Abstract
Background
PD-L1 has been reported to be expressed in diverse human malignancies. However, the prognostic value of PD-L1 in digestive system cancers remains inconclusive. Therefore, we conducted this meta-analysis to evaluate the prognostic impact of PD-L1 expression in digestive system cancers.
Materials and methods
We searched the PubMed, Embase, and the Chinese National Knowledge Infrastructure for publications concerning PD-L1 expression in digestive system cancers. Correlations of PD-L1 expression level with overall survival (OS), disease-free survival (DFS), and recurrence-free survival (RFS) were analyzed.
Results
Finally, 32 studies with 7,308 patients were included. Our results show that PD-L1 expression was significantly associated with poorer OS (hazard ratio [HR] =1.44, 95% confidence interval [CI] =1.18–1.76, P<0.001), but not DFS (HR =0.91, 95% CI =0.61–1.37, P=0.657) or RFS (HR =1.27, 95% CI =0.75–2.14, P=0.368). Moreover, in the subgroup analysis, significant associations between PD-L1 expression and OS were found in Asians (HR =1.50, 95% CI =1.19–1.89, P=0.001), gastric cancer (HR =1.43, 95% CI =1.05–1.94, P=0.021), and pancreatic carcinoma (HR =2.64, 95% CI =1.78–3.93, P<0.001).
Conclusion
These results suggest that the expression of PD-L1 is associated with worse OS in digestive system cancers, especially in gastric cancer and pancreatic cancer. In addition, PD-L1 may act as a new parameter for predicting poor prognosis and a promising target for anticancer therapy in digestive system cancers.
Introduction
Cancer is now the major cause of death in developed countries, and its incidence and mortality are increasing for several cancer types, among which the most fatal are liver and pancreatic cancer.Citation1 Liver and pancreatic cancer are digestive system cancers, which also includes esophageal cancer (EC), gastric cancer, biliary tract cancer, and colorectal cancer (CRC). Of all the cancers, digestive system cancers demonstrate the highest incidence and death rates.Citation2,Citation3 Recently, the development of multidisciplinary therapies has significantly improved treatment outcomes, but the overall prognosis for sufferers of digestive system cancers is still poor. Current research is increasingly focused on new immunotherapeutic strategies, which could be a major breakthrough in the field of cancer treatment.Citation4 In addition, certain immunologic checkpoint markers have been reported in digestive system cancers, among which PD-L1 is the focus of studies.Citation5
PD-L1, also known as CD274 and B7-H1, is a member of the B7 family of immune regulatory cell surface proteins.Citation6 It is commonly upregulated in many different human tumors. The combination of PD-L1 with the receptor PD-1, which has been reported to form and maintain an immunosuppressive microenvironment by suppressing the proliferation of activated T-cells and inducing the apoptosis of T-cells, is considered to be an important immunological escape mechanism that increases the risk of neoplasia.Citation7–Citation10 In addition, immune checkpoint blockade using PD-L1 antibodies seems to be one of the most promising immunotherapy approaches.Citation11 Although anti-PD-L1 therapies are continuously developing, the prognostic value of PD-L1 is still unclear in various digestive system cancers.
There are many studies that demonstrate the relationship between PD-L1 and survival of patients with different digestive system cancers. But, the conclusions have not reached a consensus and most studies only focused on one cancer type, and did not make an assessment on digestive system cancers. Herein, we conducted a meta-analysis to assess the impact of PD-L1 on the prognosis of digestive system cancers.
Methods
This meta-analysis complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Literature search strategy
We searched the PubMed, Embase, and the Chinese National Knowledge Infrastructure databases (up to June 2016) to obtain the relevant articles. The following keywords were used: “PD-L1,” “B7-H1,” “CD274,” “digestive system cancer,” “colorectal neoplasms,” “esophageal neoplasms,” “gastric cancer,” “hepatocellular carcinomas,” “pancreatic carcinomas,” “biliary tract neoplasms,” and “prognosis.” We also searched the references of the included studies manually to identify relevant publications. Two authors (Cong Dai and Meng Wang) performed the search strategy, and discrepancies were discussed by all the researchers in the group meeting.
Inclusion and exclusion criteria
No language restrictions were applied. If different studies published by the same investigators have overlapping data, only the most complete one was included.
Studies were included if they fulfilled the following criteria: 1) all selected cancer cases were pathologically confirmed; 2) studies evaluated the relationship of PD-L1 expression in digestive system cancer patients with detailed information of overall survival (OS), disease-free survival (DFS), or recurrence-free survival (RFS); and 3) the study provided a hazard ratio (HR) with the corresponding confidence interval (CI) or sufficient data to calculate it. Articles meeting the following criteria were excluded: 1) unrelated or duplicate publication; 2) nonhuman experiments were performed; 3) case series, case reports, reviews, or studies without original data; 4) crude data were not provided or HRs could not be calculated.
Data extraction
Two independent researchers (Zhiming Dai and Shuai Lin) extracted the detailed information of included studies with a standardized format. The results were compared, and all the researchers in the group meeting discussed the final decision in case of any discrepancy. The following information was collected: first author surname, year of publication, patient source, number of patients, tumor types, specimen types, method of detection, PD-L1 expression, median follow-up, prognostic outcomes, HR estimate, and HR with its 95% CI. If any of these data were not offered in the study, items were recorded as “–”.
Quality assessment
Methodological quality was assessed using the Newcastle–Ottawa Scale (NOS). The scale includes three domains: selection, comparability, and outcome assessment. Studies with a score of 6–9 were regarded as high quality. Two authors (Cong Dai and Meng Wang) independently graded each study, and all the researchers in the group met to make final decisions regarding any discrepancies.
Statistical analyses
The statistical analyses were performed with Stata 12.0 (StataCorp LP, College Station, TX, USA). We computed the pooled HR and its 95% CI to evaluate the relationship between the PD-L1 and the prognosis of patients with digestive system cancer. In addition, prognostic markers were classified into OS, DFS, and RFS. If HRs were provided explicitly in the studies, we used them directly. Otherwise, we calculated the HR from the Kaplan–Meier survival curve or with the available data using methods described by Parmer et al.Citation12 Data from the Kaplan–Meier survival curves were read by Engauge Digitizer version 4.1. The Q test and the I2 test were used to evaluate the heterogeneity among studies. If heterogeneity was significant (P<0.1 or I2>50%), random-effects model was used.Citation13 Otherwise, a fixed-effects Mantel–Haenszel model was applied.Citation14 We further conducted subgroup analyses by ethnicity, tumor type, and HR estimate. Sensitivity analysis was performed by omitting individual studies to examine the reliability of the results. Meta-regression analysis was conducted to identify potential factors causing heterogeneity.Citation15 Publication bias was assessed using Begg’s test, Egger’s test, and Begg’s funnel plot.Citation16,Citation17
Results
Characteristics of included studies
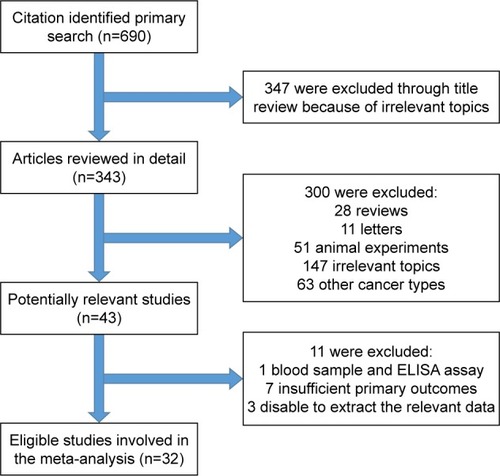
We initially identified a total of 343 studies using the search criteria listed earlier. As shown in , 311 studies were excluded owing to irrelevance to the analysis or lack of the relevant data we needed, or because they were reviews, letters, or animal experiments. Finally, there were 32 studies included in this meta-analysis.Citation18–Citation49
Figure 1 Flowchart of the selection of the studies in the meta-analysis.
We have summarized the characteristics of the 32 studies in . Of the 32 publications, 30 assessed the relationship between PD-L1 and OS in patients with digestive system neoplasms. In addition, eight studies evaluated the relationship between PD-L1 and DFS, and three studies evaluated PD-L1 and RFS. Studies with a total of 7,308 patients, from China, Korea, Japan, the UK, Switzerland, and Germany, were enrolled. In addition, the number of patients in each study ranged from 40 to 1,420. To observe the status of PD-L1 in patients with different cancers, we categorized the cancers into CRC (six studies), EC (five studies), gastric cancer (13 studies), hepatocellular cancer (four studies), pancreatic cancer (three studies), and extrahepatic bile duct cancer (one study). The median positive rate of PD-L1 was 49.6% (range 19.8%–84.8%). The median follow-up times ranged from 20.7 to 75 months. In addition, in methodological quality assessment, all of the studies, which obtained scores ranging from 6 to 9, were considered high quality ().
Table 1 Characteristics of included studies for meta-analyses
Main meta-analysis results
Overall, there were 30 studies including 6,801 patients concerning the association between OS and PD-L1 expression. The meta-analysis results show that positive expression was associated with significantly poorer OS compared to the negative expression (HR =1.44, 95% CI =1.18–1.76, P<0.001; and ). The heterogeneity among studies was statistically significant (P<0.001, I2=87.8%); therefore, a random-effects model was used.
Figure 2 Forest plot of HR for the association of PD-L1 overexpression and OS.
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival.
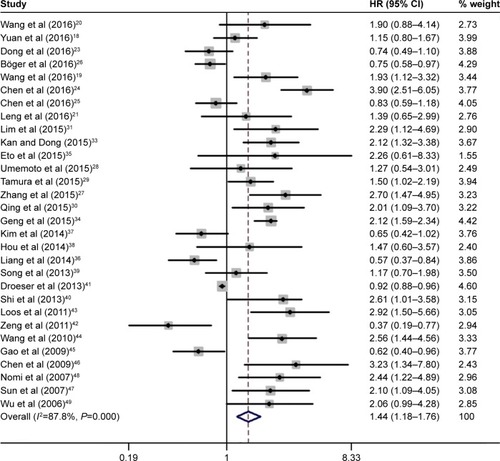
Table 2 Main meta-analysis results for OS
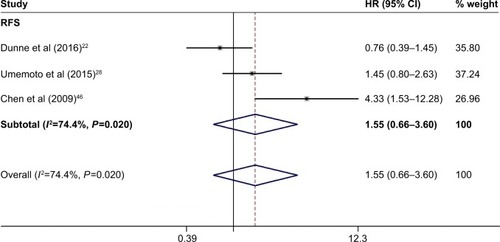
Three studies with 544 patients reported the RFS. Owing to the significant heterogeneity (P=0.023, I2=68.5%) among these studies, a random-effects model was used. Our results suggested that PD-L1 was not associated with RFS of digestive system cancers (HR =1.27, 95% CI =0.75–2.14, P=0.368; ).
Figure 3 Forest plot of HR for the association of PD-L1 overexpression and RFS.
Abbreviations: CI, confidence interval; HR, hazard ratio; RFS, recurrence-free survival.
As shown in and , there were eight studies comprising 1,566 patients which provided results regarding DFS and PD-L1 expression. The pooled data demonstrated that there was no association between them (HR =0.91, 95% CI =0.61–1.37, P=0.657, random-effects model).
Figure 4 Forest plot of HR for the association of PD-L1 overexpression and DFS.
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio.
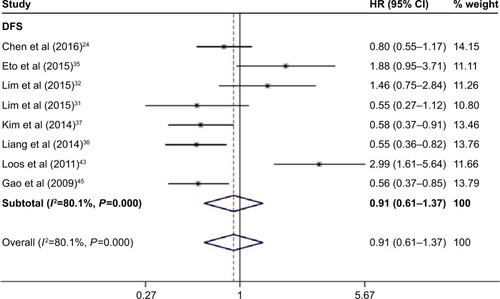
Table 3 Main meta-analysis results for DFS
Subgroup analyses, sensitivity analyses, and meta-regression in OS
To solve the heterogeneity, we performed subgroup analyses by ethnicity, tumor types, and HR estimate. The subgroup analysis by ethnicity suggested a significant association in studies based on Asians (HR =1.50, 95% CI =1.19–1.89, P=0.001) but not among other ethnicities (HR =1.07, 95% CI =0.72–1.58, P=0.740). In the subgroup analysis by tumor types, significant associations were found in gastric cancer (HR =1.43, 95% CI =1.05–1.94, P=0.021) and pancreatic carcinoma (HR =2.64, 95% CI =1.78–3.93, P<0.001). For HR estimation, subgroup analysis showed that the overall HR estimate with univariate analysis was 1.64 (95% CI =1.21–2.23, P=0.001; ). Meanwhile, it is worth mentioning that heterogeneity among most of the subgroups was statistically significant (P>0.1 or I2>50%).
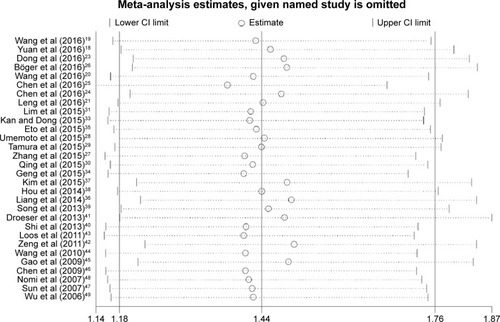
The included studies were sequentially removed to investigate whether any single study has an influence on the pooled results. As shown in , the stable pooled HR was not significantly affected by any individual study.
Figure 5 Sensitivity analysis of pooled HRs on the association between PD-L1 expression and OS.
The meta-regression was performed to identify the source of the heterogeneity. We analyzed possible factors including publication year, ethnicity, number of patients, tumor types, and PD-L1 expression. The results confirmed that the number of patients per study might be a major source of heterogeneity ().
Table 4 Meta-regression analyses of potential source of heterogeneity
Subgroup analyses in DFS
We also performed subgroup analysis for ethnicity, tumor types, and HR estimate among studies focus on DFS. Only the subgroup analysis for the HR estimate showed a significant association with DFS based on multivariate analysis (HR =0.75, 95% CI =0.59–0.97, P=0.026; ).
Publication bias
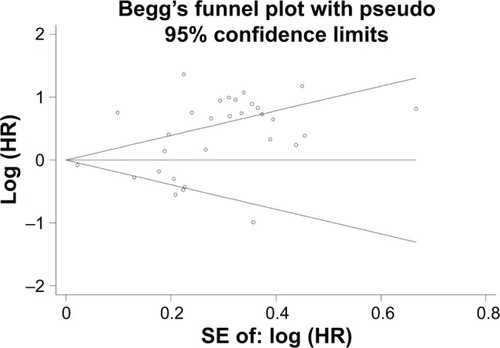
We performed Begg’s and Egger’s tests to identify whether any publication bias existed in the published literature in this meta-analysis. Publication bias was observed among studies reporting OS (P=0.498, 0.003), but no publication bias was found among studies reporting DFS (P=0.230, 0.330) or RFS (P=0.308, 0.328). The Begg’s plots for the effect of PD-L1 expression on OS are shown in .
Discussion
Recently, according to many reports, cancer cells are able to use immunosuppressive molecules to their advantage via inhibiting antitumor lymphocytes, thus evading destruction by the immune system. Therefore, immunotherapy is now considered a novel method of cancer treatment.Citation50 Cancer immunotherapy is a revolutionary cancer treatment targeting the immune checkpoint receptors such as PD-L1. PD-L1 antibodies have been proved to exert clinical activity in more than 15 types of cancers including EC, gastric cancer, hepatocellular cancer, and CRC.Citation51 These studies suggested that PD-L1 may be a prognostic biomarker and a potential target of treatment in digestive system cancers. In this meta-analysis, we investigated the association between the expression of PD-L1 and the prognosis of digestive system cancer patients by analyzing the published data.
Our results showed that PD-L1 overexpression was significantly associated with shorter OS. These results indicated that PD-L1 can serve as a novel parameter for prognostication and a promising target for anticancer therapy in digestive system cancers. When extended to subgroup analysis, no clear correlation between PD-L1 expression and OS was found in non-Asian, CRC, EC, and hepatocellular carcinoma (HC) patients, possibly owing to the insufficiently large sample size. Therefore, it is necessary for better designed studies with more patients to prove or to retort our results in the future.
There was a significant heterogeneity across the included studies. However, subgroup analyses of ethnicity, tumor types, and the methods used to estimate the HR failed to identify its source. The sensitivity analyses showed that the stable pooled HR was not significantly affected by each individual study. The meta-regression confirmed that the number of patients might be a major source of heterogeneity. However, the meta-regression adjusted R2 value of the number of patients is just 15.78% (data not shown), which could only explain a small part of heterogeneity source. Therefore, it is likely that the heterogeneity is due to the differences in the baseline characteristics of patients, the immunohistochemical methods, and the baseline referring to positive/high PD-L1 expression. However, because of lacking clinical data in these aspects, we cannot determine their contribution to the heterogeneity among studies.
Previously, some meta-analyses have been conducted to study the association between PD-L1 and some cancers in the digestive system. Huang et alCitation52 identified nine studies that involved 2,500 gastrointestinal tract cancer patients, and reported that PD-L1 is a prognostic risk factor for gastrointestinal tract cancer. The study by Wu et al,Citation53 which included seven studies with 687 patients, showed that positive PD-L1 expression status in tumor cells was associated with worse 5-year OS of EC, gastric cancer, and CRC. Compared with these studies, our meta-analysis has some differences and advantages. First of all, our study is the first meta-analysis to estimate the role of PD-L1 in the prognosis of digestive system cancers. Second, we not only analyzed OS but also evaluated DFS and RFS, which contributed to a comprehensive understanding of the issue. Third, our conclusion is more reliable because several new studies fulfilling the inclusion criteria have been included.
We attempted to conduct a comprehensive analysis of PD-L1 and prognosis of digestive system cancers, but it should be recognized that there are still some limitations in our study. First, some survival data were not reported directly; therefore, an indirect method was used to extract data from the Kaplan–Meier survival curves, which may affect the accuracy of the original data. Second, publication bias was observed among studies reporting OS. The reason may be that studies with positive results were published more easily. Third, we cannot recognize the association between subgroups of RFS and PD-L1 expression due to lack of data. More importantly, the PD-L1 expression level measured using immunohistochemistry might show high variability between studies. Hence, a baseline for positive/high PD-L1 expression should be established.
Conclusion
We found that PD-L1 expression indicated poor OS outcomes. We indicated that PD-L1 may serve as a prognostic indicator and a potential novel target for treatment in digestive system cancers.
Acknowledgments
This study was supported by the National Natural Science Foundation, China (No 81471670); China Postdoctoral Science Foundation (No 2014M560791; 2015T81037); the Fundamental Research Funds for the Central Universities, China (No 2014qngz-04); the International Cooperative Project of Shaanxi province, People’s Republic of China (No 2014KW-23-07), and Science and Technology Plan of Innovation Project, Shaanxi province, China (No 2015KTCL03-06). The authors thank all the nonauthor contributors: Yi Zhen, Yujiao Deng, and Zhe Dou.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin201060527730020610543
- MohammedFEsophageal cancerN Engl J Med20043501313631364 author reply 1363–1364
- SznolMChenLAntagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancerClin Cancer Res20131951021103423460533
- PostowMACallahanMKWolchokJDImmune checkpoint blockade in cancer therapyJ Clin Oncol201533171974198225605845
- McDermottDFAtkinsMBPD-1 as a potential target in cancer therapyCancer Med20132566267324403232
- ZouWChenLInhibitory B7-family molecules in the tumour microenvironmentNat Rev Immunol20088646747718500231
- PardollDMThe blockade of immune checkpoints in cancer immunotherapyNat Rev Cancer201212425226422437870
- ChenJLiGMengHUpregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomasCancer Immunol Immunother201261110110821853301
- DongHStromeSESalomaoDRTumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasionNat Med20028879380012091876
- LoteHCafferkeyCChauIPD-1 and PD-L1 blockade in gastrointestinal malignanciesCancer Treat Rev2015411089390326412280
- ParmarMKTorriVStewartLExtracting summary statistics to perform meta-analyses of the published literature for survival endpointsStat Med19981724281528349921604
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst195922471974813655060
- BakerWLWhiteCMCappelleriJCKlugerJColemanCIHealth Outcomes, Policy, and Economics (HOPE) Collaborative GroupUnderstanding heterogeneity in meta-analysis: the role of meta-regressionInt J Clin Pract200963101426143419769699
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- YuanJZhangJZhuYProgrammed death-ligand-1 expression in advanced gastric cancer detected with RNA in situ hybridization and its clinical significanceOncotarget2016726396713967927191996
- WangLAWeiXLiQChenLThe prediction of survival of patients with gastric cancer with PD-L1 expression using contrast-enhanced ultrasonographyTumour Biol20163767327733226671554
- WangLRenFWangQSignificance of programmed death ligand 1 (PD-L1) immunohistochemical expression in colorectal cancerMol Diagn Ther201620217518126891728
- LengCLiYQinJRelationship between expression of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the antitumor effects of CD8(+) T cellsOncol Rep201635269970826718132
- DunnePDMcArtDGO’ReillyPGImmune-derived PD-L1 gene expression defines a subgroup of stage II/III colorectal cancer patients with favorable prognosis who may be harmed by adjuvant chemotherapyCancer Immunol Res20164758259127197062
- DongMWangHYZhaoXXExpression and prognostic roles of PIK3CA, JAK2, PD-L1, and PD-L2 in Epstein-Barr virus-associated gastric carcinomaHum Pathol201653253426980034
- ChenMFChenPTChenWCLuMSLinPYLeeKDThe role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppressionOncotarget2016777913792426761210
- ChenKChengGZhangFPrognostic significance of programmed death-1 and programmed death-ligand 1 expression in patients with esophageal squamous cell carcinomaOncotarget2016721307723078027120796
- BögerCBehrensHMMathiakMKrugerSKalthoffHRockenCPD-L1 is an independent prognostic predictor in gastric cancer of Western patientsOncotarget2016717242692428327009855
- ZhangLQiuMJinYProgrammed cell death ligand 1 (PD-L1) expression on gastric cancer and its relationship with clinicopathologic factorsInt J Clin Exp Pathol201589110841109126617827
- UmemotoYOkanoSMatsumotoYPrognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomyJ Gastroenterol2015501657524509608
- TamuraTOhiraMTanakaHProgrammed death-1 ligand-1 (PDL1) expression is associated with the prognosis of patients with stage II/III gastric cancerAnticancer Res201535105369537626408698
- QingYLiQRenTUpregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancerDrug Des Devel Ther20159901909
- LimYJKohJKimKHigh ratio of programmed cell death protein 1 (PD-1)(+)/CD8(+) tumor-infiltrating lymphocytes identifies a poor prognostic subset of extrahepatic bile duct cancer undergoing surgery plus adjuvant chemoradiotherapyRadiother Oncol2015117116517026235847
- LimSHHongMAhnSChanges in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancerEur J Cancer2016521926623522
- KanGDongWThe expression of PD-L1 APE1 and P53 in hepatocellular carcinoma and its relationship to clinical pathologyEur Rev Med Pharmacol Sci201519163063307126367730
- GengYWangHLuCExpression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significanceInt J Clin Oncol201520227328124804867
- EtoSYoshikawaKNishiMProgrammed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resectionGastric Cancer201619246647126210691
- LiangMLiJWangDT-cell infiltration and expressions of T lymphocyte co-inhibitory B7-H1 and B7-H4 molecules among colorectal cancer patients in northeast China’s Heilongjiang provinceTumour Biol2014351556023873101
- KimJWNamKHAhnSHPrognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancerGastric Cancer2016191425225424150
- HouJYuZXiangRCorrelation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancerExp Mol Pathol201496328429124657498
- SongMChenDLuBPTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancerPLoS One201386e6582123785454
- ShiSJWangLJWangGDB7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cellsPLoS One2013810e7601224124529
- DroeserRAHirtCViehlCTClinical impact of programmed cell death ligand 1 expression in colorectal cancerEur J Cancer20134992233224223478000
- ZengZShiFZhouLUpregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinomaPLoS One201169e2362121912640
- LoosMLangerRSchusterTClinical significance of the costimulatory molecule B7-H1 in Barrett carcinomaAnn Thorac Surg20119141025103121440117
- WangLMaQChenXGuoKLiJZhangMClinical significance of B7-H1 and B7-1 expressions in pancreatic carcinomaWorld J Surg20103451059106520145927
- GaoQWangXYQiuSJOverexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinomaClin Cancer Res200915397197919188168
- ChenXLYuanSXChenCMaoYXXuGWangXYExpression of B7-H1 protein in human pancreatic carcinoma tissues and its clinical significanceAi Zheng2009281213281332 Chinese19958630
- SunJXuKWuCPD-L1 expression analysis in gastric carcinoma tissue and blocking of tumor-associated PD-L1 signaling by two functional monoclonal antibodiesTissue Antigens2007691192717212704
- NomiTShoMAkahoriTClinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancerClin Cancer Res20071372151215717404099
- WuCZhuYJiangJZhaoJZhangXGXuNImmunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significanceActa Histochem20061081192416530813
- MellmanICoukosGDranoffGCancer immunotherapy comes of ageNature2011480737848048922193102
- SharmaPAllisonJPThe future of immune checkpoint therapyScience20153486230566125838373
- HuangBChenLBaoCThe expression status and prognostic significance of programmed cell death 1 ligand 1 in gastrointestinal tract cancer: a systematic review and meta-analysisOnco Targets Ther201582617262526451118
- WuPWuDLiLChaiYHuangJPD-L1 and survival in solid tumors: a meta-analysisPLoS One2015106e013140326114883