Abstract
Purpose
To determine the value of radioembolization (RE) for treatment of unresectable hepatocellular carcinoma (HCC).
Patients and methods
Records of patients undergoing RE for unresectable HCC were retrospectively reviewed. Biochemical and clinical toxicities, imaging response (according to modified Response Evaluation Criteria In Solid Tumors), time-to-progression (TTP) and overall survival (OS) were analyzed. Data were stratified according to clinical and procedural parameters. Univariate and multivariate analyses were performed.
Results
One hundred and fifteen patients (89 male, mean age 69.3 years) underwent 158 REs (119 resin-, 39 glass-based) (Barcelona Clinic Liver Cancer [BCLC]-A: 6.1%, B: 33.9%, C: 60.0%). Median clinical follow-up was 5.9 (0.9–83.5) months. No grade 4 or 5 clinical toxicities were noted. Objective response rate was 35.6%; disease control rate was 76.7%. Median TTP of the treated part of the liver was 4 (0.9–45.4) months. 108/115 patients died during follow-up (median OS 8.4 [0.3–82.8] months after first RE [BCLC-A: 52.8 months, BCLC-B: 12.4 months, BCLC-C: 6.1 months]). On multivariate analysis, baseline Eastern Co-operative Oncology Group status <1, ascites prior to RE and best imaging response were predictors of longer OS. In BCLC-C patients, tumor burden, ascites prior to RE, baseline gamma-glutamyltransferase and Child–Pugh score were predictive of OS.
Conclusions
RE is safe and effective in carefully selected patients suffering from HCC with a low complication rate. Low baseline Eastern Co-operative Oncology Group status and absence of ascites prior to RE are positive prognostic factors.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy and one of the leading causes of cancer-related death worldwide.Citation1,Citation2
Complete resection, liver transplantation or – within certain limits – ablative therapies as the only curative treatment options are feasible in just 30% of patients.Citation2 In early and intermediate stage HCC, loco-regional treatments like radiofrequency ablation or transarterial chemoembolization (TACE) or combination thereof are frequently employed.Citation3–Citation11 In advanced HCC, systemic molecular targeted therapy with sorafenib has demonstrated a statistically significant, but limited increase in overall survival (OS), though this is achieved with a rather high rate of adverse events.Citation12–Citation14 Alternative treatment options are therefore necessary, especially in cases of advanced HCC associated with portal vein thrombosis, which is associated with a particularly poor prognosis (median survival 2–4 months with best supportive care).Citation15–Citation17
Radioembolization (RE) has been introduced as an alternative treatment for intermediate and advanced stage HCCCitation18 and was already shown to result in longer time- to-progression (TTP) compared with conventional TACE in early and intermediate stage HCC.Citation19 The aim of the present study was to determine the value of RE in the treatment of unresectable HCC across a range of tumor stages and to determine prognostic parameters.
Patients and methods
Patients and data acquisition
Patients suffering from HCC undergoing RE at our institution between 09/2006 and 02/2015 were identified in the clinical database. Indications to perform RE were discussed in interdisciplinary consensus conferences in accordance with published indications for RE.Citation20,Citation21 Typical indications for RE at our institution were progressive disease (PD) under a TACE treatment regime, contraindications for TACE/chemotherapy (eg, portal vein thrombosis/invasion, cardiac comorbidities) as well as impaired hemostasis (as RE usually only requires 2 angiography sessions compared with often multiple TACE sessions). All patients presented with HCC that was deemed unresectable by an experienced liver surgeon. Repeated RE of the same part of the liver was offered to selected patients showing disease progression after primary RE in interdisciplinary consensus according to the individual clinical situation of the patient. The study was approved by the local institutional review board of the University Hospital of Bonn; informed patient consent was waived due to the retrospective character of the study.
Study inclusion criteria were histologically confirmed HCC or hepatic malignancy showing imaging characteristics typical of HCC (according to the EASL–EORTC criteriaCitation22), RE performed at our institution and accessible patient data. Baseline clinical and laboratory parameters, procedural data and results of follow-up examinations were retrospectively reviewed. Baseline cross-sectional imaging was used to estimate the relative liver tumor burden as <25%, 25%–50% or >50%.
Treatment
Pre-treatment workup, including clinical/laboratory examination and cross-sectional imaging (preferentially magnetic resonance imaging [MRI] of the liver), was performed according to clinical standards.Citation21,Citation23 Pre-treatment catheter angiography with injection of Technetium-99m macroaggregated albumin into the target arteries followed by single photon emission computed tomography/CT was performed to exclude non-target sphere deposition. Vessels supplying extrahepatic tissue originating from the hepatic artery were coilembolized at the discretion of the interventionalist. Treatment activity was calculated in compliance with international consensus guidelinesCitation21 (body surface area [BSA] method for resin microspheres [SIR spheres, Sirtex Medical Limited, North Sydney, Australia] and the MIRD equation as provided by the manufacturer for glass microspheres [TheraSphere, BTG, London, UK]). RE was performed either in a single session (simultaneous bilobar or unilobar) or a sequential lobar approach depending on tumor distribution and liver function using a microcatheter (eg, Renegade, Boston Scientific, Natick, MA, USA). Glass microspheres were generally preferred in cases with portal vein thrombosis (especially if the main portal vein was affected), in patients with unilobar disease who were planned to received unilobar RE without the need for catheter repositioning, as well as for repeat RE. Peri-interventional medication included dexamethasone, ondansetron and pantoprazole. All patients were admitted to a special ward for 2 days after RE, in accordance with local radioprotection regulations of the Federal Office for Radiation Protection in Germany (BfS).
Toxicities
The Common Terminology Criteria for Adverse Events of the National Cancer Institute (CTCAE v. 4.03) were used for assessment of toxicities of biochemical parameters both before (baseline) and after RE (bilirubin, aspartate aminotransferase [AST], alanine aminotransferase, gamma glutamyl transferase [GGT]), the presence and development of ascites and clinical adverse events. Procedure-related clinical complications, for example, gastroduodenal ulceration due to non-target embolization, were noted.
Follow-up regime
Patients underwent 2 early follow-up examinations 4–6 weeks and 3 months after RE, including physical examination, laboratory liver function tests and contrast-enhanced standard MRI or CT of the liver. After that, follow-up examinations at 3-month intervals were recommended.
Hepatic response
Imaging response was assessed by a radiologist, blinded to patients’ overall outcome, on arterial-phase cross-sectional imaging according to modified Response Evaluation Criteria In Solid Tumors (mRECIST) criteria.Citation24 Two target lesions were selected in cases of single session whole liver treatment. In patients receiving sequential lobar or unilobar treatment, 2 lesions per lobe were selected to separately assess treatment response for the treated and non-treated liver lobe. Best imaging response (best response to treatment over the entire follow-up period) is reported separately for single session treatments (simultaneous bilobar or unilobar) and for sequential bilobar treatment (after completion of the treatment cycle) as well as for repeated RE of previously treated tissue.
Definitions and statistical analysis
OS was defined as the time between the first RE procedure performed in a patient and death of any cause. TTP was defined as the time between RE and first detected progression and was calculated separately for treated (time to hepatic progression [TTHP]treated) and untreated parts of the liver (TTHPuntreated) as well as for extrahepatic progression (time to extrahepatic progression [TTEP]) (first detection of new extrahepatic lesions or increase of the long axis of an existing lesion by >20%).
Statistical analyses were performed using commercially available statistical software (SPSS, version 22.0, IBM, Armonk, NY, USA). Univariate Kaplan–Meier analysis with a log-rank test for statistical significance was performed to evaluate associations of OS with clinical parameters for categorical parameters. A Cox regression model was used for continuous variables. Stepwise multivariate Cox regression was then performed to identify independent predictors of OS and to calculate hazard ratio (HR) estimates for all parameters showing significant associations on univariate analysis. All analyses were performed for the whole group and the subgroups of patients with Barcelona Clinic Liver Cancer (BCLC) stage B and C separately.
Results
Patient characteristics
Overall 115 patients (89 male, mean age 69.3±10.6 years) underwent RE for unresectable HCC. Detailed patient characteristics are given in . A total of 158 RE procedures were performed with 18 patients receiving single session whole liver treatment (16 resin-, 2 glass-based REs; 10 with lobar sphere application, 8 with application via the proper hepatic artery), 78 patients receiving unilobar treatment (56 resin, 22 glass) and 19 patients with sequential bilobar treatment (either resin [n=30] or glass [n=8]) (median time between procedures 2.5 [0.9–32.2] months). Mean administered activities are given in . In sequential bilobar treatment, cumulative activity was 3.19±1.24 GBq. Twenty-one patients received a second RE of the treated part of the liver (16 resin, 5 glass); 3 patients received a third RE (1 resin, 2 glass) (median time between primary and repeated procedures 5.5 [1.2–44.8] months).
Table 1 Patient baseline characteristics and procedural data
Median time between baseline imaging and RE was 0.7 (0–5.3) months. Twenty-five patients were lost to imaging follow-up, 5 of these did not survive until first follow-up or were terminally ill at that time. Median imaging follow-up was 5.9 (0.9–61.6) months. Clinical follow-up was available in 103/115 patients (median clinical follow-up 5.9 [0.9–83.5] months). Six out of 115 patients were alive on the date of analysis (median time after RE 17.6 [14.9–82.8] months). The status of 1 patient was unknown.
Toxicities
No access-related complications were observed. During RE, dissection of the right hepatic artery occurred in 2 cases without the need for treatment. Three patients (2.6%) presented with grade 2 gastroduodenal ulceration (1 after whole liver, 2 after right lobar treatment). The gastroduodenal artery, but not the right gastric artery was previously embolized in all 3 cases. One of the 3 cases of gastroduodenal ulceration occurred in a patient showing severe blood-flow stasis (only 55% of the intended activity administered). In 1 patient, dyspnea developed 2 months after RE, so radiation-induced pneumonitis was suspected. The patient, however, did not require any treatment. One patient developed septicemia 2 days after completion of a sequential bilobar RE cycle and died 7 days later. This patient did not present with dilated bile ducts or signs of cholangitis. Otherwise, no grade 4 or 5 clinical toxicities were noted.
During follow-up, 50 patients developed new or worsening ascites (all ≤ grade 2); 25 of these cases were observed within the first 3 months after RE (after sequential RE: 6/18 [33.3%]; single session bilobar RE: 5/19 [26.3%], unilobar RE: 8/78 [10.3%], repeated RE: 6/21 [28.6%]).
Biochemical toxicities are summarized in and . After RE, the majority of patients showed no or only grade ≤2 toxicities in investigated laboratory parameters. Patients receiving sequential lobar treatment showed a permanent increase in bilirubin levels in 61.1% (11/18), while patients receiving whole liver treatment in a single session or unilobar treatment showed permanent changes in only about 29% (21/72) of cases. Otherwise, changes in laboratory toxicity levels were comparable between the different treatment strategies. Of note, GGT levels were already elevated to grade 3 or 4 in 52/109 patients at baseline. After repeated RE, 8/21 patients (38.1%) showed an increase from grade 1 or 2 to grade 3 or 4 bilirubin toxicity.
Table 2 Laboratory and clinical toxicities according to CTCAE v. 4.0
Table 3 Increase in laboratory toxicity levels after radioembolization (according to CTCAE v. 4.0)
Tumor response and TTP
Imaging response is summarized in . An objective response (ORR) rate (complete response + partial response [CR + PR]) of 43.3% and a disease control rate (DCR) (CR + PR + stable disease [SD]) of 83.3% were observed for the treated liver area. For the whole liver (including untreated tissue), ORR and DCR were 35.6% and 76.6%, respectively. In patients undergoing repeated RE (n=21), imaging follow-up was available in 16 cases (ORR and DCR 31.3% and 68.8%, respectively).
Table 4 Best imaging response to radioembolization in patients with available imaging follow-up according to mRECIST-criteria on a per-patient basis after completion of a RE-cycle
Overall intrahepatic disease progression of the treated part of the liver was observed in 40/90 patients (44.4%) with imaging follow-up. Median TTHPtreated was 4 (0.9–45.4) months. Twenty-one out of 40 patients (52.5%) with disease progression in the treated part of the liver showed new tumor manifestations in the treated liver lobe, 7/40 (17.5%) showed growth of treated tumors and 12/40 (30%) demonstrated both.
Progression in the untreated part was observed in 32/72 patients (44.4%) with a median TTHPuntreated of 2 (0.9–45.6) months. Twenty out of 32 patients (62.5%) showed new tumor manifestations in the untreated part of the liver, 5/32 (15.6%) showed growth of existing tumor and 7/32 (21.9%) showed both.
Eleven out of 90 patients (12.2%) showed progression of extrahepatic disease during imaging follow-up (median TTEP 2.9 [1–43.7] months).
Patient survival
Thirty-day mortality rate was 2.6% (3/115 patients). A 65-year-old patient (BCLC-C, ECOG [Eastern Co-operative Oncology Group] 2, tumor burden >50%, baseline bilirubin 3 mg/dL) received resin-based unilobar treatment with 3.3 GBq early in our institutional experience with RE and died 9 days after RE. A 79-year-old patient (BCLC-C, ECOG 1, tumor burden 25%–50%, baseline bilirubin of 1.5 mg/dL) with tumor invasion into the portal vein received unilobar treatment with 2 GBq and died 21 days later. A third patient (71 years old) developed septicemia 2 days after RE and died 7 days later.
Median OS after first RE was 8.4 (0.3–82.8) months (BCLC-A: 52.8 months, BCLC-B: 12.4 months, BCLC-C: 6.1 months). Results of uni- and multivariate analyses of OS are summarized in . For the whole patient group, univariate analysis of OS showed significant interrelations with the ECOG status, liver tumor burden, BCLC status, Model for End-Stage Liver Disease (MELD) score, presence of ascites prior to RE, previous liver resection, baseline levels of bilirubin, AST, GGT and best overall response, TTP and repeated RE. On multivariate analysis, the ECOG status (p=0.001, HR for ECOG 0 vs ≥1: 0.366), the presence and extent of ascites prior to RE (p=0.029, HR for no vs extensive ascites: 0.039 and minimal vs extensive ascites: 0.044) and best overall mRECIST response (p<0.0001, HR for CR, PR and SD vs PD: 0.071, 0.331 and 1.135, respectively) were identified as independent predictors of OS. There was a trend toward longer OS in patients without baseline bilirubin toxicity (p=0.052, HR 0.543) ().
Figure 1 Kaplan–Meier curves.
Abbreviations: ECOG, Eastern Co-operative Oncology Group; OS, overall survival; RE, radioembolization.
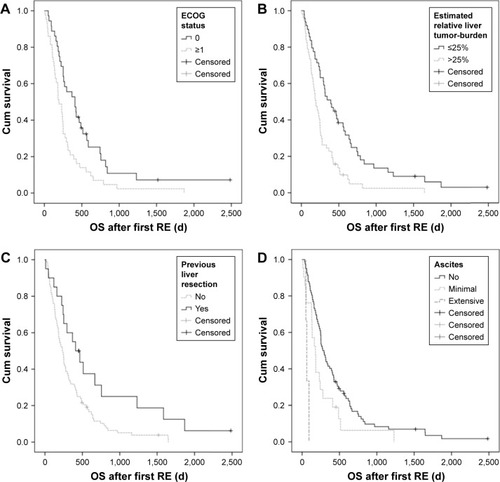
Table 5 Results of univariate analysis of overall survival after first radioembolization
Analysis of BCLC-B patients did not show any significant associations of patient-related factors and survival. In BCLC-C patients, univariate analysis showed significant interrelations of OS and patient age, liver tumor burden, Child–Pugh status, MELD score, presence of ascites prior to intervention, previous liver resection, previous application of sorafenib, baseline levels of bilirubin or GGT and repeated RE. On multivariate analysis, presence and extent of ascites prior to RE (p=0.008, HR for no vs extensive ascites: 0.156 and minimal vs extensive ascites: 0.266), baseline GGT (p=0.011, HR for ≤1 vs >1: 0.288) and Child–Pugh score (p<0.031, HR for Child–Pugh A vs B: 0.412) were identified as independent predictors of OS.
Discussion
Although TACE or sorafenib therapy can improve survival in HCC patients,Citation2,Citation12 tolerability (eg, toxicities in the majority of patients with sorafenib)Citation13 and applicability (eg, TACE in patients with portal vein thrombosis) remain problematic. Liver failure is the cause of death in about 90% of advanced HCC, even in the presence of extrahepatic metastases, which underlines the importance of liver-directed therapy.Citation2,Citation25–Citation29 RE has been investigated in several small studies with promising results. However, until now, only a limited number of studies, including >100 patients has been published.Citation30–Citation32
Clinically, disease control and ORRs of RE for HCC are ranging from 77% to 90%Citation30,Citation33,Citation34 to 40%–57%,Citation30,Citation32 respectively. Considering the dismal prognosis, especially of advanced stage HCCCitation35 with otherwise only limited therapeutic options, these are noteworthy treatment effects, comparable with response rates of TACE.Citation36 TACE, however, is relatively contraindicated in patients with portal vein occlusion, limiting its application in this subgroup of patients.Citation37 In addition, several TACE treatment sessions are usually necessary for successful therapy.
Early progression after RE primarily seems to depend on baseline characteristics like tumor distribution and extent rather than on procedural factors.Citation33 This was also true in our patients. Since a higher percentage of patients in our study suffered from intermediate and advanced stage HCC compared with previous studies (95.7% vs 80%), patients in our cohort showed an overall shorter median TTP (4 months vs 7.9–10 months).Citation30,Citation32 Hepatic progression in general may arise from an increase in known tumor masses or new manifestations in treated liver areas. In unilobar therapy, untreated contralateral disease may progress or new manifestations may become detectable. In this respect, it has been suggested that disease progression after RE of HCC is associated with new tumor manifestations rather than growth of treated tumor.Citation38–Citation40 This is corroborated by comparable rates of disease progression in treated and untreated liver lobes with progression only developing later in treated liver in our cohort. Development of new tumor nodules was associated with progression in 82.5% of treated and 84.4% of untreated liver. While, especially small tumor nodules (<3 cm) show a high rate of histological complete tumor response after RE (89%),Citation41 microscopic tumor manifestations may be insufficiently treated due to lack of neoangiogenic vessels and may, therefore, give rise to post RE disease progression.Citation40
Extrahepatic disease was already present in 35% of our patients at the time of RE, with only 12% showing extrahepatic progression on follow-up. Moreover, as liver failure is known to be the cause of death in the majority of patients,Citation2,Citation25–Citation29 extrahepatic disease does not seem to be a contraindication for RE.
The importance of liver-directed treatment is reflected in the fact that OS can be considerably increased by implementing RE into the treatment algorithm. Patients with untreated HCC have a median survival of only 9 months after initial diagnosis (BCLC-A: 25 months, B: 10 months, C: 7 months, D: 6 months).Citation35 As RE was often performed rather late in the sequence of treatment options in our cohort, patients had already survived longer after initial diagnosis than they would have without any treatment (time from diagnosis to RE: BCLC A: 26.4 months, B: 17.3 months, C: 12 months). The added value of RE is reflected in considerably longer cumulative OS after initial diagnosis that amounted to a total of 77 months in BCLC-A, 29.7 months in BCLC-B and 18.1 months in BCLC-C in our patients. This is particularly interesting, since the added value of sorafenib in advanced stage HCC is a prolongation of mean OS of only 6 weeks.Citation12–Citation14
Patients receiving either resin- or glass-based RE are reported to have a median OS of 13–16 months after treatment (BCLC-A: 24.4–26.9 months, B: 16.9–17.2 months, C: 6.0–10.0 months).Citation30–Citation32 Patients in our cohort showed a shorter OS of 8.4 months, which was mainly influenced by the high rate of advanced stage HCC (median OS 6.1 months). Our patient cohort is comparable with the recently published prospective randomized controlled SARAH trial as far as a high percentage of BCLC-C patients was concerned. A slightly longer median OS in the SARAH trial (9.9 months vs 8.4 months) may be explained by the fact that we included patients with an ECOG status of 2 in the first years of our experience with RE over 10 years ago (about 19% of the patient cohort) that were excluded in the SARAH trail. According to current inclusion criteria for RE, patients with an ECOG status of 2 would no longer be treated by RE. Compared with the SIRveNIB trail that included less BCLC-C patients (35% vs 60%) and also did not include ECOG 2 patients, OS was also longer in the SIRveNIB trial (11.3 months vs 8.4 months).Citation42,Citation43 Of note, median OS in the small group of patients with BCLC stage A in our cohort was considerably higher compared with previous reports (median OS 50.6 months). High tumor response rates do not generally translate into longer OS: while response rates are comparable for HCC treated with RE and TACE, OS is known to be significantly longer after RE.Citation36 This can be due to different tumor and patient characteristics. In our patients, especially a low ECOG status and the absence of ascites prior to RE were predictive of longer OS. Furthermore, already identified prognostic factors include patient age, gender, presence of portal hypertension, extend and distribution of tumor burden, several laboratory values and extrahepatic disease.Citation31,Citation32 Considering these factors, lower OS in our study may be explained by the fact that our patients showed adverse prognostic parameters in a larger percentage than in previously published reports. Among other factors, patients had a higher ECOG status (ECOG 0: 45.2% vs 54%–56% in previous reports), a higher tumor burden (≥25%: 53.1% vs 23.4% or >5 tumor nodules: 67.8% vs 38.6%) and a higher incidence of extrahepatic disease (37.4% vs 9.2%–16%).Citation31,Citation32
Especially in BCLC-C patients, adequate patient selection is imperative. Patients with complete portal vein thrombosis, who are typically no candidates for TACE,Citation44 have a dismal prognosis (median OS 2–4 months with best supportive care, 3–6 months after RE).Citation15,Citation17,Citation34,Citation45 We also found a median OS of 6.0 months in patients presenting with macroscopic vascular invasion. However, selected BCLC-C patients with a low tumor burden, absence of ascites, low baseline GGT levels and Child–Pugh status A showed an OS of up to 18.9 months after RE, which is even above the previously reported median OS of intermediate stage patients.
There is ongoing debate on how to deal with recurrent progression after successful RE, especially as impaired hepatic function has to be taken into account when considering repeated liver-directed treatment. We offered repeated RE to patients with preserved liver function,Citation21 developing recurrent disease progression after initial RE. We found that tumor control could be achieved in nearly 70% (11/16) of repeated REs with a significantly longer OS in patients receiving repeat RE. Although disease progression can be stopped in a substantial percentage of patients receiving repeat RE, the fact that liver function had to be preserved to be considered for repeat RE certainly also influenced OS. However, appropriately selected BCLC-C patients seem to benefit from repeat RE (OS 17.1 vs 5.2 months). Further systematic investigation into adequate patient selection for repeated RE, therefore, seems to be warranted.
Positive treatment effects are usually associated with a certain degree of adverse events. This holds especially true in advanced stage HCC patients treated with sorafenib who experience adverse events in up to 80%, often warranting dose reduction, which, in turn, may have a detrimental effect on treatment efficacy.Citation12–Citation14 By comparison, RE is associated with a rather low toxicity profile in appropriately selected patients.Citation46,Citation47 Severe adverse events ≥ grade 3 (eg, gastroduodenal ulceration) occur in <5% of procedures.Citation48,Citation49 We observed grade 2 gastroduodenal ulceration in 2.6% of cases although the gastroduodenal artery was coil-embolized. Additional vessels not identified on angiography and MAA test injection (eg, newly formed collaterals after coil-embolization of the gastroduodenal artery) may have been the cause of extrahepatic non-target embolization in these cases. Early stasis during RE, a risk factor for gastroduodenal ulceration,Citation50 is rare in HCC treatmentCitation51 and was only observed in 1 patient presenting with ulceration in our cohort.
Special care must be taken in cirrhotic patients, due to a reduced functional liver reserve and an increased risk of liver failure or RE-induced liver disease (REILD).Citation52,Citation53 We found no or only minor biochemical toxicities in the majority of cases. Grade ≥3 bilirubin toxicities within 3 months after RE (an indicator of REILD) occurred in 8.3% of patients, which is in agreement with reported values of 6%–14%.Citation31,Citation32 Development or worsening of ascites, another hallmark of REILD, occurred in 21.7%, but was ≤ grade 2 in all cases. Sequential lobar therapy is thought to be better tolerated than whole liver treatment.Citation54 However, we observed a higher rate of bilirubin toxicities in sequential bilobar compared with unilobar or simultaneous bilobar treatment, which may be due a higher cumulative administered dose in sequential treatment (3.2 GBq vs 1.5–1.8 GBq).
The results of our analysis are limited by the retrospective character of our study with inherent methodological problems. Several parameters, for example, the ECOG status or the exact cause of death could not be reconstructed in many patients, especially in those treated before systematic electronic archiving was implemented at our institution. Baseline alpha-fetoprotein (AFP) values were only available in a minority of patients and were therefore not included into the analysis. As decisions to perform RE were reached in interdisciplinary tumor boards based on the individual patient history, the patient cohort was rather heterogenic, especially concerning therapy regimes prior to and after RE. Although data of retrospectively published cohorts generally seem to be in line with each other, prospectively conducted, preferably randomized controlled trials are imperative for implementation of RE into HCC therapy algorithms.
Conclusion
The results of our study add to the growing literature on RE of HCC, demonstrating that RE is safe and effective in carefully selected patients. It stops progression of HCC in a large percentage of patients even in advanced stages. Progression after RE is associated rather with the development of new HCC nodules than the growth of treated tumors. Especially patients with a low baseline ECOG status without ascites may benefit from treatment. In addition, patients with advanced HCC should be carefully selected according to the presence of ascites, baseline GGT and Child–Pugh class. Repeated RE can prolong survival in BCLC-C patients with adequate liver function who demonstrate recurrent progression after first RE.
Acknowledgments
Jennifer Nadal (Institute for Medical Biometry, Informatics and Epidemiology, University of Bonn) provided statistical advice.
Disclosure
CM is a consultant for SIRTEX Medical, PharmaCept and GoreMedical. The authors report no other conflicts of interest in this work.
References
- BoschFXRibesJDíazMClériesRPrimary liver cancer: worldwide incidence and trendsGastroenterology20041275 Suppl 1S5S1615508102
- LlovetJMDucreuxMEuropean Association For The Study Of The Liver; European Organization For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinomaJ Hepatol201256490894322424438
- LencioniRCioniDCrocettiLEarly-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablationRadiology2005234396196715665226
- BruixJShermanMPractice Guidelines Committee, American Association for the Study of Liver DiseasesManagement of hepatocellular carcinomaHepatology20054251208123616250051
- ElnekaveEErinjeriJPBrownKTLong-term outcomes comparing surgery to embolization-ablation for treatment of solitary HCC<7 cmAnn Surg Oncol20132092881288623563960
- ZhaoMWangJPPanCCCT-guided radiofrequency ablation after with transarterial chemoembolization in treating unresectable hepatocellular carcinoma with long overall survivalimprovementEur J Radiol201281102717272522245655
- VeltriAMorettoPDoriguzziAPaganoECarraraGGandiniGRadiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC)Eur Radiol200616366166916228211
- KagawaTKoizumiJKojimaSTokai RFA Study GroupTranscatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resectionCancer2010116153638364420564097
- MalagariKPomoniMKelekisAProspective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinomaCardiovasc Intervent Radiol201033354155119937027
- GolfieriRGiampalmaERenzulliMRandomized controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinomaBr J Cancer2014111225526424937669
- LlovetJMRealMIMontañaXArterial embolisation or chemoembolisation vs symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trialLancet200235993191734173912049862
- LlovetJMRicciSMazzaferroVSHARP Investigators Study GroupSorafenib in advanced hepatocellular carcinomaN Engl J Med2008359437839018650514
- ChengALKangYKChenZEfficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomized, double-blind, placebo-controlled trialLancet Oncol2009101253419095497
- LiYGaoZHQuXJThe adverse effects of sorafenib in patients with advanced cancersBasic Clin Pharmacol Toxicol2015116321622125495944
- LlovetJMBustamanteJCastellsANatural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trialsHepatology199929162679862851
- MinagawaMMakuuchiMTreatment of hepatocellular carcinoma accompanied by portal vein tumor thrombusWorld J Gastroenterol200612477561756717171782
- Schöniger-HekeleMMüllerCKutilekMOesterreicherCFerenciPGanglAHepatocellular carcinoma in Central Europe: prognostic features and survivalGut200148110310911115830
- SalemRMazzaferroVSangroBYttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectivesHepatology20135862188219723512791
- SalemRGordonACMouliSY90 Radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinomaGastroenterology2016151611551163.e227575820
- SalemRThurstonKGRadioembolization with 90Yttrium micro-spheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerationsJ Vasc Interv Radiol20061781251127816923973
- KennedyANagSSalemRRecommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortiumInt J Radiat Oncol Biol Phys2007681132317448867
- European Association For The Study Of The LiverEuropean Organization For Research And Treatment Of CancerEASL-EORTC clinical practice guidelines: management of hepatocellular carcinomaJ Hepatol201256490894322424438
- MahnkenAHSpreaficoCMaleuxGHelmbergerTJakobsTFStandards of practice in transarterial radioembolizationCardiovasc Intervent Radiol201336361362223511991
- LencioniRLlovetJMModified RECIST (mRECIST) assessment for hepatocellular carcinomaSemin Liver Dis2010301526020175033
- RaoulJLGilabertMPianaGHow to define transarterial chemoembolization failure or refractoriness: a European perspectiveLiver Cancer20143211912424945002
- Peck-RadosavljevicMDrug therapy for advanced-stage liver cancerLiver Cancer20143212513124945003
- KudoMBiomarkers and personalized sorafenib therapyLiver Cancer201433–439940426280001
- FornerAGilabertMBruixJRaoulJLTreatment of intermediate-stage hepatocellular carcinomaNat Rev Clin Oncol201411952553525091611
- UkaKAikataHTakakiSClinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinomaWorld J Gastroenterol200713341442017230611
- HilgardPHamamiMFoulyAERadioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survivalHepatology20105251741174921038413
- SangroBCarpaneseLCianniREuropean Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY)Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluationHepatology201154386887821618574
- SalemRLewandowskiRJMulcahyMFRadioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomesGastroenterology20101381526419766639
- IñarrairaeguiMMartinez-CuestaARodríguezMAnalysis of prognostic factors after yttrium-90 radioembolization of advanced hepatocellular carcinomaInt J Radiat Oncol Biol Phys20107751441144820056355
- KulikLMCarrBIMulcahyMFSafety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosisHepatology2008471718118027884
- GianniniEGFarinatiFCiccareseFItalian Liver Cancer (ITA.LI.CA) groupPrognosis of untreated hepatocellular carcinomaHepatology201561118419025234419
- ZhangYLiYJiHZhaoXLuHTransarterial Y90 radioembolization vs chemoembolization for patients with hepatocellular carcinoma: a meta-analysisBiosci Trends20159528929826559021
- LauWYSangroBChenPJTreatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90Oncology201384531131823615394
- LauWYHoSLeungTWSelective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90yttrium microspheresInt J Radiat Oncol Biol Phys19984035835929486608
- SangroBBilbaoJIBoanJRadioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinomaInt J Radiat Oncol Biol Phys200666379280016904840
- SangroBIñarrairaeguiMBilbaoJIRadioembolization for hepatocel-lular carcinomaJ Hepatol201256246447321816126
- RiazAKulikLLewandowskiRJRadiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheresHepatology20094941185119319133645
- VilgrainVBouattourMSibertASARAH: a randomized controlled trial comparing efficacy and safety of selective internal radiation therapy (with yttrium-90 microspheres) and sorafenib in patients with locally advanced hepatocellular carcinoma. The International Liver Congress™ 2017 52nd Annual Meeting of the European Association for the Study of the LiverJ Hepatol201766Suppl 1 Abs GS-012
- ChowPKHGandhiMPhase III multi-center open-label randomized controlled trial of selective internal radiation therapy (SIRT) versus sorafenib in locally advanced hepatocellular carcinoma: The SIRveNIB study. 2017. ASCO Annual MeetingJ Clin Oncol20173535 Abs 4002
- BruixJShermanMAmerican Association for the Study of Liver DiseasesManagement of hepatocellular carcinoma: an updateHepatology20115331020102221374666
- WoodallCEScogginsCREllisSFIs selective internal radioembolization safe and effective for patients with inoperable hepatocellular carcinoma and venous thrombosis?J Am Coll Surg2009208337538219317999
- GoinJESalemRCarrBITreatment of unresectable hepato-cellular carcinoma with intrahepatic yttrium 90 microspheres: factors associated with liver toxicitiesJ Vasc Interv Radiol2005162 Pt 120521315713921
- SalemRLewandowskiRJAtassiBTreatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survivalJ Vasc Interv Radiol200516121627163916371529
- ChenSWLinLCKuoYCLiangJAKuoCCChiouJFPhase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinomaInt J Radiat Oncol Biol Phys20148851041104724661657
- RiazALewandowskiRJKulikLMComplications following radioembolization with yttrium-90 microspheres: a comprehensive literature reviewJ Vasc Interv Radiol20092091121113019640737
- LamMGBanerjeeSLouieJDRoot cause analysis of gastroduodenal ulceration after yttrium-90 radioembolizationCardiovasc Intervent Radiol20133661536154723435742
- PieperCCWillinekWAThomasDIncidence and risk factors of early arterial blood flow stasis during first radioembolization of primary and secondary liver malignancy using resin microspheres: an initial single-center analysisEur Radiol20162682779278926560720
- EdelineJGilabertMGarinEBoucherERaoulJLYttrium-90 microsphere radioembolization for hepatocellular carcinomaLiver Cancer201541162526020026
- FuruseJIshiiHNagaseMKawashimaMOginoTYoshinoMAdverse hepatic events caused by radiotherapy for advanced hepatocellular carcinomaJ Gastroenterol Hepatol200520101512151816174067
- SeidenstickerRSeidenstickerMDammRHepatic toxicity after radioembolization of the liver using (90)Y-microspheres: sequential lobar versus hole liver approachCardiovasc Intervent Radiol20123551109111822037709
