Abstract
Purpose
Abnormal expression of miR-223 in cancerous tissue has confirmed it as an important player in tumorigenesis of cancers, such as hepatocellular carcinoma, colorectal carcinoma, osteosarcoma, gastric cancer, and chronic lymphocytic leukemia. The present meta-analysis aimed to explore the association between circulating miR-223 and prognosis of cancers.
Methods
The studies were accessed by an electronic search of multiple databases. RevMan5.3 and STATA14.0 were used to estimate the heterogeneity among studies, pooled effects, and publication bias.
Results
Ten studies with data of 1,002 patients with cancer were included in this meta-analysis. The risk of metastasis from stages 3 to 4 of TNM did not decrease when high versus low circulating expression of miR-223 were compared (pooled odds ratio =0.50, 95% CI: 0.24–1.03). In case of prognosis, the overall survival time was not significantly longer with high circulating miR-223 expression (pooled hazard ratio [HR] =0.64, 95% CI: 0.38–1.11) in all cancer types. However, the overall survival time of chronic lymphocytic leukemia (pooled HR =0.19, 95% CI: 0.07–0.54) increased in subgroup analysis. Moreover, the treatment-free survival of chronic lymphocytic leukemia was significantly increased with high circulating miR-223 expression (pooled HR =0.38, 95% CI: 0.23–0.64).
Conclusion
Circulating miR-223 was not an effective biomarker in prognosis surveillance in all cancers but in chronic lymphocytic leukemia.
Background
MicroRNAs (miRNAs) are small noncoding RNA molecules (20–24 nucleotides) with negative regulation in gene expression at the posttranscriptional level.Citation1 Experiments have shown that miRNA broadly impact hundreds of mRNA targets based on the ~7 nt complementary base-pairing to the “seed sequence” of an miRNA.Citation2 The dysregulation of homeostatic control of miRNA biogenesis, such as miR-155, miR-17~92, miR-16, miR-10b, and miR-373, could be associated with multiple pathological cancers, especially tumors of the breast, lung, liver, pancreas, and bone marrow.Citation3 Furthermore, the regulating miRNAs have shown aberrant expression in tumor tissues and patient serum and participate in the onset and progression of cancer.Citation4,Citation5 Because of the involvement of miRNAs in many cellular cancer pathways including development, cell proliferation, differentiation, and apoptosis,Citation6–Citation8 miRNAs were expected to play crucial roles in cancer diagnosis and therapy as well as in prognosis surveillance.
MiR-223, a common tumor-associated microRNA, has been reported to be dysregulated in various human tumors, such as hepatocellular carcinoma,Citation9 colorectal carcinoma,Citation10 osteosarcoma,Citation11 gastric cancer,Citation12 and chronic lymphocytic leukemia.Citation13 Abnormal expression of miR-223 in cancerous tissue has confirmed it as an important player in tumorigenesis of cancers.Citation14 Similar to the tissue, miR-223 could be steadily detected in patient’s circulating blood, and be used as a noninvasive biomarker for early cancer detection and diagnosis.Citation9 Moreover, circulating miR-223 was found to be associated with metastasis and prognosis of cancersCitation15–Citation20 and it might be a diagnostic biomarker and prognostic factor. However, the effect of circulating miR-223 on cancer prognosis is controversial, and no meta-analysis has investigated the relationship between circulating miR-223 expression and prognosis.
Therefore, the present meta-analysis aimed to explore the association between circulating miR-223 level and clinical outcome of patients with cancer to further determine the biomarker role of circulating miR-223 in metastasis and prognosis of cancers.
Materials and methods
Literature search strategy
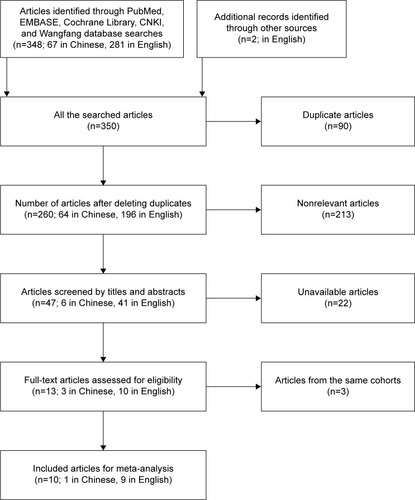
Reports of studies in Language of English or Chinese on the role of circulating miR-223 expression in the development of human cancer were searched in PubMed, EMBASE, the Cochrane Library, China National Knowledge Infrastructure, and Wanfang databases with the following keywords: (“cancer” or “tumor” or “neoplasm” or “malignant” or “carcinoma”) and (“prognos*” or “surviv*” or “follow-up” or “mortality” or “predict” or “outcome” or “metastas*”) and (“microRNA-223” or “miRNA-223”, or “miR-223”) and (“serum” or “plasma” or “blood” or “circulating”). The last search date was September 23, 2016. References of retrieved papers and conference reports were also searched to identify relevant studies.
Selection criteria
After removing the duplicate entries, titles and abstracts of articles were checked by 4 authors (YZ, JL, YC, YL). The full text of eligible articles was retrieved. The eligible articles had the following criteria: 1) the expression of circulating miR-223 was analyzed by metastasis or survival, 2) patients were divided by high and low expression of circulating miR-223, 3) odd ratios (ORs) for metastasis (tumor node metastasis [TNM] stage, international staging system) or hazard ratios (HRs) for survival (overall survival [OS], disease-free survival, event-free survival, progression-free survival, cause-specific survival, and treatment-free survival [TFS]) were provided or could be calculated from the available data; and 4) the expression of circulating miR-223 was tested in patients’ peripheral blood by real-time PCR or fluorescence in situ hybridization. Studies not fulfilling the criteria, reviews, and animal/cell-line studies were excluded. Furthermore, if more than 1 study of the same cohort was published, only the most recent English publication was included. Consensus on searching and exclusion was resolved by discussion and with 2 other investigators (TW, XC) if needed.
Data extraction and quality assessment
The general data was extracted by 4 authors (WH, WZ, DW, RM) using the following form: first author’s name, published year, region of cohort, sample size, cancer type, method to test miR-223, cases in each expression group (high/low), cases of metastasis (TNM/ISS) in each group, and survival results (OS, disease-free survival, event-free survival, progression-free survival, cause-specific survival, and TFS). Furthermore, the reference for all effects (ORs or HRs) was reformatted as low circulating miR-223 expression, and the multivariate analysis effects were used for pooled analysis. The quality of each eligible study was assessed by the Newcastle-Ottawa Scale, consisting of selection, outcome, and comparability, with scores from 0 to 9. A study with Newcastle-Ottawa Scale score ≥6 was considered at high quality.
Statistical methods
This meta-analysis involved the use of Review Manager 5.3 (Cochrane network) and STATA 14.0. When HRs and 95% CIs were not provided directly in some studies, Engauge Digitizer 4.1 was used to analyze them from Kaplan–Meier curve. The heterogeneity among eligible studies was tested by Inconsistency (I2) and Q tests (chi-squared test). If no statistical heterogeneity was found (PQ>0.05, I2<50%), a fixed-effects model was used to estimate the pooled OR and HR. Otherwise, a random-effects model was used. Moreover, Begg’s and Egger’s tests were used to assess publication bias. All tests were two-sided, and P<0.05 was considered statistically significant.
Results
Characteristics of eligible studies
The literature search included 10 studies eligible in the meta-analysis (), 6 from ChinaCitation13,Citation17,Citation18,Citation21–Citation23 and 1 each from France,Citation24 United States,Citation15 Japan,Citation19 and Belgium.Citation20 The studies involved a total of 1,002 patients with cancer, with mean sample size of 1,002 patients (range: 46–180). Six different types of cancers were evaluated: nonsmall cell lung carcinoma (n=3); esophageal squamous cell carcinoma and chronic lymphocytic leukemia (n=2 each); and pancreatic cancer, osteosarcoma, and multiple myeloma (n=1 each). The level of miR-223 was detected in patients’ plasma or serum by real-time PCR and the negative control was healthy plasma or serum. Furthermore, the group cutoff value determined by the original research depended on the median/mean value of miR-223 level receiver operating characteristic curve analysis. The main characteristics of each study are summarized in .
Table 1 Basic data for all included studies in the meta-analysis
Association between circulating miR-223 and metastasis
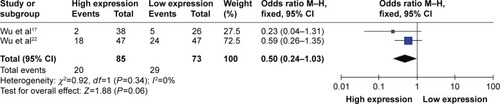
Although three studies reported the cases among TNM stage, only two in esophageal squamous cell carcinoma and nonsmall cell lung carcinoma had the same classification between high and low expression of circulating miR-223 with 158 cases in stages 3 and 4 of TNM. Because of severe heterogeneity with 2 studies (I2=0%, PQ=0.34), the fixed-effects model was used to calculate the pooled effect. High expression of circulating miR-223 did not significantly decrease the risk of stage 3 developing to 4, with pooled OR =0.50 (95% CI: 0.24–1.03) ().
Association between circulating miR-223 and OS
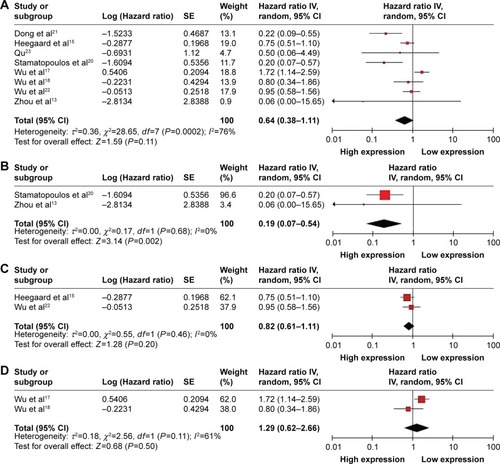
Eight studies showed data for OS in terms of circulating miR-223 level for 904 patients with cancer. Because of significant heterogeneity (I2=76%, PQ=0.0002), the random-effects model was used. The pooled HR of OS was 0.64 (95% CI: 0.38–1.11, P=0.11) for high versus low circulating miR-223 expression (); therefore, high miR-223 expression did not significantly increase the OS time. Because of the different types of cancer involved in this meta-analysis, we also calculated the pooled HR for OS by cancer-type. For studies of chronic lymphocytic leukemia, high miR-223 expression significantly increased the OS time with pooled HR of 0.19 (95% CI: 0.07–0.54) under the fixed-effects model (I2=0%, PQ=0.68) (). For studies of nonsmall cell lung carcinoma, the pooled HR was 0.82 (95% CI: 0.61–1.11) under the fixed-effects model (I2=0%, PQ=0.46) () and for esophageal squamous cell carcinoma, it was 1.29 (95% CI: 0.62–2.66) under the random-effects model (I2=61%, PQ=0.11) ().
Figure 3 Forest plot showing the association between circulating miR-223 expression and OS. (A) Pooled HR of circulating miR-223 on OS of all types of cancer under random-effects model. In subgroup analyses, (B) pooled HR of circulating miR-223 on OS of chronic lymphocytic leukemia under the fixed-effects model; (C) pooled HR of circulating miR-223 on OS of nonsmall cell lung carcinoma under fixed-effects model; (D) pooled HR of circulating miR-223 on OS of esophageal squamous cell carcinoma under the random-effects model. Data are HRs and 95% CIs.
Association between circulating miR-223 and TFS
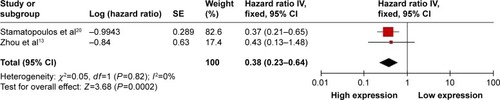
Two studies showed data for TFS with respect to circulating miR-223 level for 163 patients of chronic lymphocytic leukemia. These studies showed no significant heterogeneity (I2=0%, PQ=0.82) and therefore a fixed-effects model was used. High miR-223 expression significantly increased TFS time in patients with chronic lymphocytic leukemia (OR =0.38, 95% CI: 0.23–0.64) ().
Sensitivity analysis
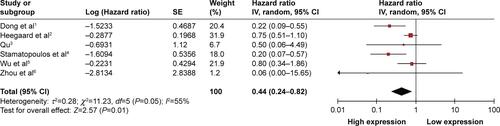
Sensitivity analysis was conducted to test the association between circulating miR-223 and OS. Each study was deleted in turn to examine the influence of the deleted data on the overall HR. Only exclusion of Wu et al’s data significantly changed the results of pooled HR of 0.54 (95% CI: 0.33–0.89) (), which suggested that the role of circulating miR-223 was differential or reverse in OS of esophageal squamous cell carcinoma. Therefore, we pooled the data on effect of circulating miR-223 on OS of all cancers except esophageal squamous cell carcinoma, which significantly increased the OS time under a random-effects model (HR =0.44, 95% CI: 0.24–0.82) (Figure S1).
Table 2 Sensitivity analysis for the studies that resulted in loss of significance after omission
Publication bias
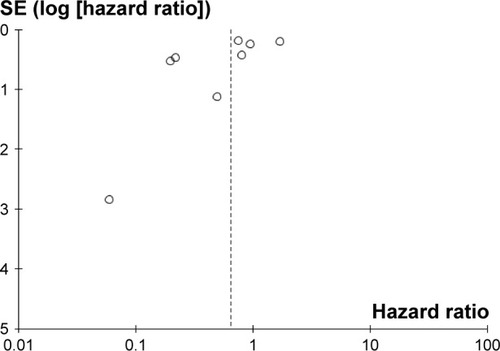
Publication bias for the association between circulating miR-223 and OS was checked by a Begg’s funnel plot under the random-effects model (). Begg’s test showed no significant rank correlation with Kendall score (Z=-1.24, Pr>|z| =0.216). Given this result, we performed Egger’s test where evidence of significance of publication bias was not found (r=−2.11, 95% CI: −5.09–0.87, P>|t| =0.133).
Discussion
This current study aimed to assess the pooled effect of circulating miR-223 expression on prognosis with cancer. High miR-223 expression was not significantly associated with decreased cancer metastasis and increased OS time of reported cancers. However, high miR-223 expression significantly increased the OS time as well as TFS time of patients with chronic lymphocytic leukemia.
MiR-223, initially identified as part of the hematopoietic system,Citation25 has been widely reported to show association with different cancers. Research in the mechanism of miR-223 found that it could target C/EBPβ, E2F1, FOXO1, NFI-A, and MAFB to inhibit tumor cell proliferation, migration, and invasive capability in vitro;Citation26–Citation30 however, it could also deregulate the expression of tumor suppressors.Citation31–Citation33 As miR-223 affects multiple targets simultaneously that are involved in key processes,Citation14 the role of miR-223 in cancer depends on the type of cancer and the total level in patient’s body. Although, circulating miR-223 was not always positively correlated with the level expressed in tumor tissue,Citation19,Citation34 the level of circulating miR-223 was proven as a high-quality biomarker for early cancer detection and diagnosis.Citation9,Citation35,Citation36 Moreover, circulating miR-223 could contribute to the epithelial-to-mesenchymal transition, required for cancer metastasis and invasion,Citation37 and could enhance response to preoperative chemo-radiotherapy in patients with cancer.Citation38 Therefore, circulating miR-223 is an important cancer-associated miRNA and participates in tumor development and progression.
In our meta-analysis, although the risk of progression of stages 3 to 4 of TNM was not significantly decreased with high circulating miR-223 expression for the small sample size and few cancer types, it still suggested that high circulating miR-223 might be epidemiologically associated with decreased metastasis of cancers. For prognosis of patients, circulating miR-223 was not significantly associated with OS in all reported cancers; however, their association in subgroup analysis was not coincident. It epidemiologically supported the fact that the role of circulating miR-223 in cancers depends on the mechanism and character of cancer-type. It suggested that the circulating miR-223 could not be used as biomarker for prognosis surveillance of cancer but chronic lymphocytic leukemia.
Some meta-analyses focused on the association of miR-223 with cancer; all analyzed the role of miR-223 in the early detection and diagnosis of cancer.Citation9,Citation39–Citation41 To search for an applicable biomarker for therapy, we focused on the association of circulating miR-223 on metastasis and prognosis. To the best of our knowledge, this is the first meta-analysis of the effect of circulating miR-223 on outcomes of patients with cancer.
Our study has some limitations. First, the studies of each cancer-type and respective patient samples were few in number. Second, because of heterogeneity of different cancer-type in the included studies, the results need to be further identified with more randomized clinical trials. Finally, because of using aggregated group data in the meta-analysis, some confounding factors could not be controlled.
Conclusion and recommendations
This meta-analysis is the first to demonstrate that high expression of circulating miR-223 is related to prognosis for patients with cancer. The expression of circulating miR-223 might be a biomarker for prognosis surveillance of some cancer types, especially chronic lymphocytic leukemia.
Author contributions
XLC conceived the study; YFZ, JBL, YC, and YL conducted the review and screened records for eligibility; WJH, WYZ, DLW, and RRM extracted data and conducted statistical analysis under the supervision of XLC and TQW. JBL prepared the initial report, which was read and edited by XLC. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was funded by the Shenzhen Technology Research and Development Funds (JCYJ20150403095530583 and 201604130086), and the Science & Technology Project of Shenzhen Longgang District (201406063001026 and 20160607153104624).
Supplementary material
Figure S1 Forest plot of the pooled HRs for OS with esophageal squamous cell carcinoma excluded under the random-effects model.
Abbreviations: HR, hazard ratio; OS, overall survival; IV, inverse variance; CI, confidence interval; SE, standard error.
References
- DongJLiuYLiaoWLiuRShiPWangLmiRNA-223 is a potential diagnostic and prognostic marker for osteosarcomaJ Bone Oncol201652747927335775
- HeegaardNHSchetterAJWelshJAYonedaMBowmanEDHarrisCCCirculating micro-RNA expression profiles in early stage nonsmall cell lung cancerInt J Cancer201213061378138621544802
- QuYThe clinical significance of serum microRNA, long non-coding RNA and CRBN protein in multiple myeloma [master’s thesis]ShanghaiThe Second Military Medical University2014
- StamatopoulosBMeulemanNHaibe-KainsBmicroRNA-29c and microRNA-223 down-regulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratificationBlood2009113215237524519144983
- WuCLiMHuCDuanHClinical significance of serum miR-223, miR-25 and miR-375 in patients with esophageal squamous cell carcinomaMol Biol Rep20144131257126624390317
- ZhouKYiSYuZMicroRNA-223 expression is uniformly down-regulated in B cell lymphoproliferative disorders and is associated with poor survival in patients with chronic lymphocytic leukemiaLeuk Lymphoma20125361155116122145958
Disclosure
The authors report no conflicts of interest in this work.
References
- ShenJHungMCSignaling-mediated regulation of MicroRNA processingCancer Res201575578379125660948
- KimVNHanJSiomiMCBiogenesis of small RNAs in animalsNat Rev Mol Cell Biol200910212613919165215
- VenturaAJacksTMicroRNAs and cancer: short RNAs go a long wayCell2009136458659119239879
- LeeJYRyuDSKimWJKimSJAberrantly expressed microRNAs in the context of bladder tumorigenesisInvestig Clin Urol201657Suppl 1S52S59
- GulyaevaLFKushlinskiyNERegulatory mechanisms of microRNA expressionJ Transl Med20161414327197967
- PileczkiVCojocneanu-PetricRMaralaniMNeagoeIBSandulescuRMicroRNAs as regulators of apoptosis mechanisms in cancerClujul Med2016891505527004025
- KwanJYPsarianosPBruceJPYipKWLiuFFThe complexity of microRNAs in human cancerJ Radiat Res201657Suppl 1i106i11126983984
- PinwehaPRattanapornsompongKCharoensawanVJitrapakdeeSMicroRNAs and oncogenic transcriptional regulatory networks controlling metabolic reprogramming in cancersComput Struct Biotechnol J20161422323327358718
- LiGShenQLiCLiDChenJHeMIdentification of circulating MicroRNAs as novel potential biomarkers for hepatocellular carcinoma detection: a systematic review and meta-analysisClin Transl Oncol201517968469325956842
- EarleJSLuthraRRomansAAssociation of microRNA expression with microsatellite instability status in colorectal adenocarcinomaJ Mol Diagn201012443344020413677
- XuJYaoQHouYMiR-223/Ect2/p21 signaling regulates osteosarcoma cell cycle progression and proliferationBiomed Pharmacother201367538138623601845
- ShresthaSHsuSDHuangWYA systematic review of microRNA expression profiling studies in human gastric cancerCancer Med20143487888824902858
- ZhouKYiSYuZMicroRNA-223 expression is uniformly down-regulated in B cell lymphoproliferative disorders and is associated with poor survival in patients with chronic lymphocytic leukemiaLeuk Lymphoma20125361155116122145958
- HaneklausMGerlicMO’NeillLAMastersSLmiR-223: infection, inflammation and cancerJ Intern Med2013274321522623772809
- HeegaardNHSchetterAJWelshJAYonedaMBowmanEDHarrisCCCirculating micro-RNA expression profiles in early stage nonsmall cell lung cancerInt J Cancer201213061378138621544802
- JoergerMBatyFFrühMCirculating microRNA profiling in patients with advanced non-squamous NSCLC receiving bevacizumab/erlotinib followed by platinum-based chemotherapy at progression (SAKK 19/05)Lung Cancer201485230631324928469
- WuCLiMHuCDuanHClinical significance of serum miR-223, miR-25 and miR-375 in patients with esophageal squamous cell carcinomaMol Biol Rep20144131257126624390317
- WuCWangCGuanXDiagnostic and prognostic implications of a serum miRNA panel in oesophageal squamous cell carcinomaPLoS One201493e9229224651474
- KomatsuSIchikawaDMiyamaeMMalignant potential in pancreatic neoplasm; new insights provided by circulating miR-223 in plasmaExpert Opin Biol Ther201515677378525819175
- StamatopoulosBMeulemanNHaibe-KainsBmicroRNA-29c and microRNA-223 down-regulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratificationBlood2009113215237524519144983
- DongJLiuYLiaoWLiuRShiPWangLmiRNA-223 is a potential diagnostic and prognostic marker for osteosarcomaJ Bone Oncol201652747927335775
- WuCCaoYHeZSerum levels of miR-19b and miR-146a as prognostic biomarkers for non-small cell lung cancerTohoku J Exp Med20142322859524531034
- QuYThe clinical significance of serum microRNA, long non-coding RNA and CRBN protein in multiple myeloma [master’s thesis]ShanghaiThe Second Military Medical University2014
- SanfiorenzoCIlieMIBelaidATwo panels of plasma microRNAs as non-invasive biomarkers for prediction of recurrence in resectable NSCLCPLoS One201381e5459623342174
- JohnnidisJBHarrisMHWheelerRTRegulation of progenitor cell proliferation and granulocyte function by microRNA-223Nature200845171821125112918278031
- ZhouHXiaoJWuNMicroRNA-223 Regulates the differentiation and function of intestinal dendritic cells and macrophages by targeting C/EBPbetaCell Rep20151361149116026526992
- PulikkanJADenglerVPeramangalamPSCell-cycle regulator E2F1 and microRNA-223 comprise an autoregulatory negative feedback loop in acute myeloid leukemiaBlood201011591768177820029046
- WuLLiHJiaCYMicroRNA-223 regulates FOXO1 expression and cell proliferationFEBS Lett201258671038104322569260
- FaziFRosaAFaticaAA minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesisCell2005123581983116325577
- YangWLanXLiDLiTLuSMiR-223 targeting MAFB suppresses proliferation and migration of nasopharyngeal carcinoma cellsBMC Cancer20151546126055874
- LiXZhangYZhangHmiRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3Mol Cancer Res20119782483321628394
- LiZWYangYMDuLTOverexpression of miR-223 correlates with tumor metastasis and poor prognosis in patients with colorectal cancerMed Oncol2014311125625270282
- KurashigeJWatanabeMIwatsukiMOverexpression of microRNA-223 regulates the ubiquitin ligase FBXW7 in oesophageal squamous cell carcinomaBr J Cancer2012106118218822108521
- CinpolatOUnalZNIsmiOGorurAUnalMComparison of microRNA profiles between benign and malignant salivary gland tumors in tissue, blood and saliva samples: a prospective, case-control studyBraz J Otorhinolaryngol Epub2016427
- TachibanaHShoRTakedaYCirculating miR-223 in oral cancer: its potential as a novel diagnostic biomarker and therapeutic targetPLoS One2016117e015969327441818
- LiBSZhaoYLGuoGPlasma microRNAs, miR-223, miR-21 and miR-218, as novel potential biomarkers for gastric cancer detectionPLoS One201277e4162922860003
- TangYWangYChenQQiuNZhaoYYouXMiR-223 inhibited cell metastasis of human cervical cancer by modulating epithelial-mesenchymal transitionInt J Clin Exp Pathol201589112241122926617846
- NakaoTIwataTHotchiMPrediction of response to preoperative chemoradiotherapy and establishment of individualized therapy in advanced rectal cancerOncol Rep20153441961196726260776
- FiorinoSBacchi-ReggianiMLVisaniMMicroRNAs as possible biomarkers for diagnosis and prognosis of hepatitis B- and C-related-hepatocellular-carcinomaWorld J Gastroenterol201622153907393627099435
- ZhouXJiGChenHJinWYinCZhangGClinical role of circulating miR-223 as a novel biomarker in early diagnosis of cancer patientsInt J Clin Exp Med201589168901689826629240
- KimSYJeonTYChoiCIValidation of circulating miRNA biomarkers for predicting lymph node metastasis in gastric cancerJ Mol Diagn201315566166923806809