Abstract
Objective
We previously reported that the staging system and epidermal growth factor receptor (EGFR) mutation status are key factors for treatment strategy and predicting survival. However, the significance of these factors as predictors of overall survival (OS) and postoperative recurrence survival (PRS) has not been sufficiently elucidated. The objective here was to investigate EGFR mutation status and p-stage, which affect PRS and OS in patients with completely resected lung adenocarcinoma, using a different database.
Patients and methods
We retrospectively reviewed 56 consecutive lung adenocarcinoma patients with disease recurrence in St. Marianna University Hospital between January 2010 and December 2014.
Results
EGFR mutants (M) were detected in 16/56 patients (29%). The patients with EGFR M had a better OS than those with EGFR wild-type (WT) status (5-year survival: 50.3% vs 43.1, P=0.133). There was no significant difference in the 3-year recurrence-free survival rate between patients with M and WT (6.3% vs 7.7%, P=0.656), and the patients with EGFR M had a significantly better 3-year PRS than those with WT (77.4% vs 51.7%, P=0.033). The 3-year PRS rate for patients with M/pathologic stage (p-stage) I–II (87.5%) was better than that for patients with M/p-stage III (60.0%), WT/p-stage I–II (52.7%), and WT/p-stage III (43.8%). There was a significant difference between patients with M/p-stage I and WT/p-stage I–II or WT/p-stage III (P=0.021 and 0.030, respectively). During the study period, of the 16 patients with mutants, 12 patients (75%) received EGFR-tyrosine kinase inhibitor (TKI) therapy and among the 40 patients with WT, no patient received EGFR-TKI therapy. Multivariate survival analysis showed that patients with EGFR-TKI therapy had a statistically significant association with favorable PRS (hazard ratio 0.271; 95% confidence interval 0.074–1.000; P=0.050).
Conclusion
EGFR status and p-stage were found to be essential prognostic factors for estimating PRS using this database. The recurrent patients with EGFR M and EGFR-TKI therapy had a statistically significant association with favorable PRS.
Introduction
Surgical resection with a curative intent is considered to be the standard treatment for early-stage non-small-cell lung cancer (NSCLC). However, recurrence rates after complete resection for stage I to III of NSCLC are from 30% to 75% and depend on pathologic staging and follow-up period. Even with combined modality treatment, including chemotherapy, radiotherapy, or other therapeutic modalities, most patients with recurrence after resection have little possibility of cure.Citation1–Citation5 With the advancement of local and systemic therapies for recurrent NSCLC, survival after disease recurrence, which we defined as postoperative recurrence survival (PRS), has extensively improved.Citation1,Citation6 However, conclusive factors that affect PRS in NSCLC have not been discussed as widely as those that affect overall survival (OS) or recurrence-free survival (RFS) from the time of initial treatment.
The epidermal growth factor receptor (EGFR) mutation, which has been detected mostly in lung adenocarcinoma, is a predictor of response to EGFR tyrosine kinase inhibitors (TKIs), with 70%–80% of NSCLC patients receiving substantial benefits from these targeted therapies. EGFR mutation is reported to be a predictive and prognostic factor of EGFR-TKI therapy outcome.Citation7–Citation9 Testing for these mutations in all patients with recurrent/metastatic lung adenocarcinoma is, therefore, recommended in standard practice.
We previously reported that the staging system and EGFR mutation status are key factors for treatment strategy and survival prediction.Citation5 However, the significance of these factors as predictors of OS and PRS has not been sufficiently elucidated. This study aims to further investigate EGFR mutation status and pathologic stage (p-stage), which affect PRS and OS in patients with completely resected lung adenocarcinoma, using a different database during the standard treatment with EGFR-TKIs in patients with NSCLC with EGFR mutations.
Patients and methods
Patient selection
We retrospectively reviewed a total of 340 consecutive patients with adenocarcinoma who underwent lobectomy, bilobectomy, or pneumonectomy with systematic lymph node dissection at St. Marianna University Hospital from January 2010 to December 2014. Of these, 75 (22%) patients had recurrence of disease after surgery during median follow-up time of 36.6 months (range, 6.0–77.0 months). We excluded six patients who had received preoperative chemotherapy, radiotherapy, or both, or patients whose tumors had EGFR mutation in exon 20 (two patients because of resistance to EGFR-TKIs) or the echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase fusion gene (two patients). We excluded 11 patients who received good supportive care, including only palliative radiotherapy for controlling pain from bone metastasis, and no treatments such as radiotherapy, chemotherapy, or surgical treatment after disease recurrence. We enrolled the remaining 56 patients with postoperative recurrent disease in this study.
Data collection
Preoperative evaluation included physical examination, chest radiography, computed tomography (CT) scan of the chest and abdomen, blood examination, and positron emission tomography-CT scan. Histologic subtypes of lung cancer were determined according to the World Health Organization classification.Citation10 Determination of the tumor stages was based on the Seventh TNM Classification for Lung and Pleural Tumors.Citation11
All patients were followed up at our hospital every 3 months in the first year, every 6 months from the second to the fifth year, and annually thereafter on an outpatient basis, and were aimed at continuing follow-up for 10 years. Follow-up procedures included physical examination, chest radiography, and blood examination (including serum tumor markers). CT scans of chest and upper abdomen were routinely done in every scheduled outpatient department visit at our hospital for follow-up. Whenever any symptoms or signs of recurrence were detected, magnetic resonance imaging of the brain and bone scintigraphy were performed. Once a metastasis was discovered by physical examination and diagnostic imaging, histologic or cytologic confirmation of the metastatic site was performed when clinically feasible. Local recurrence was defined as disease recurrence at the surgical margin, ipsilateral hemithorax, or mediastinum. Distant metastasis was defined as disease recurrence in the contralateral lung or outside the hemithorax and mediastinum. A second primary tumor was recorded when a patient presented with a new histologic type and with clinical features consistent with a new primary tumor.
The hospital charts of all patients were reviewed to collect clinicopathologic data including age, sex, smoking history, tumor location, operative procedure, histologic type, tumor size, vascular invasion, lymphatic permeation, visceral pleural invasion, EGFR mutation status, administration of EGFR-TKI therapy, recurrence pattern, OS, RFS, and PRS. The institutional review board of the St Marianna University School of Medicine approved the protocols for data collection and analyses. The requirement for written informed consent from the patients was waived because of the retrospective study design. All patient data were maintained confidentially throughout this research.
Statistical analysis
OS, RFS, and PRS were estimated using the Kaplan–Meier method, and survival differences between patient groups were determined using log-rank analysis. OS was defined as the time elapsed from the date of pulmonary resection to the date of death. The length of PRS was defined as the interval between the date of initial recurrence identification and the date of either death or the last follow-up. RFS was defined as the time elapsed from the date of pulmonary resection to the date of the first tumor recurrence.
Univariate analysis was conducted between the groups. Significance in a 2×2 contingency table was tested by using the χ2 test. P-values and hazard ratios in the multivariate analyses were calculated using the Cox regression model. All P-values <0.05 were considered to indicate a statistically significant difference. All statistical calculations were performed using the SPSS statistical software package (version 21.0; SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
The characteristics of the patients are listed in . Of the 56 patients, EGFR mutants (M) were detected in 16 patients (29%). The most common EGFR mutation was in-frame deletion in exon 19 (8/16; 50%). The second most common mutation was missense mutation (mostly L858R) in exon 21 in seven patients (44%). Regarding the initial recurrence patterns, 35 patients (64%) had distant or local and distant recurrence and 21 patients (36%) had only local recurrence. Of the 16 patients with mutants, 12 patients (75%) received EGFR-TKI therapy. Among the 40 patients with wild-type (WT) status, no patient received EGFR-TKI therapy.
Table 1 Patient characteristics (N=56)
Survival
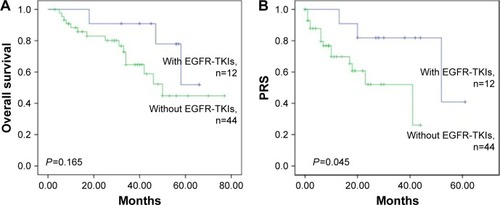
The median follow-up time for all 56 patients was 36.6 months (range, 6.0–77.0 months). The median PRS time for all 56 patients was 16.7 months (range, 0.7–61.0 months). The 5-year OS rates of patients with EGFR M and WT were 50.3% and 43.1%, respectively (). There was no significant difference between them (P=0.133). The 3-year RFS rates of patients with EGFR M and WT were 6.3% and 7.7%, respectively (P=0.656; ). The 3-year PRS of patients with EGFR M was significantly longer than that of patients with WT (77.4% vs 51.7%, P=0.033; ). shows the PRS curves stratified according to the EGFR status and the p-stage. The 3-year PRS rates for the patients with EGFR M with p-I–II, EGFR M with p-III, WT with p-I–II, and WT with p-III were 87.5%, 60.0%, 52.7%, and 43.8%, respectively. There were statistically significant differences in the PRS rates between the patients with EGFR M with p-I–II and those with WT with p-I–II (P=0.030) and between the patients with EGFR M with p-I–II and those with WT with p-III (P=0.021). There were no significant differences between the patients with EGFR M with p-III and those with WT with p-I–II (P=0.468). shows that there was a significant difference in PRS between patients with and without EGFR-TKIs (P=0.045), but no significant difference in OS between the two groups (P=0.165).
Figure 1 (A) Overall survival stratified according to EGFR mutants and WT. (B) RFS stratified according to EGFR mutants and WT. (C) PRS stratified according to EGFR mutants and WT. Significant differences in the PRS rates were observed between the patients with mutants and WT (P=0.030). (D) PRS stratified according to EGFR status and p-stage. Significant differences in the PRS rates were observed between the patients with mutants/p-stage I–II and WT/p-stage I–II (P=0.030) and between the patients with mutants/p-stage I–II and WT/p-stage III (P=0.021).
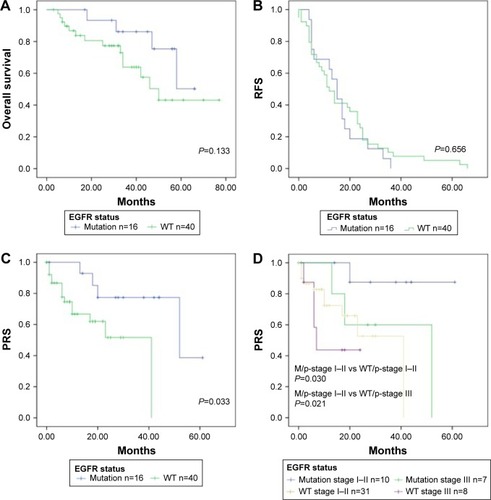
Figure 2 (A) Overall survival stratified according to whether EGFR-TKIs were administered or not. (B) RFS stratified according to whether EGFR-TKIs were administered or not. Significant differences in the PRS rates were observed between the patients with and without EGFR-TKIs (P=0.045).
Prognostic factors
Of the 16 patients with mutants, 12 patients (75%) received EGFR-TKI therapy. Among the 40 patients with WT, no patient received EGFR-TKI therapy. During the study period, EGFR-TKIs were recommended for most recurrent or metastatic patients with lung adenocarcinoma with EGFR M as the standard practice; we, therefore, selected administration of EGFR-TKI for this prognostic analysis. The potential prognostic factors for PRS were analyzed using univariate survival analysis (), which showed that sex and EGFR-TKI therapy were significant prognostic factors. Multivariate survival analysis () showed that patients with EGFR-TKI therapy had a statistically significant association with favorable PRS (hazard ratio 0.271; 95% confidence interval 0.074–1.000; P=0.050).
Table 2 Univariate and multivariate analyses for PRS (N=56)
Discussion
Personalization of lung cancer treatment requires predictive biomarkers that have been validated by correlation between tumor features and outcomes after therapy. Several mutations have been identified in EGFR in NSCLC. Mutation status in this gene is thought to be an important predictor of response to EGFR-TKIs, with 70%–80% of NSCLC patients receiving substantial benefits from this targeted therapy.Citation7 EGFR mutation is both a predictive and prognostic factor of EGFR-TKI therapy outcome.Citation7,Citation12 Testing for these mutations in all patients with recurrent or metastatic lung adenocarcinoma is, therefore, recommended in standard practice.
We aimed to 1) identify the prognostic factors of PRS in patients who underwent complete resection of lung adenocarcinoma, 2) clarify whether EGFR mutation status and p-stage can be considered as conclusive indicators of OS or PRS, and 3) elucidate whether the EGFR-TKI therapy and p-stage at surgery affect prediction of PRS. We found that having EGFR mutation itself was a favorable PRS factor, but neither a predicting OS factor nor a disease free survival factor. Although p-stage at surgery itself may not affect the prediction of PRS, PRS outcomes were stratified by EGFR mutation status and p-stage. Moreover, whether EGFR-TKI therapy was administered was the most independent prognostic factor in patients who underwent complete resection of lung adenocarcinoma and relapsed.
Although several studies have reported on the PRS of patients with NSCLC,Citation1–Citation3,Citation6,Citation13,Citation14 no standard treatment strategy has been established for the patients with recurrence based on prospective studies. The concept of PRS of surgically treated patients has been the focus of much attention recently because encouraging new treatments such as EGFR-TKIs,Citation7–Citation9 anaplastic lymphoma kinase inhibitors,Citation15 vascular endothelial growth factor antibody,Citation16 or recently, immune check point inhibitorsCitation17,Citation18 have provided long-term PRS and better quality of life to selected patients with recurrent or metastatic disease. Several retrospective studies, in addition to our own recently published study, have reported that shorter relapse-free interval, initial recurrence pattern, lymph node metastasis pattern, tumor differentiation, EGFR status, and administration of EGFR-TKIs and the p-stage are independent prognostic factors for PRS.Citation1,Citation5,Citation6,Citation13,Citation19–Citation23 In our current series, EGFR-TKI administration was shown to be a strong independent prognostic factor for estimating PRS. Of the 16 relapse patients with EGFR mutants, 12 patients (75%) received EGFR-TKI therapy. Because our series enrolled from 2010 when analysis of EGFR status are common and EGFR-TKIs are already thought as a standard first-line therapy in practice setting for metastatic lung cancer patients with EGFR mutant.Citation7–Citation9,Citation24 Our results, however, demonstrated that p-stage at surgery may not affect PRS, suggesting that p-stage at surgery does not correlate with survival interval after relapse.
Two studies have reported that EGFR mutations have an impact on prognosis after surgical resection of NSCLCCitation25,Citation26 and that the mutation status of EGFR can serve as an independent prognostic marker associated with decreased recurrence and improved progressionfree survival and OS in patients with stage I lung adenocarcinoma.Citation25 This study, however, restricted to patients who had no adjuvant or neoadjuvant systemic therapy administered and focused solely on the postoperative prognostic differences among different genotypes, showed that the significant prognostic impact of EGFR mutation was lost after adjusting for other confounding prognostic factors.Citation26 Other studies in the literature corroborate this finding.Citation27,Citation28 Additionally, we showed in our previous study that EGFR mutation status and the use of EGFR-TKI therapy particularly affected the PRS among patients who underwent surgical resection for lung cancers,Citation5 and that the results could also affect OS in general. In this study of a small sample and a short median follow-up time, however, we found that the EGFR mutation status could affect only PRS, but not OS.
There are several limitations and biases of this study that should be taken into account for interpreting the results. As a retrospective single-institute study, patient selection bias and time trend bias regarding the treatment for recurrent diseases were inevitable. Because follow-up examination of the patients after the initial resection was comparatively uniform, systematic follow-up is suggested to increase the early detection of recurrent lesions to which we could apply intensive treatment. Recently, it has been shown there are some differences in response to EGFR-TKIs among EGFR mutation subtypes.Citation29 Because of the small size of sample in our study, we could not analyze these differences.
Conclusion
We retrospectively reviewed 56 consecutive lung adenocarcinoma patients with disease recurrence in our hospital between January 2010 and December 2014. EGFR status and p-stage were also found to be essential prognostic factors for estimating PRS using this database. The recurrent lung adenocarcinoma patients with EGFR M and EGFR-TKI therapy had a statistically significant association with favorable PRS. Advances in treatment strategy for PRS may provide improvement in OS among patients who undergo surgery.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research, Japan Society for the Promotion of Science (24592104), Ministry of Education, Culture, Sports, Science and Technology, Japan.
Disclosure
The authors report no conflicts of interest in this work.
References
- YoshinoIYohenaTKitajimaMSurvival of non-small cell lung cancer patients with postoperative recurrence at distant organsAnn Thorac Cardiovasc Surg20017420420911578260
- HungJJHsuWHHsiehCCPost-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrenceThorax200964319219619252018
- HungJJJengWJHsuWHPrognostic factors of postrecurrence survival in completely resected stage I non-small cell lung cancer with distant metastasisThorax201065324124520335294
- ShimadaYSajiHYoshidaKPrognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancerChest201314361626163423348916
- KudoYShimadaYSajiHPrognostic factors for survival after recurrence in patients with completely resected lung adenocarcinoma: important roles of epidermal growth factor receptor mutation status and the current staging systemClin Lung Cancer2015166e213e22125986624
- SugimuraHNicholsFCYangPSurvival after recurrent nonsmall-cell lung cancer after complete pulmonary resectionAnn Thorac Surg2007832409417 discussioin 417–41817257962
- MokTSWuYLThongprasertSGefitinib or carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J Med20093611094795719692680
- MaemondoMInoueAKobayashiKGefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFRN Engl J Med2010362252380238820573926
- MitsudomiTMoritaSYatabeYGefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trialLancet Oncol201011212112820022809
- International Agency for Research on CancerTravisWDBrambillaEBurkeAMarxANicholsonAGWHO Classification of Tumours of the Lung, Pleura, Thymus and Heart4th edGeneva, SwitzerlandWorld Health Organization2015
- SobinLHGospodarowiczMKWittekindCInternational Union against Cancer, ebrary IncTNM Classification of Malignant Tumours7th edChichester, West Sussex, UK; Hoboken, NJWiley-Blackwell2009 Available from: http://site.ebrary.com/lib/yale/Doc?id=10342913Accessed June 10, 2017
- EberhardDAJohnsonBEAmlerLCMutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinibJ Clin Oncol200523255900590916043828
- WilliamsBASugimuraHEndoCPredicting postrecurrence survival among completely resected nonsmall-cell lung cancer patientsAnn Thorac Surg20068131021102716488713
- NakagawaTOkumuraNOhataKIgaiHMatsuokaTKameyamaKPostrecurrence survival in patients with stage I non-small cell lung cancerEur J Cardiothorac Surg200834349950418579404
- KwakELBangYJCamidgeDRAnaplastic lymphoma kinase inhibition in non-small-cell lung cancerN Engl J Med2010363181693170320979469
- SandlerAGrayRPerryMCPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med2006355242542255017167137
- BrahmerJReckampKLBaasPNivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancerN Engl J Med2015373212313526028407
- ReckMRodriguez-AbreuDRobinsonAGKEYNOTE-024 InvestigatorsPembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancerN Engl J Med2016375191823183327718847
- OkamiJTaniguchiKHigashiyamaMPrognostic factors for gefitinib-treated postoperative recurrence in non-small cell lung cancerOncology2007723–423424218176089
- SaishoSYasudaKMaedaAPost-recurrence survival of patients with non-small-cell lung cancer after curative resection with or without induction/adjuvant chemotherapyInteract Cardiovasc Thorac Surg201316216617223143203
- JeonJHKangCHKimHSSeongYWParkIKKimYTPrognostic and predictive role of epidermal growth factor receptor mutation in recurrent pulmonary adenocarcinoma after curative resectionEur J Cardiothorac Surg201547355656224760387
- SonobeMYamadaTSatoMIdentification of subsets of patients with favorable prognosis after recurrence in completely resected non-small cell lung cancerAnn Surg Oncol20142182546255424633668
- TakenakaTTakenoyamaMYamaguchiMImpact of the epidermal growth factor receptor mutation status on the post-recurrence survival of patients with surgically resected non-small-cell lung cancerEur J Cardiothorac Surg201547355055524894095
- JiangHOverview of gefitinib in non-small cell lung cancer: an Asian perspectiveJpn J Clin Oncol200939313715019088154
- IzarBSequistLLeeMThe impact of EGFR mutation status on outcomes in patients with resected stage I non-small cell lung cancersAnn Thorac Surg201396396296823932319
- KosakaTYatabeYOnozatoRKuwanoHMitsudomiTPrognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinomaJ Thorac Oncol200941222919096302
- MarksJLBroderickSZhouQPrognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinomaJ Thorac Oncol20083211111618303429
- KimYTSeongYWJungYJThe presence of mutations in epidermal growth factor receptor gene is not a prognostic factor for long-term outcome after surgical resection of non-small-cell lung cancerJ Thorac Oncol20138217117823287850
- YangJCWuYLSchulerMAfatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trialsLancet Oncol201516214115125589191
