Abstract
Purpose
MicroRNAs (miRNAs) play important roles in tumorigenesis and metastasis by regulating genes expression. MiRNA-148b (miR-148b) had been reported to inhibit tumor progression in some kinds of cancers, but the functions of miR-148b in nasopharyngeal carcinoma (NPC) remain largely unknown. The aim of this study was to investigate the functional role of miR-148b in NPC.
Methods
Expression of miR-148b in NPC tissues and cell lines was detected by quantitative reverse transcription polymerase chain reaction. MiR-148b was overexpressed in CNE2 and C666-1 cells by miR-148b mimic transfection. The effects of miR-148b on cell proliferation, migration, and invasion were determined by colony formation assays, cell viability assays, and transwell assays. The target gene of miR-148b was investigated by luciferase assays, and the rescue experiment was performed.
Results
MiR-148b was downregulated in NPC tissues and cell lines. Ectopic miR-148b expression significantly inhibited proliferation, migration, and invasion of CNE2 and C666-1 cells. We identified that metastasis-associated gene 2 (MTA2) is a direct target of miR-148b. Rescue experiment demonstrated that the tumor-suppressive effects of miR-148b on C666-1 cell were partly reversed by restoration of MTA2 expression. Moreover, miR-148b expression was negatively related to mRNA level of MTA2 in NPC tissues.
Conclusion
Our findings elucidate that miR-148b negatively regulates the growth, migration, and invasion of NPC cells, at least in part, by targeting MTA2. The present study indicates that miR-148b is a potential therapeutic agent for NPC.
Introduction
Nasopharyngeal carcinoma (NPC), a unique type of the head and neck cancer, is most prevalent in Southeast Asia and Southern China. In some regions of these endemic areas, the incidence rate of NPC is more than 10/100,000. However, the incidence rate of NPC in non-endemic areas is less than 1/100,000.Citation1 Radiation therapy is effective in the treatment of NPC, especially for early-stage NPC patients. However, a large proportion of NPC patients are diagnosed at advanced stage. Each year in China, about 20,000 people die of NPC, mainly due to tumor recurrence and metastasis.Citation2 It is of great urgency to elucidate the molecular mechanisms of metastasis in NPC, which is critical for identifying new biomarkers and treatment strategies.
MicroRNAs (miRNAs) are endogenous small noncoding RNA, which can regulate genes expression by binding to the specific recognition sequences in the 3′-untranslated region (UTR) of target mRNAs.Citation3 MiRNAs have been shown to play important roles in tumorigenesis, progression and metastasis in NPC and other cancers by regulating the expression of target genes.Citation4,Citation5 Exploration of dysregulated miRNAs in NPC might contribute to the discovery of new biomarkers valuable for diagnosis or therapy of NPC.
MiRNA-148b (miR-148b) had been reported to be downregulated, and it acts as a tumor suppressor in human lung cancer,Citation6 glioma,Citation7 pancreatic cancerCitation8 and hepatocellular carcinoma.Citation9 However, miR-148b is found to be upregulated in human ovarian carcinoma.Citation10 In normal Schwann cells, the upregulation of miR-148b promoted cell migration.Citation11 These studies suggest that miR-148b may play different roles in different situations.
To date, the functional role of miR-148b in NPC is still largely unknown. In this study, we found that miR-148b was downregulated in NPC tissues and cell lines. Ectopic miR-148b expression significantly inhibited proliferation, migration, and invasion of NPC cells. Metastasis-associated gene 2 (MTA2), which is associated with malignant behaviors of tumor cells,Citation12 is identified to be a direct target of miR-148b. Our findings demonstrated, for the first time, that miR-148b could suppress NPC cell growth, migration, and invasion by targeting MTA2.
Materials and methods
Patients and tissue samples
Samples of 20 noncancerous nasopharyngeal tissues and 25 NPC tissues were obtained from the Affiliated Hospital of Guangdong Medical University, Zhanjiang, China, between 2013 and 2014. The use of the clinical materials was approved by the medical ethical committee of the hospital. All the patients enrolled in this study had signed the informed consent documents. The NPC patients had not received any radiotherapy or chemotherapy before biopsy. The patients comprise 17 men and 8 women with an average age of 45.7 years (range, 22–70 years). The clinical stage of patients was based on the pathology tumor–node–metastasis (pTNM) system issued by American Joint Committee on Cancer ⁄ Union for International Cancer Control in 2002 (stage I: one case; stage II: six cases; stage III: 16 cases; stage IV: three cases). All samples of NPC patients were diagnosed to be nasopharyngeal nonkeratinizing carcinoma.
Cell culture
Four human NPC cell lines (CNE1, CNE2, C666-1 and HNE1) and an immortalized nasopharyngeal epithelium cell, NP69, were obtained from the Cancer Research Institute of Guangdong Medical University. The application of these cell lines in this study was approved by the Medical Ethics Committee of Guangdong Medical University. NPC cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS). NP69 cell was cultured in keratinocyte serum-free medium (Thermo Fisher Scientific, Waltham, MA, USA). All the cells were grown in a humidified atmosphere at 37°C with 5% CO2.
RNA isolation and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA of cells or tissues was extracted using TRIzol reagent (Thermo Fisher Scientific). The cDNA was synthesized using the PrimeScript RT Reagent Kit (Takara, Dalian, China). For the detection of miR-148b level, miRNAs were reversely transcribed using the One Step PrimeScript miRNA cDNA Synthesis Kit (Takara) and the amplification processes were performed using the SYBR Premix Ex Taq II Kit (Takara) by a Light Cycler machine (Hoffman-La Roche Ltd, Basel, Switzerland). The primers for miR-148b and U6 small nuclear RNA (snRNA) were purchased from Ambion (Thermo Fisher Scientific). U6 snRNA was used as an internal control of miR-148b. For the detection of MTA2 mRNA level, the cDNA was synthesized using the Prime-Script RT Reagent Kit (Takara) and then amplified using an SYBR Green PCR Kit (Takara). The primers for MTA2 and β-actin were described previously,Citation13 and β-actin was used as an internal control of MTA2. The PCR results were normalized to internal controls and analyzed according to the 2−ΔΔCt method. Three independent experiments were performed.
Transfection of miRNA mimic and MTA2 expression plasmid
MiR-148b mimic or control RNA (miR-Ctrl) were designed and synthesized by RiboBio (Guangzhou, China). The pcDNA3.1 plasmid encoding full-length cDNA sequence of MTA2 (pcMTA2) and the empty vector were purchased from Biogot Biotechnology (Nanjing, China). For cell transfection, a total of 2×105 cells were plated in six-well plates. After 24 hours, miR-148b mimic or pcMTA2 or control was transfected to cells with Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Western blot analysis
Western blot assays were performed as previously described.Citation14,Citation15 Briefly, 48 hours after transfection, cells were lysed with radioimmunoprecipitation assay buffer (Thermo Fisher Scientific) and proteins were quantified by bicinchoninic acid method. Proteins were then separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. The membranes were incubated with 5% nonfat milk and probed with the antibodies against β-actin (1:10,000 diluted; Santa Cruz Biotechnology Inc., Dallas, TX, USA) or MTA2 (1:2,000 diluted; Santa Cruz Biotechnology), followed by horseradish peroxidase (HRP)-labeled secondary antibodies. The signals were detected by the enhanced chemiluminescence detection system (Pierce, Rockford, IL, USA). The protein levels of MTA2 were normalized to β-actin. Three independent experiments were performed.
Cell Counting Kit-8 (CCK8) cell counting assay
Cells transfected with miR-148b mimic or miR-Ctrl were seeded in 96-well plates at 5×103 cells/well. At 24 and 48 hours after plating, 20 µL of CCK-8 solutions (Beyotime, Shanghai, China) were added. After incubation for 2 hours, the absorbance at 450 nm was measured with a microplate reader. Three independent experiments were conducted with six replicates in each experiment.
Colony formation assay
Cells were seeded in six-well plates. miR-148b mimic or miR-Ctrl was transfected to cells and cultured for 48 hours. The cells were then washed three times with PBS and cultured in DMEM containing 10% FBS for 2 weeks in an incubator. The colonies were fixed with methanol and stained with crystal violet. The number of colonies was counted under an inverse microscope. Three independent experiments were performed.
Transwell migration and invasion assays
For transwell migration assays, 5×104 cells transfected with miR-148b mimic or miR-Ctrl were suspended in 100 µL of serum-free DMEM and seeded in the upper chamber of transwell inserts (with 8.0 µm pores; Corning Inc., Corning, NY, USA). For invasion assays, the upper chambers were pre-coated with Matrigel (BD Biosciences, San Jose, CA, USA). A total of 500 µL of complete medium (DMEM with 10% FBS) was loaded to the lower chamber as a chemoattractant. After 20 hours of incubation, the migrated or invaded cells in the lower surfaces of the membranes were fixed with methanol and stained with crystal violet. Cells were observed with a light microscope and counted in five randomly selected fields. Three independent experiments were performed.
Luciferase assay
A total of 40,000 cells of CNE2 and C666-1 were cultured in 24-well plates and incubated for 24 hours before transfection. The pGL3-MTA2 3′UTR-wild type (WT) plasmid or pGL3-MTA2 3′UTR-mutant (Mut) plasmid together with the control vector pRL-TK (Promega Corporation, Fitchburg, WI, USA), and miR-148b mimic or miR-Ctrl, were co-transfected to cells with Lipofectamine 2000 (Thermo Fisher Scientific). After incubation for 48 hours, the cells were harvested, and the firefly and renilla luciferase activities were determined by the dual luciferase assay (Promega Corporation). Renilla luciferase activity was used for normalization. Three independent experiments were performed.
Statistical analysis
Results are presented as mean ± standard deviation of at least three independent experiments and analyzed by SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Student’s t-test or one-way analysis of variance with least significant difference (LSD) test for multiple comparisons was performed for comparison between groups. Pearson’s correlation assay was used to analyze the correlation. Differences were regarded to be statistically significant at P<0.05.
Results
MiR-148b is downregulated in NPC tissues and cell lines
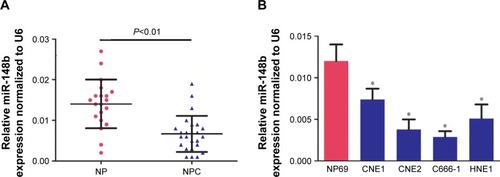
To investigate the exact function of miR-148b in NPC, we detected the expression of miR-148b by qRT-PCR in 20 cases of noncancerous nasopharyngeal tissues and 25 cases of NPC tissues. MiR-148b expression was greatly decreased in NPC tissues compared with nasopharyngeal tissues (). Meanwhile, the expression of miR-148b was downregulated in NPC cell lines (CNE1, CNE2, C666-1 and HNE1) compared with nasopharyngeal epithelium cell, NP69 (). Among the cell lines detected, C666-1 and CNE2 cells had the lowest miR-148b expression so that they were chosen for the subsequent gain of function experiment.
Figure 1 Downregulation of miR-148b in NPC tissues and cell lines.
Abbreviations: miR, microRNA; NP, nasopharyngeal; NPC, nasopharyngeal carcinoma; qRT-PCR, quantitative reverse transcription polymerase chain reaction; U6, U6 snRNA.
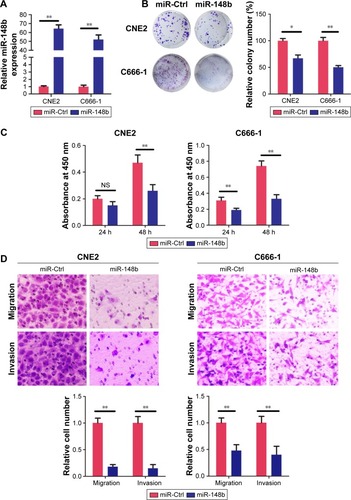
Ectopic miR-148b expression suppressed proliferation, migration, and invasion in NPC cells
To explore the functional role of miR-148b in NPC, miR-148b mimic or mimic control (miR-Ctrl) was transfected to C666-1 and CNE2 cells. As shown in , miR-148b mimic transfection dramatically increased miR-148b expression in CNE2 and C666-1 cells. Colony formation assays demonstrated that miR-148b transfection resulted in the formation of fewer and smaller colonies, compared with control group (). CCK-8 assays demonstrated that miR-148b inhibited the growth of NPC cells in vitro, especially in C666-1 cell (). Transwell assays revealed that miR-148b transfection significantly inhibited the migration and invasion of CNE2 and C666-1 cells, as shown in . These results showed that miR-148b could inhibit the growth, migration, and invasion of NPC cells in vitro.
Figure 2 Inhibition effects of miR-148b on proliferation, migration, and invasion of CNE2 and C666-1 cells.
Abbreviations: CCK-8, Cell Counting Kit-8; Ctrl, control; h, hours; miR, microRNA; NS, no significance; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
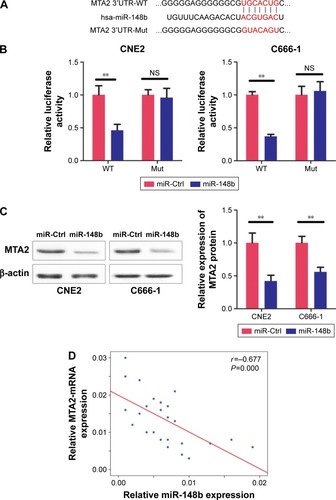
MTA2 is a direct target of miR-148b
We had previously reported that MTA2 promoted proliferation and metastasis of NPC cells and acted as an oncogene in NPC.Citation13 Using a miRNA targeting prediction program TargetScan (http://www.targetscan.org/), we found that the 3′UTR of MTA2 mRNA had a complementary sequence for the seed region of miR-148b (), suggesting that MTA2 is a potential target of miR-148b. To verify whether MTA2 was a true target of miR-148b, we conducted luciferase assays in CNE2 and C666-1 cells. We found that, compared with control, miR-148b mimic transfection inhibited the luciferase activity when cells were co-transfected with WT 3′UTR of MTA2. However, no differences in luciferase activity between miR-148b mimic and control group were found when cells were co-transfected with Mut type 3′UTR of MTA2 (). Western blot analysis revealed that miR-148b remarkably downregulated MTA2 protein expression in CNE2 and C666-1 cells (). Notably, we found that the mRNA level of MTA2 was negatively related to miR-148b level in NPC tissues (r=−0.677, P<0.01, ). Overall, these experiments identified that MTA2 was a direct target of miR-148b.
Figure 3 MTA2 is a target of miR-148b.
Abbreviations: Ctrl, control; miR, microRNA; MTA2, metastasis-associated gene 2; Mut, mutant; NPC, nasopharyngeal carcinoma; NS, no significance; WT, wild type; UTR, untranslated region.
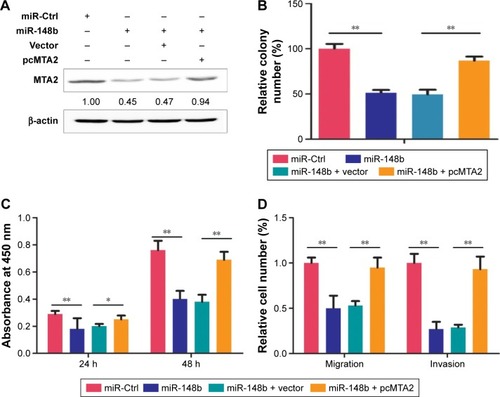
The tumor suppressive effects of miR-148b on C666-1 cell are partially reversed by MTA2 restoration
To verify whether the tumor suppressive role of miR-148b in NPC cells was mediated by MTA2, the expression of MTA2 was rescued in miR-148b-overexpressing cells (). The highly metastatic C666-1 cell, which was more sensitive to miR-148b treatment demonstrated in CCK-8 and colony formation assays, was used in the rescue experiment. We found that the restoration of MTA2 expression in C666-1 cell partially reversed the inhibitive effects on cell proliferation, migration, and invasion induced by miR-148b overexpression ().
Figure 4 The tumor-suppression effects of miR-148b on C666-1 cell were partially reversed by MTA2 restoration.
Abbreviations: CCK-8, Cell Counting Kit-8; Ctrl, control; miR, microRNA; h, hours; MTA2, metastasis-associated gene 2; pcMTA2, MTA2 expression plasmid pcDNA3.1.
Discussion
Dysregulated expression of miRNAs had been shown to play important roles in regulating biological behaviors of NPC.Citation16 The tumor-suppressive miR-29c, which was frequently reduced in NPC tissues, suppressed NPC progression and metastasis by targeting multiple genes, including TIAM1,Citation17 Mcl-1 and Bcl-2.Citation18 Moreover, miR-29c increased the sensitivities of NPC to chemotherapy and radiotherapy.Citation18 The oncogenic miR-155 was upregulated in NPC by LMP1 and LMP2A, two proteins encoded by Epstein–Barr virus.Citation19,Citation20 miR-155 promoted the malignant biological behaviors of NPC cells by targeting JMJD1ACitation20 and ZDHHC2.Citation21
MiR-148b, as a member of the miR-148/152 family, plays important roles in development and diseases.Citation22 MiR-148b had been reported to be downregulated in some human cancers.Citation7,Citation8,Citation23 Dysregulated miR-148b in pancreatic cancer was correlated with increased tumor size, advanced clinical stage, distant metastasis and poor prognosis. Ectopic miR-148b expression inhibited the growth and metastasis of pancreatic cancer cells by targeting AMPKα1.Citation8 In hepatocellular carcinoma, miR-148b negatively regulated WNT1/β-catenin pathway by targeting WNT.Citation19 Moreover, miR-148b was critical in the maintenance of properties of cancer stem cells by targeting Neuropilin-1 in hepatocellular carcinoma, suggesting that the restoration of miR-148b may be a new therapeutic strategy by targeting cancer stem cells.Citation24 miR-148b inhibits malignant behaviors of glioma cells through targeting HOTAIR.Citation7 These studies, overall, revealed that miR-148b may play important roles in NPC. However, the functions of miR-148b in NPC are still largely unknown.
In the current study, we found that miR-148b expression was frequently decreased in NPC tissues and cell lines. In the NPC cell lines detected, the highly metastatic C666-1 cell had the lowest miR-148b expression, suggesting that miR-148b may be related to the metastasis of NPC. Besides C666-1 cell, another NPC cell line, CNE2, with relatively low miR-148b expression, were transfected with miR-148b mimic for the gain of function study. The results showed that the overexpression of miR-148b significantly inhibited proliferation, colony formation, migration, and invasion of C666-1 and CNE2 cells. These experiments revealed that miR-148b acts as a tumor suppressor in NPC.
MTA2 had been reported to promote tumor progression.Citation25 MTA2 overexpression promoted the proliferation and invasion of gastric cancer cells through stimulating the secretion of interleukin 11.Citation26 MTA2 promoted the migration of breast cancer cells by activating the RhoA signaling pathway.Citation27 We had previously found that MTA2 was upregulated in NPC. MTA2 promoted the growth and metastasis of NPC cells in vitro and in vivo.Citation13 In silico analysis by TargetScan reveals that MTA2 is a potential target of miR-148b. Subsequently, the luciferase assays were performed and MTA2 was identified to be a novel target of miR-148b. In addition, we found that the mRNA level of MTA2 was negatively related to miR-148b in NPC tissues, indicating that miR-148b can negatively regulate MTA2 expression in NPC. The rescue experiment was performed to explore the functional role of miR-148b/MTA2 axis in C666-1 cell. We found that the tumor-suppressive effects of miR-148b were partially reversed. The results indicated that reduced miR-148b expression promoted proliferation, migration, and invasion of NPC cells by targeting MTA2. The current in vitro study reveals the function of miR-148b in NPC. However, further investigation is needed to fully understand the role of miR-148b in NPC, such as in vivo study and exploring the interaction between miR-148b and its potential targets in NPC.
Conclusion
Our study demonstrates that miR-148b acts as a tumor suppressor in NPC. MTA2 is a novel target of miR-148b. MiR-148b inhibits malignant behaviors of NPC cells, at least partly, through the repression of MTA2 expression.
Acknowledgments
This study was supported by the Medical Scientific Research Foundation of Guangdong Province, China (grants A2016529 and A2015105), Administration of Traditional Chinese Medicine of Guangdong Province, China (grant A20171148), National Natural Science Foundation of China (grant 81201763), National Natural Science Foundation of Guangdong, China (grant 2016A030310357) and the Science Research Foundation of Guangdong Medical University, China (grant M2015011).
Disclosure
The authors report no conflicts of interest in this work.
References
- CarioliGNegriEKawakitaDGaravelloWLa VecchiaCMalvezziMGlobal trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: focus on low-risk areasInt J Cancer2017140102256226428224615
- YoshizakiTItoMMuronoSWakisakaNKondoSEndoKCurrent understanding and management of nasopharyngeal carcinomaAuris Nasus Larynx201239213714421592702
- BartelDPMicroRNAs: genomics, biogenesis, mechanism, and functionCell2004116228129714744438
- AleckovicMKangYRegulation of cancer metastasis by cell-free miRNAsBiochim Biophys Acta201518551244225450578
- TanGTangXTangFThe role of microRNAs in nasopharyngeal carcinomaTumour Biol2015361697925427638
- LiuGLLiuXLvXBWangXPFangXSSangYmiR-148b functions as a tumor suppressor in non-small cell lung cancer by targeting carcinoembryonic antigen (CEA)Int J Clin Exp Med2014781990199925232379
- WangGLiZTianNmiR-148b-3p inhibits malignant biological behaviors of human glioma cells induced by high HOTAIR expressionOncol Lett201612287988627446363
- ZhaoGZhangJGLiuYmiR-148b functions as a tumor suppressor in pancreatic cancer by targeting AMPKalpha1Mol Cancer Ther2013121839323171948
- ZhangJGShiYHongDFMiR-148b suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting WNT1/beta-catenin pathwaySci Rep20155808725627001
- ChangHZhouXWangZNIncreased expression of miR-148b in ovarian carcinoma and its clinical significanceMol Med Rep2012551277128022344713
- QianTMZhaoLLWangJmiR-148b-3p promotes migration of Schwann cells by targeting cullin-associated and neddylation-dissociated 1Neural Regen Res20161161001100527482232
- CovingtonKRFuquaSARole of MTA2 in human cancerCancer Metastasis Rev201433492192825394532
- WuMYeXDengXWuYLiXZhangLUpregulation of metastasis-associated gene 2 promotes cell proliferation and invasion in nasopharyngeal carcinomaOnco Targets Ther201691647165627051300
- MaLDengXWuMZhangGHuangJDown-regulation of miRNA-204 by LMP-1 enhances CDC42 activity and facilitates invasion of EBV-associated nasopharyngeal carcinoma cellsFEBS Lett201458891562157024613926
- DengXMaLWuMmiR-124 radiosensitizes human glioma cells by targeting CDK4J Neurooncol2013114326327423761023
- BruceJPLiuFFMicroRNAs in nasopharyngeal carcinomaChin J Cancer2014331153954425367334
- LiuNTangLLSunYMiR-29c suppresses invasion and metastasis by targeting TIAM1 in nasopharyngeal carcinomaCancer Lett2013329218118823142282
- ZhangJXQianDWangFWMicroRNA-29c enhances the sensitivities of human nasopharyngeal carcinoma to cisplatin-based chemotherapy and radiotherapyCancer Lett20133291919823142283
- ZhuXWangYSunYZhengJZhuDMiR-155 up-regulation by LMP1 DNA contributes to increased nasopharyngeal carcinoma cell proliferation and migrationEur Arch Otorhinolaryngol201427171939194524241359
- DuZMHuLFWangHYUpregulation of MiR-155 in nasopharyngeal carcinoma is partly driven by LMP1 and LMP2A and downregulates a negative prognostic marker JMJD1APLoS One201164e1913721541331
- JiangYXDuZMJiaoLInhibition of MiR-155 suppresses cell migration in nasopharyngeal carcinoma through targeting ZDHHC2Int J Clin Exp Med2015868472848426309499
- ChenYSongYXWangZNThe microRNA-148/152 family: multifaceted playersMol Cancer2013124323683438
- LiaoYHChangYHSungLYOsteogenic differentiation of adipose-derived stem cells and calvarial defect repair using baculovirus-mediated co-expression of BMP-2 and miR-148bBiomaterials201435184901491024674465
- LiuQXuYWeiSmiRNA-148b suppresses hepatic cancer stem cell by targeting neuropilin-1Biosci Rep2015354e0022925997710
- TohYNicolsonGLThe role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implicationsClin Exp Metastasis200926321522719116762
- ZhouCJiJCaiQMTA2 enhances colony formation and tumor growth of gastric cancer cells through IL-11BMC Cancer20151534325929737
- CovingtonKRBruscoLBaroneIMetastasis tumor-associated protein 2 enhances metastatic behavior and is associated with poor outcomes in estrogen receptor-negativeBreast Cancer Res Treat2013141337538424077732
