Abstract
Foxj2, a novel member of Forkhead box family, has been reported to play an important role in tumorigenesis, progression, and metastasis of certain cancers. However, the expression status and effects of Foxj2 on nasopharyngeal carcinoma (NPC) progression and metastasis remain debated. In this study, we first examined the expression of Foxj2 in NPC by immunohistochemistry and Western blotting analysis. We confirmed significantly elevated expression of Foxj2 in NPC tissues and cell lines. Next, the relationships between Foxj2 expression levels and the clinicopathological factors were investigated. Its expression level correlated with T-classification (P=0.026), distant metastasis (P=0.004), and clinical stage (P=0.029). In addition, high expression of Foxj2 was associated with poor prognosis in NPC patients. The effects of Foxj2 on cell proliferation and migration were explored by RNA interference (RNAi) with CCK-8 assay, cell cycle analyses, wound healing, and transwell assay. In conclusion, our data indicate that Foxj2 upregulation promotes the progression and migration of NPC. It makes Foxj2 serve as a potential therapeutic target for the treatment of NPC.
Introduction
Nasopharyngeal carcinoma (NPC) is the most common head and neck cancer with dramatically different geographical and ethnic distribution patterns.Citation1 It has high rates of local recurrence and distant metastasis, and 90% of patients show cervical lymph node metastasis at the time of diagnosis.Citation2 Therefore, understanding the mechanisms associated with NPC progression, metastasis, and prognosis is crucial.
Forkhead box (Fox) family, which includes more than 80 members of transcription factors, share a common forkhead DNA-binding domain, termed the “fork-head” or “winged-helix” motif.Citation3 Most of the members of the Fox family have been reported to be widely present in different organs of different species ranging from yeast to humans and play extraordinarily diverse roles in embryonic development by regulating the expression of different sets of target genes.Citation4 The role played by these transcription factors of the Fox family is important for a wide spectrum of biological processes such as signal transduction, metabolism, development, cell differentiation, proliferation, apoptosis, migration, invasion, and even longevity.Citation5–Citation11 Currently, mounting evidence suggests that Fox family members are abnormally expressed in many cancers, including NPC, and are involved in a variety of cellular processes that are abnormal in cancer cells, such as proliferation, differentiation, adhesion, migration, and invasion.Citation12–Citation17 Forkhead box J2 (Foxj2), a member of this family formerly named as FHX, has dual DNA-binding specificity.Citation18 Previous studies have shown its biological importance in mouse embryogenesis and tumorigenesis.Citation19,Citation20 The expression patterns of Foxj2 have been reported to be aberrant in a variety of cancers, such as breast cancer, extrahepatic cholangiocarcinoma, glioma, non-small cell lung cancer.Citation21–Citation24 In addition, it is reported that Foxj2 appears to be involved in regulating the progression and metastasis of the cancers, as well as aiding in tumorigenesis.Citation21,Citation24 However, its possible roles in NPC are still unknown.
The purpose of this article was demonstrating the expression levels and roles of Foxj2 in the prognosis, progression, and metastasis of NPC. We first determined the expression of Foxj2 in NPC. Then, its associations with clinical and pathologic factors were evaluated. Furthermore, we explored the biological functions, such as proliferation and migration of Foxj2 in NPC, by silencing its expression. All the data suggested that Foxj2 might be a positive regulator in NPC progression.
Materials and methods
Tissue specimens
A total of 57 NPC sections from patients who underwent biopsy at the Affiliated Hospital of Nantong University, China, were fixed in formalin and embedded in paraffin for histopathologic diagnosis and immunohistochemistry (IHC). Noncancerous nasopharyngeal tissues were collected from patients with clinical symptoms suggestive of NPC but ruled out by biopsy. Before biopsy, none of the patients with newly diagnosed NPC had received any antitumor therapy. The main clinical and pathologic characteristics are listed in . The fresh tissues were frozen in liquid nitrogen immediately after biopsy and stored at −80°C until use. This study was approved by the ethics committee of the Affiliated Hospital of Nantong University and all participants gave informed consent.
Table 1 Sequences of siRNAs targeting Foxj2
IHC staining
IHC analysis was carried out as previously reported.Citation25,Citation26 The primary antibody used was ab22857 against Foxj2 (1:50; Santa Cruz Biotechnology Inc, Dallas, TX, USA) and slides were incubated overnight at 4°C. Negative control slides were processed in parallel with nonspecific immunoglobulin G (IgG) (Sigma Chemical Co, St Louis, MO, USA) at the same concentration as the primary antibody. Sections were then washed and treated with horseradish peroxidase-conjugated goat anti-rabbit antibody (DakoCytomation, Carpinteria, CA, USA) for 15 min. For assessment of Foxj2, 2 blinded pathologists evaluated the immunostaining (including the staining intensity and percentage of positive cells). The intensity was estimated as follows: strong staining (3), moderate staining (2), weak staining (1), or negative staining (0). The scale of positive cells was graded as follows: 0% of positive cells (0), 1%–10% of positive cells (1), 10%–50% of positive cells (2), 51%–75% of positive cells (3), 76%–100% of positive cells (4). The results of intensity and extent were added as described.Citation24 The samples with a final score of 0 were considered to represent negative expression; 2–3 indicated weak expression; 4–5 was considered moderate expression; and 6–7 was indicative of strong expression. For statistical analysis, scores of 0–3 were regarded as Foxj2 negative or weak expression and 4–7 were determined as overexpression.
Western blot analysis
Western blot analysis was performed as described previously.Citation25,Citation26 The primary antibodies used for Western blot analysis were as follows: Foxj2 polyclonal antibody (1:1,000; Santa Cruz Biotechnology) and β-actin polyclonal antibody (1:2,000; Santa Cruz Biotechnology).
Cell cultures
The process for obtaining the conditioned medium from the cells was the same as that in our previous study.Citation27 In brief, NPC cell lines (CNE-1, CNE-2, 5-8F, and 6-10B) were maintained in RPMI 1640 (Gibco BRL, Grand Island, NY, USA) containing additionally 10% fetal bovine serum (FBS; Gibco BRL), while NP69 (normal nasopharyngeal epithelial cell line) was cultured in Keratinocyte-SFM (Invitrogen, Carlsbad, CA, USA). All the cells were incubated at 37°C in 5% CO2 incubator.
Small interfering RNA (siRNA) transfection
The Foxj2 siRNA and negative control siRNA were designed and obtained from Biomics Biotechnologies Co, Ltd (Nantong, China). The sequences of siRNAs are shown in . Before transfection, cells were plated and allowed to grow to 30%–50% confluence in 6-well plates. According to the manufacturer’s suggestion, the siRNAs were transfected with Lipofectamine 2000 (Invitrogen).
Cell proliferation assay
The cell proliferation was assessed using the cell counting kit-8 (CCK-8) assay. Cells transfected with Foxj2 or control siRNA were seeded in 96-well plates (20,000 cells per well) and grown overnight. At time points of 0, 6, 12, 24, 36, and 48 h, 10 μL per well CCK-8 solution was added and incubated for 2 h at 37°C. The absorbance was measured at 450 nm using a microplate reader.
Cell cycle analyses
For cell cycle analysis, cells transfected with Foxj2 or control siRNA were fixed and incubated with RNase A. Subsequently, the cells were stained with Cell Cycle Detection Kit (Key-Gene, Wageningen, the Netherlands) according to the manufacturer’s instructions. Cells were analyzed using a BD FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA) and Cell Quest software. All samples were assayed in triplicate.
Transwell assays
Cell migration was measured using cell culture inserts (24-well type, 8 μm pore size; Corning Inc, Corning, NY, USA). Subsequently, 1×105 cells transfected with Foxj2 or control siRNA were added into the upper chambers with the serum-free RPMI 1640 medium, while the lower chambers were filled with 500 μL complete RPMI 1640. After 16 h of incubation, the cells that had migrated to the undersurface of the membrane were fixed in 100% methanol and stained with crystal violet. Cells were visualized and 10 random fields were counted under a microscope.
Wound-healing assay
Cells transfected with Foxj2 or control siRNA were plated in 6-well plates. After the cells reached 80% confluence, scratches were made using a 100 μL pipette tip. Wound healing was observed and the migration distance was imaged at different time points.
Calculation and statistical analysis
All the quantified data from 3 independent experiments are expressed as the mean values ± SDs and analyzed using SPSS17.0 statistical software. The χ2 test was used to determine the significance of Foxj2 expression and the clinicopathological features of NPC. Survival data were calculated by the Kaplan–Meier method and compared by the log-rank test. Statistical significance was determined by the 2-tailed Student’s t-test for 2 groups and 1-way analysis of variance (ANOVA) for more than 2 groups. The value of P<0.05 was considered statistically significant.
Results
Foxj2 was overexpressed in NPC
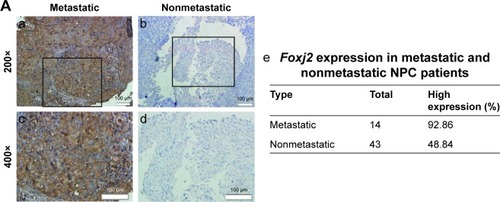
In order to characterize the role of Foxj2 in NPC, we first detected the expression and subcellular localization of Foxj2 in NPC tissues by IHC analysis. We found that Foxj2 had higher expression in NPC (34/57, 59.65%) than in non-tumor tissues (4/26, 15.38%) (). A combination of nuclear and cytoplasmic positive staining was observed in most of the NPC samples (). The results of IHC staining are summarized in . Western blotting was further performed to assess the expression level of Foxj2 (). In agreement with these data, Foxj2 was found to be markedly overexpressed in NPC tissues (). These data raised the possibility that high levels of Foxj2 might be a potential biomarker for NPC.
Figure 1 Expression pattern and prognosis role of Foxj2 in NPC tissues.
Abbreviation: NPC, nasopharyngeal carcinoma.
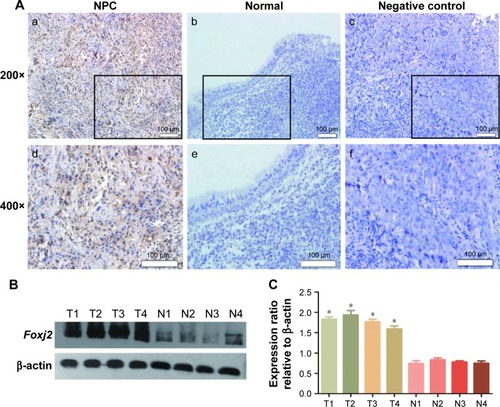
Table 2 The association between Foxj2 expression and clinicopathological features in 57 NPC patients
Correlation between Foxj2 expression and clinicopathological parameters
We further explored the association of Foxj2 expression with clinicopathologic variables in NPC. The clinicopathological data of Foxj2 expression are summarized in . As shown, high Foxj2 expression was significantly associated with T-classification (P=0.026), distant metastasis (P=0.004), and clinical stage (P=0.029).
Overexpression of Foxj2 was indicative of poor survival for NPC patients
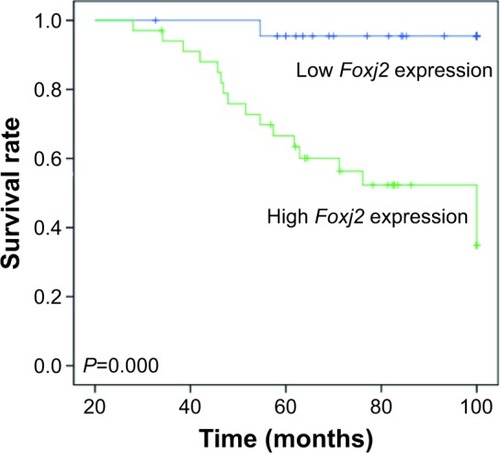
To know whether the overexpression of Foxj2 was correlated with survival, Kaplan–Meier analysis was performed to study the prognostic significance of Foxj2. The survival curves indicated that patients with Foxj2 overexpression had lower overall survival rate than those with negative expression () (P<0.001).
Figure 2 Prognosis role of Foxj2 in NPC.
Abbreviation: NPC, nasopharyngeal carcinoma.
Moreover, univariate and multivariate analyses showed that NPC patients’ overall survival rate were significantly related to Foxj2 expression and that Foxj2 expression (P=0.031) was an independent prognostic factor in NPC patients ( and ). These results strengthen our conjecture that there may be a correlation between the overexpression of Foxj2 and clinical survival in NPC patients.
Table 3 Survival status and clinicopathological parameters in 57 human NPC tissues
Table 4 Contribution of various potential prognostic factors to survival, determined by Cox regression analysis, in 57 human NPC tissues
Expression of Foxj2 in NPC cells
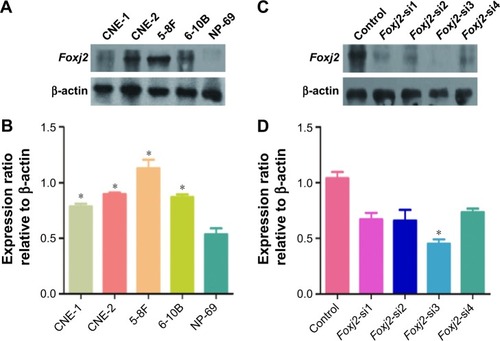
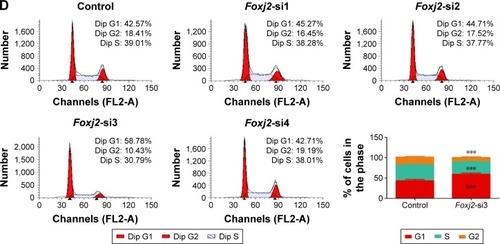
To further assess the biological role of Foxj2 in NPC, we carried out cell-based experiments. First, the expression of Foxj2 in NPC cell lines was investigated. As shown in , Foxj2 were overexpressed in all 4 kinds of NPC cell lines compared with the normal nasopharyngeal epithelial cell line NP69 (). To clarify the function of Foxj2 clearly, 4 siRNAs were designed and transfected into CNE-2 cells to knock down the Foxj2 expression. Foxj2-si3 showed the highest knockdown efficiency, and the expression level was inhibited by Foxj2-si3 up to 56.6% ().
Figure 3 Expression of Foxj2 in NPC cells.
Abbreviations: NPC, nasopharyngeal carcinoma; siRNA, small interfering RNA.
Foxj2 siRNA inhibits cell proliferation
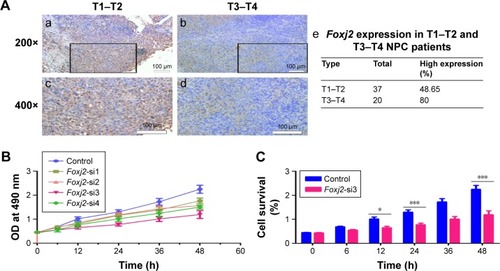
As IHC analysis showed overexpression of Foxj2 in T3–T4 tissues versus T1–T2, we hypothesized that Foxj2 was closely related to NPC proliferation (). Next, CCK-8 and flow cytometry assays were carried out to assess the cell proliferation function of Foxj2 on NPC. CCK-8 assays showed that after downregulating the Foxj2 expression, the CNE-2 cells showed a significant decrease of the cell proliferation rate (). Consistent with these results, flow cytometry analysis revealed that CNE-2 cells accumulated in the G0/G1 phase (from 42.57% to 58.78%), whereas those in the S phase decreased (from 39.01% to 30.79%) after downregulating Foxj2 expression with Foxj2-si3 (), suggesting that Foxj2 could promote accumulation of the cells in the S phase and thus promote the cell growth. These results suggested that Foxj2 might play a crucial role in regulation of NPC cell proliferation.
Figure 4 Knockdown of Foxj2 reduces CNE-2 cell proliferation.
Abbreviations: CCK-8, cell counting kit 8; IHC, immunohistochemistry; NPC, nasopharyngeal carcinoma; OD, optical density; siRNA, small interfering RNA.
Foxj2 siRNA inhibits cell migration
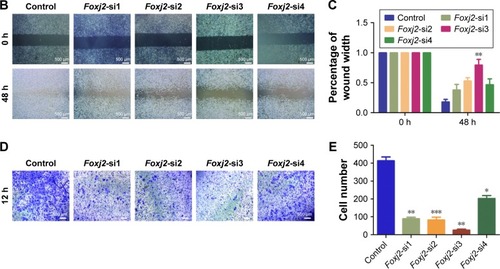
As IHC analysis showed that Foxj2 overexpression is significantly associated with distant metastasis in NPC (), we investigated the cell migration effects of Foxj2. Wound-healing and transwell assays were carried out to examine the migration impact of Foxj2 knockdown on CNE-2 cells. Interestingly, both the assays suggested that knockdown of Foxj2 significantly inhibited cell migration (). These data indicated that downregulation of Foxj2 reduced the migration of CNE-2 cells.
Figure 5 Knockdown of Foxj2 decreases the migration of CNE-2 cells.
Abbreviations: IHC, immunohistochemistry; NPC, nasopharyngeal carcinoma; siRNA, small interfering RNA.
Discussion
NPC is the most common head and neck cancer and continues to be one of the leading causes of cancer-related death worldwide. It has long been acknowledged that gene changes play crucial roles in NPC development and metastasis and affect patients’ outcome. Therefore, it is imperative for us to understand the gene changes that govern its progression and enable early diagnosis. This study provides the first evidence that overexpressed Foxj2 might be a potential regulator in NPC progression and migration.
Foxj2 belongs to the human Forkhead box (Fox) family of proteins, which are implicated in carcinogenesis through their action of transcriptional regulation.Citation28,Citation29 Upregulation of these genes, caused by various mechanisms such as mutation, amplification, and gene fusion, leads to congenital disorders, diabetes mellitus, or carcinogenesis.Citation30 Many Fox family genes, such as FoxA, FoxQ, FoxC, FoxM, and FoxO, have been shown to play an important role in the genesis, progression, and cell dissemination of certain cancers.Citation12–Citation17,Citation31,Citation32 Previous evidences suggest that Foxj2 is abnormally expressed in various malignancies and is involved in positively regulating the progression of the cell cycle or actively participating in the metastatic process.Citation20 The current study extended these findings by investigating Foxj2 expression and functions in NPC tissues and cell lines. We found that Foxj2 was remarkably increased in NPC by IHC and Western blot. The association of Foxj2 expression with NPC clinicopathologic variables was further explored. As shown, overexpressed Foxj2 was significantly associated with T-classification, distant metastasis, and clinical stage. These results indicated that the overexpressed Foxj2 might play an important role in NPC progression and migration. Several Fox family genes have been reported in the literature to influence NPC patients’ prognosis. For example, Huang et alCitation33 recently observed that elevated expression of FoxM1 predicts poor survival in patients with NPC. Our research confirmed that patients with Foxj2 overexpression had a shorter survival time and overexpressed Foxj2 was a remarkable independent predictor of poor prognosis for NPC. Therefore, Foxj2 expression in NPC might serve as a predictor for the clinical outcome.
Chen et alCitation34 established that Foxj2 was colocalized with proliferating cell nuclear antigen (PCNA), which had been used as a general marker of cell proliferative activity. However, the contribution of Foxj2 to malignant proliferation of cancers including NPC remained largely unidentified and need to be determined. In order to explore this role, a series of experiments were performed in cell lines. We used siRNA to knock down Foxj2 expression in NPC cells. After Foxj2 was silenced, we found that the proliferation of CNE-2 cells was significantly inhibited. Cell cycle analysis demonstrated that downregulation of Foxj2 decreased the cell population in the S phase as well as augmented G1 cycle arrest in CNE-2 cells, leading to inability to complete cell division. As Foxj2 participates in regulating the cell cycle, this might partly explain the mechanism by which Foxj2 accelerated cell proliferation. Taken together, these findings indicated that Foxj2 might contribute to carcinogenesis by regulating cell proliferation through the regulation of cell cycle distribution.
Increasing evidence suggests that Foxj2 might actively participate in the metastatic process of various cancers, including glioma, extrahepatic cholangiocarcinoma, breast cancer, and lung cancer,Citation21,Citation22,Citation24,Citation35 as NPC patients with metastasis had a higher rate of treatment failure and mortality.Citation26 As NPC has the characteristics of highly malignant local invasion and early distant metastasis and metastasis is an important character that influences NPC patients’ prognosis, we tried to explore the relationship between Foxj2 and metastasis in NPC. Wound-healing and transwell migration assays demonstrated that silencing of Foxj2 inhibited the migration of CNE-2 cells. These data suggested that Foxj2 overexpression promoted metastasis of NPC.
Conclusion
This study indicated that Foxj2 functioned as a potential oncogene and played an important role in NPC progression and migration. In this research, we confirmed that Foxj2 was upregulated in NPC and the level of Foxj2 is associated with the clinical progression and poor prognosis. Furthermore, our data obtained by downregulation of Foxj2 expression establish the role of Foxj2 in modulating the biological properties of NPC cells, including proliferation and migration. We believe that precise understanding of the biological functions of Foxj2 will provide specific targets for cancer therapeutic intervention.
Acknowledgments
This study was funded by the Chinese National Natural Science Foundation (grant numbers 81672682 and 81602385) and the Natural Science Foundation of Jiangsu Province (grant number SBK2015022581).
Disclosure
The authors report no conflicts of interest in this work.
References
- PeterssonFNasopharyngeal carcinoma: a reviewSemin Diagn Pathol2015321547325769204
- LeungTWTungSYSzeWKTreatment results of 1070 patients with nasopharyngeal carcinoma: an analysis of survival and failure patternsHead Neck200527755556515880410
- HannenhalliSKaestnerKHThe evolution of Fox genes and their role in development and diseaseNat Rev Genet200910423324019274050
- LehmannOJSowdenJCCarlssonPJordanTBhattacharyaSSFox’s in development and diseaseTrends Genet200319633934412801727
- ZhouSZawelLLengauerCKinzlerKWVogelsteinBCharacterization of human FAST-1, a TGF beta and activin signal transducerMol Cell1998211211279702198
- KaestnerKHSilbergDGTraberPGSchutzGThe mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiationGenes Dev19971112158315959203584
- KumeTDengKYWinfreyVGouldDBWalterMAHoganBLThe forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalusCell19989369859969635428
- LinKDormanJBRodanAKenyonCDaf-16: an HNF-3/fork-head family member that can function to double the life-span of Caenorhabditis elegansScience19972785341131913229360933
- OggSParadisSGottliebSThe fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C elegansNature199738966549949999353126
- MyattSSLamEWThe emerging roles of forkhead box (Fox) proteins in cancerNat Rev Cancer200771184785917943136
- BenayounBACaburetSVeitiaRAForkhead transcription factors: key players in health and diseaseTrends Genet201127622423221507500
- YuCChenLYieLTargeting FoxM1 inhibits proliferation, invasion and migration of nasopharyngeal carcinoma through the epithelial-to-mesenchymal transition pathwayOncol Rep20153352402241025738652
- WangWYiMChenSSignificance of the NOR1-FOXA1/HDAC2-slug regulatory network in epithelial-mesenchymal transition of tumor cellsOncotarget2016713167451675926934447
- PengXHHuangHRLuJMiR-124 suppresses tumor growth and metastasis by targeting Foxq1 in nasopharyngeal carcinomaMol Cancer20141318625098939
- LiYChenXMiR-4792 inhibits epithelial-mesenchymal transition and invasion in nasopharyngeal carcinoma by targeting FOXC1Biochem Biophys Res Commun2015468486386926585487
- Ou-YangLXiaoSJLiuPForkhead box C1 induces epithelial–mesenchymal transition and is a potential therapeutic target in nasopharyngeal carcinomaMol Med Rep20151268003800926461269
- ZhouZZhangLXieBFOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transitionCancer Lett2015363213714525896630
- Perez-SanchezCGomez-FerreriaMAde La FuenteCAFHX, a novel fork head factor with a dual DNA binding specificityJ Biol Chem200027517129091291610777590
- GranadinoBArias-de-la-FuenteCPerez-SanchezCFhx (Foxj2) expression is activated during spermatogenesis and very early in embryonic developmentMech Dev2000971–215716011025217
- KehnKBerroRAlhajAFunctional consequences of cyclin D1/BRCA1 interaction in breast cancer cellsOncogene200726355060506917334399
- WangYYangSNiQOverexpression of forkhead box J2 can decrease the migration of breast cancer cellsJ Cell Biochem201211382729273722441887
- QiangYWangFYanSAbnormal expression of forkhead box J2 (FOXJ2) suppresses migration and invasion in extrahepatic cholangiocarcinoma and is associated with prognosisInt J Oncol20154662449245825873280
- YangQCaoXTaoGEffects of FOXJ2 on TGF-beta1-induced epithelial-mesenchymal transition through Notch signaling pathway in non-small lung cancerCell Biol Int2017411798327611107
- QiuXJiBYangLThe role of FoxJ2 in the migration of human glioma cellsPathol Res Pract2015211538939725661068
- YouYYaoHYouBClinical significance of HAX-1 expression in laryngeal carcinomaAuris Nasus Larynx201542429930425554539
- ShiSCaoXGuMYouBShanYYouYUpregulated expression of SOX4 is associated with tumor growth and metastasis in nasopharyngeal carcinomaDis Markers2015201565814126578818
- YouBShanYShiSLiXYouYEffects of ADAM10 upregulation on progression, migration, and prognosis of nasopharyngeal carcinomaCancer Sci2015106111506151426310711
- CofferPJBurgeringBMForkhead-box transcription factors and their role in the immune systemNat Rev Immunol200441188989915516968
- Perez-SanchezCArias-de-la-FuenteCGomez-FerreriaMAGranadinoBRey-CamposJFHX.L and FHX.S, two isoforms of the human fork-head factor FHX (FOXJ2) with differential activityJ Mol Biol2000301479580610966786
- KatohMKatohMHuman FOX gene family (review)Int J Oncol20042551495150015492844
- DuJLiLOuZFOXC1, a target of polycomb, inhibits metastasis of breast cancer cellsBreast Cancer Res Treat20121311657321465172
- LouKChenNLiZMicroRNA-142-5p overexpression inhibits cell growth and induces apoptosis by regulating FOXO in hepatocellular carcinoma cellsOncol Res2017251657328081734
- HuangPYLiYLuoDHExpression of aurora-B and FOXM1 predict poor survival in patients with nasopharyngeal carcinomaStrahlenther Onkol2015191864965525986250
- ChenXCaoXTaoGFOXJ2 expression in rat spinal cord after injury and its role in inflammationJ Mol Neurosci201247115816522246994
- DubeyRSainiNSTAT6 silencing up-regulates cholesterol synthesis via miR-197/FOXJ2 axis and induces ER stress-mediated apoptosis in lung cancer cellsBiochim Biophys Acta201518491324325451482
