJ Clin Oncol200927182983299019414673
- KreitmanRJPastanIAntibody fusion proteins: anti-CD22 recombinant immunotoxin moxetumomab pasudotoxClin Cancer Res201117206398640522003067
- WayneASKreitmanRJFindleyHWAnti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: preclinical studies and phase I clinical trialClin Cancer Res20101661894190320215554
- SalvatoreGBeersRMarguliesIKreitmanRJPastanIImproved cytotoxic activity toward cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage displayClin Cancer Res200284995100211948105
- AldersonRFKreitmanRJChenTCAT-8015: a second-generation Pseudomonas exotoxin A–based immunotherapy targeting CD22-expressing hematologic malignanciesClin Cancer Res200915383283919188153
- KreitmanRJTallmanMSRobakTPhase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemiaJ Clin Oncol201230151822182822355053
- AronsEStetler-StevensonMWilsonWHFitzGeraldDJPPastanIPharmacokinetic analysis of response in hairy cell leukemia treated by anti-CD22 recombinant immunotoxin moxetumomab pasudotoxBlood2013122212871
- KreitmanRJAronsETallmanMSHigh response rate of Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia includes eradication of minimal residual disease: potential importance for outcomeBlood2015126234161
- VitettaESStoneMAmlotPPhase I immunotoxin trial in patients with B-cell lymphomaCancer Res19915115405240581855219
- AmlotPLStoneMJCunninghamDA phase I study of an anti-CD22-deglycosylated ricin A chain immunotoxin in the treatment of B-cell lymphomas resistant to conventional therapyBlood1993829262426338219217
- SausvilleEAHeadleeDStetler-StevensonMContinuous infusion of the anti-CD22 immunotoxin IgG-RFB4-SMPT-dgA in patients with B-cell lymphoma: a phase I studyBlood19958512345734657780133
- KreitmanRJImmunotoxins for targeted cancer therapyAAPS J200683E532E55117025272
- PezzuttoADörkenBRabinovitchPSLedbetterJAMoldenhauerGClarkEACD19 monoclonal antibody HD37 inhibits anti-immunoglobulin-induced B cell activation and proliferationJ Immunol19871389279327992437199
- GhetieMAPickerLJRichardsonJATuckerKUhrJWVitettaESAnti-CD19 inhibits the growth of human B-cell tumor lines in vitro and of Daudi cells in SCID mice by inducing cell cycle arrestBlood1994835132913367509655
- MessmannRAVitettaESHeadleeDA phase I study of combination therapy with immunotoxins IgG-HD37-deglycosylated ricin A chain (dgA) and IgG-RFB4-dgA (Combotox) in patients with refractory CD19 (+), CD22 (+) B cell lymphomaClin Cancer Res2000641302131310778955
- HerreraLFarahRAPellegriniVAImmunotoxins against CD19 and CD22 are effective in killing precursor-B acute lymphoblastic leukemia cells in vitroLeukemia200014585385810803517
- HerreraLBostromBGoreLA phase 1 study of combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemiaJ Pediatr Hematol Oncol2009311293694119875969
- SchindlerJGajavelliSRavandiFA phase I study of a combination of anti-CD19 and anti-CD22 immunotoxins (Combotox) in adult patients with refractory B-lineage acute lymphoblastic leukaemia: combotox in Adult ALLBr J Haematol2011154447147621732928
- SchwabUSteinHGerdesJProduction of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin’s disease and a subset of normal lymphoid cellsNature198229965677110326
- AndreesenROsterholzJLohrGWBrossKJA Hodgkin cell-specific antigen is expressed on a subset of auto-and alloactivated T (helper) lymphoblastsBlood1984636129913026202342
- FaliniBPileriSPizzoloGCD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapyBlood19958517803786
- SchwartingRGerdesJDurkopHFaliniBPileriSSteinHBER-H2: a new anti-Ki-1 (CD30) monoclonal antibody directed at a formol-resistant epitopeBlood1989745167816892477085
- WileySRGoodwinRGSmithCAReverse signaling via CD30 ligandJ Immunol19961578363536398871664
- SchneixRLinnartzCKatouziAADevelopment of new ricin A-chain immunotoxins with potent anti-tumor effects against human Hodgkin cells in vitro and disseminated Hodgkin tumors in SCID mice using high-affinity monoclonal antibodies directed against the CD30 antigenInt J Cancer19956322382447591211
- SchnellRStaakOBorchmannPA phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4. dgA) in patients with refractory CD30+ Hodgkin’s and non-Hodgkin’s lymphomaClin Cancer Res2002861779178612060617
- AndrewsRGTakahashiMSegalGMPowellJSBernsteinIDSingerJWThe L4F3 antigen is expressed by unipotent and multipotent colony-forming cells but not by their precursorsBlood1986685103010353768529
- AndrewsRGTorok-StorbBBernsteinIDMyeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodiesBlood19836211241326190518
- FreemanSDKelmSBarberEKCrockerPRCharacterization of CD33 as a new member of the sialoadhesin family of cellular interaction moleculesBlood1995858200520127718872
- BernsteinIDSingerJWAndrewsRGTreatment of acute myeloid leukemia cells in vitro with a monoclonal antibody recognizing a myeloid differentiation antigen allows normal progenitor cells to be expressedJ Clin Invest1987794115311593470307
- DeanATalpazMKantarjianHPhase I clinical trial of the anti-CD33 immunotoxin HuM195/rgel in patients (pts) with advanced myeloid malignanciesASCO Meet Abstr20102815_suppl6549
- ChoudharySMathewMVermaRSTherapeutic potential of anticancer immunotoxinsDrug Discov Today20111611–1249550321511052
- BorthakurGRosenblumMGTalpazMPhase 1 study of an anti-CD33 immunotoxin, humanized monoclonal antibody M195 conjugated to recombinant gelonin (HUM-195/rGEL), in patients with advanced myeloid malignanciesHaematologica201398221722122875630
- van AgthovenATerhorstCReinherzESchlossmanSCharacterization of T cell surface glycoproteins T1 and T3 present on all human peripheral T lymphocytes and functionally mature thymocytesEur J Immunol198111118216260508
- BorstJPrendivilleMATerhorstCComplexity of the human T lymphocyte-specific cell surface antigen T3J Immunol19821284156015656174606
- BorstJAlexanderSElderJTerhorstCThe T3 complex on human T lymphocytes involves four structurally distinct glycoproteinsJ Biol Chem19832588513551416220014
- FrankelAZuckeroSMankinAAnti-CD3 recombinant diphtheria immunotoxin therapy of cutaneous T cell lymphomaCurr Drug Targets200910210410919199905
- MadhumathiJDevilakshmiSSrideviSVermaRSImmunotoxin therapy for hematologic malignancies: where are we heading?Drug Discov Today201621232533226049016
- FrankelALiuJ-SRizzieriDHoggeDPhase I clinical study of diphtheria toxin-interleukin 3 fusion protein in patients with acute myeloid leukemia and myelodysplasiaLeuk Lymphoma200849354355318297533
- KreitmanRJPastanIRecombinant toxins containing human granulocyte-macrophage colony-stimulating factor and either Pseudomonas exotoxin or diphtheria toxin kill gastrointestinal cancer and leukemia cellsBlood19979012522599207460
- FrankelAEPowellBLHallPDCaseLDKreitmanRJPhase I trial of a novel diphtheria toxin/granulocyte macrophage colony-stimulating factor fusion protein (DT388GMCSF) for refractory or relapsed acute myeloid leukemiaClin Cancer Res2002851004101312006512
- ChangKPaiLHPassHMonoclonal antibody K1 reacts with epithelial mesothelioma but not with lung adenocarcinomaAm J Surg Pathol19921632592681599018
- HassanRLaszikZGLernerMRaffeldMPostierRBrackettDMesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitisAm J Clin Pathol2005124683884516416732
- ArganiPIacobuzio-DonahueCRyuBMesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas identification of a new pancreatic cancer marker by Serial Analysis of Gene Expression (SAGE)Clin Cancer Res20017123862386811751476
- OrdóñezNGApplication of mesothelin immunostaining in tumor diagnosisAm J Surg Pathol200327111418142814576474
- HassanRKreitmanRJPastanIWillinghamMCLocalization of mesothelin in epithelial ovarian cancerAppl Immunohistochem Mol Morphol200513324324716082249
- TchouJWangL-CSelvenBMesothelin, a novel immunotherapy target for triple negative breast cancerBreast Cancer Res Treat2012133279980422418702
- BabaKIshigamiSArigamiTMesothelin expression corre-lates with prolonged patient survival in gastric cancerJ Surg Oncol2012105219519921780126
- EinamaTHommaSKamachiHLuminal membrane expression of mesothelin is a prominent poor prognostic factor for gastric cancerBr J Cancer2012107113714222644300
- ChangKPastanIWillnghamMCFrequent expression of the tumor antigen cak1 in squamous-cell carcinomasInt J Cancer19925145485541351045
- HoMBeraTKWillinghamMCMesothelin expression in human lung cancerClin Cancer Res20071351571157517332303
- BeraTKPastanIMesothelin is not required for normal mouse development or reproductionMol Cell Biol20002082902290610733593
- HassanRBullockSPremkumarAPhase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancersClin Cancer Res200713175144514917785569
- KreitmanRJHassanRFitzGeraldDJPastanIPhase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1PClin Cancer Res200915165274527919671873
- HassanRSharonEThomasAPhase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125Cancer2014120213311331924989332
- VogelzangNJRusthovenJJSymanowskiJPhase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesotheliomaJ Clin Oncol200321142636264412860938
- HassanRMillerACSharonEMajor cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppressionSci Transl Med20135208208ra147
- PaiLHBookmanMAOzolsRFClinical evaluation of intraperitoneal Pseudomonas exotoxin immunoconjugate OVB3-PE in patients with ovarian cancerJ Clin Oncol1991912209521031960550
- PowellDJFelipe-SilvaAMerinoMJAdministration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivoJ Immunol200717974919492817878392
- OlayioyeMANeveRMLaneHAHynesNEThe ErbB signaling network: receptor heterodimerization in development and cancerEMBO J200019133159316710880430
- YardenYSliwkowskiMXUntangling the ErbB signalling networkNat Rev Mol Cell Biol20012212713711252954
- SlamonDGodolphinWJonesLStudies of the HER-2/neu proto-oncogene in human breast and ovarian cancerScience198924449057077122470152
- SchneiderPMHungM-CChioccaSMDifferential expression of the c-erbB-2 gene in human small cell and non-small cell lung cancerCancer Res19894918496849712569928
- ParkJ-BRhimJSParkS-CKimmS-WKrausMHAmplification, overexpression, and rearrangement of the erbB-2 protooncogene in primary human stomach carcinomasCancer Res19894923660566092573419
- PressMFCordon-CardoCSlamonDJExpression of the HER-2/neu proto-oncogene in normal human adult and fetal tissuesOncogene1990579539621973830
- Pai-ScherfLHVillaJPearsonDHepatotoxicity in cancer patients receiving erb-38, a recombinant immunotoxin that targets the erbB2 receptorClin Cancer Res1999592311231510499598
- WelsWHarwerthI-MMuellerMGronerBHynesNESelective inhibition of tumor cell growth by a recombinant single-chain antibody-toxin specific for the erbB-2 receptorCancer Res19925222631063171358432
- SpyridonidisASchmidtMBernhardtWPurging of mammary carcinoma cells during ex vivo culture of CD34+ hematopoietic progenitor cells with recombinant immunotoxinsBlood1998915182018279473251
- SchmidtMMcWattersAWhiteRASynergistic Interaction between an anti-p185HER-2 Pseudomonas exotoxin fusion protein [scFv(FRP5)–ETA] and ionizing radiation for inhibiting growth of ovarian cancer cells that overexpress HER-2Gynecol Oncol200180214515511161852
- WangLLiuBSchmidtMLuYWelsWFanZAntitumor effect of an HER2-specific antibody–toxin fusion protein on human prostate cancer cellsProstate2001471212811304726
- WelsWBeerliRHellmannPEGF receptor and p185erbB-2-specific single-chain antibody toxins differ in their cell-killing activity on tumor cells expressing both receptor proteinsInt J Cancer19956011371447814146
- AzemarMSchmidtMArltFRecombinant antibody toxins specific for ErbB2 and EGF receptor inhibit the in vitro growth of human head and neck cancer cells and cause rapid tumor regression in vivoInt J Cancer200086226927510738256
- AzemarMDjahansouziSJägerERegression of cutaneous tumor lesions in patients intratumorally injected with a recombinant single-chain antibody-toxin targeted to ErbB2/HER2Breast Cancer Res Treat200382315516414703062
- HellströmIGarriguesHJGarriguesUHellströmKEHighly tumor-reactive, internalizing, mouse monoclonal antibodies to Ley-related cell surface antigensCancer Res1990507218321901690595
- PaiLHWittesRSetserAWillinghamMCPastanITreatment of advanced solid tumors with immunotoxin LMB–1: an antibody linked to Pseudomonas exotoxinNat Med1996233503538612238
- FriedmanPNMcAndrewSJGawlakSLBR96 sFv-PE40, a potent single-chain immunotoxin that selectively kills carcinoma cellsCancer Res19935323343398417827
- FriedmanPNChaceDFTrailPASiegallCBAntitumor activity of the single-chain immunotoxin BR96 sFv-PE40 against established breast and lung tumor xenograftsJ Immunol19931507305430618454873
- SiegallCBTargeted therapy of carcinomas using BR96 sFv-PE40, a single-chain immunotoxin that binds to the LeyantigenSemin Cancer Biol1995652892958562906
- PoseyJAKhazaeliMBBookmanMAA phase I trial of the single-chain immunotoxin SGN-10 (BR96 sFv-PE40) in patients with advanced solid tumorsClin Cancer Res20028103092309912374676
- PuriRKLelandPKreitmanRJPastanIHuman neurological cancer cells express interleukin-4 (IL-4) receptors which are targets for the toxic effects of IL4-Pseudomonas exotoxin chimeric proteinInt J Cancer19945845745818056454
- LiuHPraysonRAEstesMLIn vivo eepression of the interleukin 4 receptor alpha by astrocytes in epilepsy cerebral cortexCytokine200012111656166111052816
- JoshiBHPlautzGEPuriRKInterleukin-13 receptor α chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomasCancer Res20006051168117210728667
- PuriRKHoonDSLelandPPreclinical development of a recombinant toxin containing circularly permuted interleukin 4 and truncated Pseudomonas exotoxin for therapy of malignant astrocytomaCancer Res19965624563156378971168
- HusainSRBehariNKreitmanRJPastanIPuriRKComplete regression of established human glioblastoma tumor xenograft by interleukin-4 toxin therapyCancer Res19985816364936539721874
- RandRWKreitmanRJPatronasNVarricchioFPastanIPuriRKIntratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade gliomaClin Cancer Res2000662157216510873064
- WeberFAsherABucholzRSafety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant gliomaJ Neurooncol2003641–212513712952293
- BalzarMWinterMJde BoerCJLitvinovSVThe biology of the 17–1A antigen (Ep-CAM)J Mol Med1999771069971210606205
- LitvinovSVBalzarMWinterMJEpithelial cell adhesion molecule (Ep-CAM) modulates cell–cell interactions mediated by classic cadherinsJ Cell Biol19971395133713489382878
- ZorzosJZiziABakirasAExpression of a cell surface antigen recognized by the monoclonal antibody AUA1 in bladder carcinoma: an immunohistochemical studyEur Urol1994283251254
- BrunnerAPrelogMVerdorferITzankovAMikuzGEnsingerCEpCAM is predominantly expressed in high grade and advanced stage urothelial carcinoma of the bladderJ Clin Pathol200861330731017586680
- PaoloCDWilludaJKubetzkoSA recombinant immunotoxin derived from a humanized epithelial cell adhesion molecule-specific single-chain antibody fragment has potent and selective antitumor activityClin Cancer Res2003972837284812855664
- KowalskiMEntwistleJCizeauJA phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCG-refractory and BCG-intolerant patientsDrug Des Devel Ther20104313320
- AlewineCHassanRPastanIAdvances in anticancer immunotoxin therapyOncologist201520217618525561510
- YounesABartlettNLLeonardJPBrentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomasN Engl J Med2010363191812182121047225
- VermaSMilesDGianniLTrastuzumab emtansine for HER2-positive advanced breast cancerN Engl J Med2012367191783179123020162
- KreitmanRJHansenHJJonesALFitzGeraldDJPGoldenbergDMPastanIPseudomonas exotoxin-based immunotoxins containing the antibody LL2 or LL2-Fab′ induce regression of subcutaneous human B-cell lymphoma in miceCancer Res19935348198258428363
- KuanCTPaiLHPastanIImmunotoxins containing Pseudomonas exotoxin that target LeY damage human endothelial cells in an antibody-specific mode: relevance to vascular leak syndromeClin Cancer Res1995112158915949815960
- BalunaRRizoJGordonBEGhetieVVitettaESEvidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndromeProc Natl Acad Sci U S A19999673957396210097145
- SmallshawJEGhetieVRizoJGenetic engineering of an immunotoxin to eliminate pulmonary vascular leak in miceNat Biotechnol200321438739112627168
- WangHSongSKouGTreatment of hepatocellular carcinoma in a mouse xenograft model with an immunotoxin which is engineered to eliminate vascular leak syndromeCancer Immunol Immunother200756111775178317431617
- WeldonJEXiangLZhangJA recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicityMol Cancer Ther2013121485723136186
- SchindlerJSausvilleEMessmannRVitettaESThe toxicity of deglycosylated ricin A chain-containing immunotoxins in patients with non-Hodgkin’s lymphoma is exacerbated by prior radiotherapy: a retrospective analysis of patients in five clinical trialsClin Cancer Res2001725525811234876
- SiegallCBLiggittDChaceDTepperMAFellPHPrevention of immunotoxin-mediated vascular leak syndrome in rats with retention of antitumor activityProc Natl Acad Sci U S A19949120951495187937798
- BüttelICChamberlainPChowersYTaking immunogenicity assessment of therapeutic proteins to the next levelBiologicals201139210010921353596
- LeeJWKelleyMKingLEBioanalytical approaches to quantify “Total” and “Free” therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug developmentAAPS J20111319911021240643
- BeraTKOndaMKreitmanRJPastanIAn improved recombinant Fab-immunotoxin targeting CD22 expressing malignanciesLeuk Res201438101224122925127689
- AlewineCXiangLYamoriTNiederfellnerGBossletKPastanIEfficacy of RG7787, a next-generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancersMol Cancer Ther201413112653266125239937
- BenharIPadlanEAJungS-HLeeBPastanIRapid humanization of the Fv of monoclonal antibody B3 by using framework exchange of the recombinant immunotoxin B3 (Fv)-PE38Proc Natl Acad Sci U S A1994912512051120557991583
- NagataSPastanIRemoval of B cell epitopes as a practical approach for reducing the immunogenicity of foreign protein-based therapeuticsAdv Drug Deliv Rev2009611197798519679153
- BirdREHardmanKDJacobsonJWSingle-chain antigen-binding proteinsScience198824248774234273140379
- WardESGüssowDGriffithsADJonesPTWinterGBinding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coliNature198934162425445462677748
- ReddyKRDevelopment and pharmacokinetics and pharmacodynamics of pegylated interferon alfa-2a (40 kD)Semin Liver DisNew York, NYThieme Medical Publishers, Inc200424333815346244
- GrahamMLPegaspargase: a review of clinical studiesAdv Drug Deliv Rev200355101293130214499708
- MazorROndaMPastanIImmunogenicity of therapeutic recombinant immunotoxinsImmunol Rev2016270115216426864110
- InadaYFurukawaMSasakiHBiomedical and biotechnological applications of PEG-and PM-modified proteinsTrends Biotechnol199513386917766222
- LiuWOndaMLeeBRecombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopesProc Natl Acad Sci U S A201210929117821178722753489
- HollevoetKMason-OsannELiuXImhof-JungSNiederfellnerGPastanIIn vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancerMol Cancer Ther20141382040204924928849
- MazorREberleJAHuXRecombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human T-cell epitopesProc Natl Acad Sci U S A2014111238571857624799704
- MazorRZhangJXiangLRecombinant immunotoxin with T-cell epitope mutations that greatly reduce immunogenicity for treatment of mesothelin-expressing tumorsMol Cancer Ther201514122789279626443804
- OndaMBeersRXiangLNagataSWangQCPastanIAn immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopesProc Natl Acad Sci U S A200810532113111131618678888
