Abstract
Background
Alcohol dehydrogenase (ADH) isoenzymes have been reported as a potential diagnostic marker for pancreatic cancer, but their prognostic value in pancreatic cancer remains unclear. The aim of this investigation was to identify the prognostic value of ADH genes in human patients with pancreatic adenocarcinoma (PAAD).
Materials and methods
An RNA sequencing dataset and corresponding survival profiles of PAAD were obtained from The Cancer Genome Atlas. Survival analysis and gene set enrichment analysis were used to investigate the prediction value and potential mechanism of ADH genes in PAAD prognosis.
Results
Survival analysis of ADH genes suggests that a high expression of ADH1A (adjusted P=0.037, adjusted hazard ratio [HR] =0.627, 95% CI =0.404–0.972) and ADH6 (adjusted P=0.018, adjusted HR =0.588, 95% CI =0.378–0.914) were associated with a significantly decreased risk of death, while a high expression of ADH5 was associated with a significantly increased risk of death (adjusted P=0.043, adjusted HR =1.564, 95% CI =1.013–2.414). Joint effects analysis of three ADH gene prognostic markers suggests that the prognosis difference for any marker combination was more significant than that for any individual marker. The potential mechanism of ADH1A and ADH6 in PAAD prognosis was that a high expression of ADH1A and ADH6 was involved in the P450 pathway and biological processes, while high ADH5 expression was involved in transforming growth factor β regulation-related pathways and biological processes, Wnt, the cell cycle, ErbB, and mitogen-activated protein kinase signaling pathways.
Conclusion
Our data suggest that ADH1A, ADH5, and ADH6 expression may be potential prognostic markers of PAAD and in combination have a strong interaction and better predictive value for PAAD prognosis.
Introduction
Pancreatic cancer presents as highly lethal malignant tumors, for which mortality closely parallels incidence, with an estimated 330,400 deaths occurring worldwide in 2012.Citation1,Citation2 It is estimated that about 79,400 Chinese will die from pancreatic cancer in 2015, and it is predicted that there will be about 90,100 newly diagnosed pancreatic cancer cases in China in 2015.Citation3 The age-standardized mortality rates of pancreatic cancer in the Chinese male population have been shown to have an upward trend.Citation3 Pancreatic cancer patients always have a relatively poor prognosis in China with an age-standardized 5-year relative survival rate of 11.7%.Citation4 Therefore, it is very important to find markers and prognostic predictive indicators that detect malignant cell transformation at an early stage.
Studies have demonstrated that alcohol dehydrogenase (ADH) is present in the pancreatic tissue and plays an important role in multiple biological processes of the pancreas.Citation5,Citation6 A study by Jelski et al reported that class III ADH activity was markedly higher in pancreatic cancer tissue than in healthy tissue.Citation7 ADH isoenzymes have also been reported as a potential diagnostic marker for pancreatic cancer, and the combination of circulating ADH and macrophage inhibitory cytokine to carbohydrate antigen 19–9 can improve the overall quality of diagnosis for this lethal disease.Citation8,Citation9 Although the diagnostic value of ADH in pancreatic cancer has been identified, the prognostic value of ADH in pancreatic cancer remains unclear. The aim of this investigation was to identify the prognostic value of ADH gene expression in pancreatic cancer patients.
The Cancer Genome Atlas (TCGA) has generated comprehensive, multidimensional maps of the key genomic changes in 33 types of cancer including pancreatic adenocarcinoma (PAAD), which is available as open access. In the present study, we utilized the TCGA database to investigate the prognostic prediction value and potential mechanism of ADH genes in patients with PAAD.
Materials and methods
RNA sequencing dataset
An RNA sequencing dataset including 177 PAAD patient transcriptome and corresponding survival profiles was obtained from the TCGA web server (https://portal.gdc.cancer.gov/, accessed March 20, 2017). RNA sequencing datasets of 177 tumor tissues and four adjacent normal tissues were downloaded from the TCGA database; information on overall survival (OS), as well as the status of events, was available for all of these patients. Normalization of the PAAD RNA sequencing dataset was performed using DESeq, an R package for transcriptome profiling, according to the user guide.Citation10 Genes that were 0 in >10% of all subjects were eliminated.
Association analysis
The comparison of ADH gene expression between pancreatic tumor tissue and adjacent normal tissues was done by an analysis using Metabolic gEne RApid VisualizerCitation11 (http://merav.wi.mit.edu/) and TCGA dataset, respectively. Pearson correlation coefficient was used to evaluate correlations among genes in coexpression analysis.
Survival analysis
All patients were divided into two groups according to gene expression levels in tumor tissue for survival analysis. The high-expression group consisted of patients in which gene expression levels were above the median value, and a low-expression group comprised the remaining patients. We also stratified the analysis on the basis of associations between gene expression and clinical features in OS. Alcohol history and tumor stage were adjusted in multivariate Cox proportion hazard regression analysis. On the basis of the results of coexpression analysis, we also investigated the joint effects of significant prognostic-related ADH genes in PAAD.
Gene set enrichment analysis
Differences of pathways and biological process in transcrip-tome levels between high and low ADH genes expression were analyzed using gene set enrichment analysis (GSEA) v2–2.2.3,Citation12,Citation13 with reference to gene sets from the Molecular Signatures Database (MSigDB) of c2 (KEGG gene sets: c2.cp.kegg.v5.2.symbols.gmt) and c5 (GO gene sets: c5.bp. v5.2.symbols.gmt, c5.cc.v5.2.symbols.gmt, and c5.mf. v5.2.symbols.gmt), respectively. The number of permutations was set at 1,000. Enrichment results satisfying a nominal P-value <0.05 and a false discovery rate (FDR) <0.25 were considered statistically significant.
Statistical analysis
The mRNA expression of ADH genes in tumor and adjacent nontumor tissue was analyzed using an independent sample t-test. The Pearson correlation coefficient was used to assess the coexpression correlation at the mRNA level, and the coexpression heat map was constructed by the corrplot package in the R platform. Survival analysis was carried out using the Kaplan–Meier method with the log-rank test to compare clinical factors and gene expression groups. Cox proportional hazards regression analysis was used to calculate the crude or adjusted hazard ratio (HR) and 95% CI in uni- and multivariate analyses. The FDR in GSEA was adjusted for multiple testing with the Benjamini–Hochberg procedure to control FDR.Citation14,Citation15 Kaplan–Meier survival curves were plotted using GraphPad Prism 6.0. A value of P<0.05 was considered statistically significant. Data were analyzed with the help of SPSS v.20.0 software (IBM, Chicago, IL, USA).
Results
Data processing
Seven ADH genes were available in the TCGA PAAD mRNA expression dataset, ADH1A, ADH1B, ADH1C, ADH4, ADH5, ADH6, and ADH7. After normalization of the RNA sequencing data, we found that the expression level of ADH7 was very low in PAAD tumor tissue. Therefore, ADH7 data were excluded from the present study. After normalization by DESeq, 177 pancreatic tumor tissues and 4 adjacent normal tissues expression data with six ADH genes were used for further analysis.
Association analysis
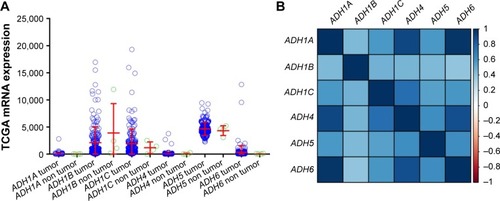
Distribution of ADH gene expression between pancreatic tumor tissue and adjacent normal tissues using MERAV () showed that ADH1A and ADH1B were markedly downregulated in pancreatic tumor tissue (), whereas ADH5 was significantly upregulated in tumor tissue (). Similar results were also observed in ADH1B and ADH5 of patients with TCGA PAAD (), but the difference did not reach statistical significance among these genes. Coexpression analysis of ADH genes indicated that ADH1A (r=0.581, P<0.001, ), ADH1C (r=0.371, P<0.001, ), and ADH6 (r=0.502, P<0.001, ) had a significantly positive correlation with ADH4 in PAAD tumor tissue, while ADH1A (r=0.761, P<0.001, ) and ADH5 (r=0.176, P=0.019, ) had a significantly positive correlation with ADH6.
Figure 1 Distribution of ADH genes expression between pancreatic tumor tissue and adjacent normal tissues using the MERAV web server.
Abbreviations: ADH, alcohol dehydrogenase; MERAV, metabolic gEne RApid visualizer.
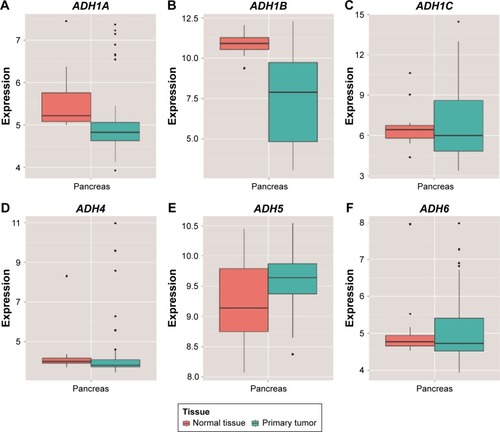
Figure 2 Gene expression distribution and coexpression heat map of ADH genes using the TCGA dataset.
Abbreviations: ADH, alcohol dehydrogenase; TCGA, The Cancer Genome Atlas; PAAD, pancreatic adenocarcinoma.
Survival analysis
The clinical characteristics of PAAD are summarized in . Information only with regard to age, sex, alcohol history, tumor stage, and clinical outcomes of the PAAD can be obtained from the TCGA website. OS stratified by the clinical characteristics indicate that advanced tumor stage was associated with a significantly increased risk of death in PAAD patients. Survival analysis of ADH genes are showed in , and suggested that a high expression of ADH1A (adjusted P=0.037, adjusted HR =0.627, 95% CI =0.404–0.972; ) and ADH6 (adjusted P=0.018, adjusted HR =0.588, 95% CI =0.378–0.914; ) was significantly associated with a decreased risk of death and a long median survival time (MST; 545 vs 913 days for low ADH1A vs high ADH1A, log-rank P=0.073, ; 592 vs 691 days for low ADH6 vs high ADH6, log-rank P=0.03, ; respectively) in PAAD patients, after adjusting for alcohol history and tumor stage. In contrast, a high expression of ADH5 was significantly associated with a poor clinical outcome (MST: 702 vs 511 days for low ADH5 vs high ADH5, log-rank P=0.0079, ) and an increased risk of death (adjusted P=0.043, adjusted HR =1.564, 95% CI =1.013–2.414; ). The associations between other ADH genes and PAAD OS did not show statistical significance.
Figure 3 The prognostic value of ADH genes expression in PAAD patients.
Abbreviations: ADH, alcohol dehydrogenase; PAAD, pancreatic adenocarcinoma; OS, overall survival.
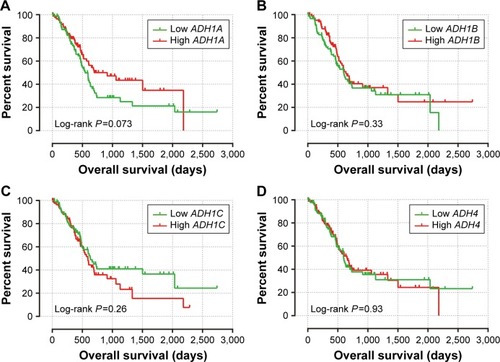
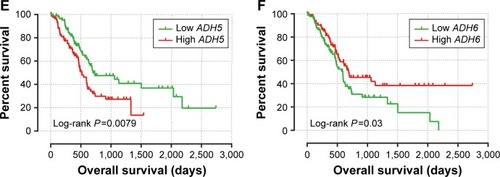
Table 1 Clinical characteristics of PAAD patients
Table 2 Associations between ADH genes and OS in PAAD patients
Stratification analysis
Results of the stratified analysis of ADH1A, ADH5, and ADH6 with OS in different strata of clinical characteristics are shown in . A high expression of ADH1A (adjusted P=0.017, adjusted HR =0.468, 95% CI =0.251–0.873) and ADH6 (adjusted P=0.026, adjusted HR =0.498, 95% CI =0.270–0.918) has a protective effect in male PAAD patients, while high ADH5 expression significantly increases the risk of death (adjusted P=0.028, adjusted HR =1.977, 95% CI =1.075–3.636). Similar protective effects can also be found with age >60 years (adjusted P=0.006, adjusted HR =0.488, 95% CI =0.293–0.811) and tumor stage II (adjusted P=0.049, adjusted HR =0.627, 95% CI =0.394–0.997) in patients with high ADH6 expression. However, high ADH5 expression also increased the risk of death in patients without a history of alcohol (adjusted P=0.014, adjusted HR =2.574, 95% CI =1.213–5.464).
Table 3 Stratified analysis of ADH genes and OS in PAAD patients
Joint effects analysis
Coexpression analysis indicates that ADH1A and ADH5 were positively correlated with ADH6 at the mRNA expression level. We further investigated the joint effects of these genes in the prediction of PAAD prognosis. The combination of ADH1A and ADH5 was divided into three groups (Table S1) for assessing the prognostic value in PAAD according to the associations between the genes and OS. Similar classification methods were also used for the combinations of ADH1A+ADH6, ADH5+ADH6, and ADH1A+ADH5+ADH6, and the detailed grouping information is shown in Table S1. Joint effects analysis in the combination of ADH1A and ADH5 demonstrated that group 2 (adjusted P=0.011, adjusted HR =0.525, 95% CI =0.320–0.862, ; ) and group 3 (adjusted P=0.003, adjusted HR =0.379, 95% CI =0.198–0.723, ; ) were associated with a significantly decreased risk of death in PAAD, compared to group 1. In , a protective effect can also be observed in the combination of ADH1A+ADH6 (group ii vs group i, adjusted P=0.029, adjusted HR =0.576, 95% CI =0.351–0.946; group iii vs group i, adjusted P=0.003, adjusted HR =0.408, 95% CI =0.225–0.741, ), ADH5+ADH6 (group C vs group A, adjusted P=0.004, adjusted HR =0.378, 95% CI =0.196–0.727, ) and ADH1A+ADH5+ADH6 (group III vs group I, adjusted P=0.014, adjusted HR =0.475, 95% CI =0.262–0.861; group IV vs group I, adjusted P=0.003, adjusted HR =0.221, 95% CI =0.082–0.597, ), respectively. Our joint effects analysis of the three ADH gene prognostic markers suggests that the prognosis difference for any marker combination was more significant than that for any individual marker.
Figure 4 Kaplan–Meier survival curve for joint effects analysis among ADH1A, ADH5, and ADH6 in PAAD patients.
Abbreviations: ADH, alcohol dehydrogenase; PAAD, pancreatic adenocarcinoma.
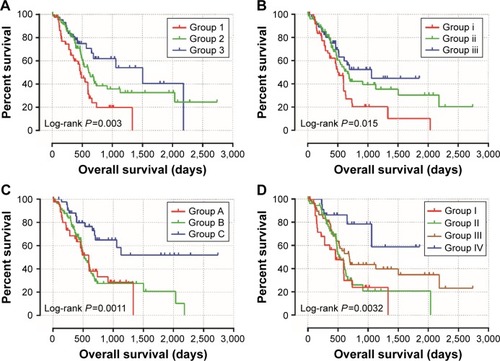
Table 4 Joint effects analysis of ADH genes and OS in PAAD patients
Gene set enrichment analysis
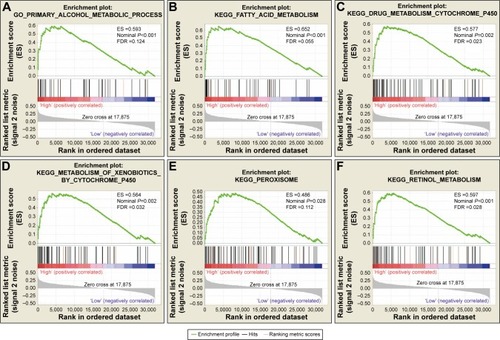
To further explore the potential mechanism of ADH genes in PAAD prognosis, we used the PAAD genome-wide RNA sequencing dataset for GSEA. GSEA results of the c5 reference gene set suggest that high ADH1A expression was involved in the epoxygenase P450 pathway and cell differentiation regulation biological processes (; Table S2), while the enrichment of c2 indicates that high ADH1A is also involved in drug metabolism, cytochrome P450, fatty acid metabolism, and peroxisome proliferator-activated receptor signaling pathway (; Table S3). GSEA results reveal that the high expression of ADH5 was correlated to the transforming growth factor β (TGF-β) regulation-related biological process (; Table S4), whereas the c2 enrichments suggest that high ADH5 expression is involved in TGF-β, Wnt, cell cycle, ErbB, mitogen-activated protein kinase (MAPK), and the pancreatic cancer signaling pathway (; Table S5). We also investigated the potential mechanism in ADH6 and demonstrated that high ADH6 expression was related to primary alcohol metabolic processes (; Table S6), fatty acid and retinol metabolism, and drug metabolism cytochrome P450 (; Table S7).
Figure 5 GSEA results of ADH1A expressed in PAAD patients.
Abbreviations: ADH, alcohol dehydrogenase; ES, enrichment score; FDR, false discovery rate; GSEA, gene set enrichment analysis; PAAD, pancreatic adenocarcinoma.
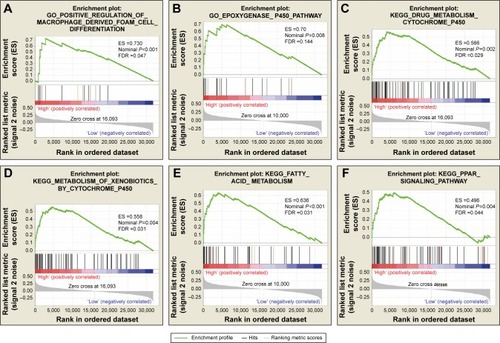
Figure 6 GSEA results of ADH5 expressed in PAAD patients.
Abbreviations: ADH, alcohol dehydrogenase; ES, enrichment score; FDR, false discovery rate; GSEA, gene set enrichment analysis; PAAD, pancreatic adenocarcinoma.
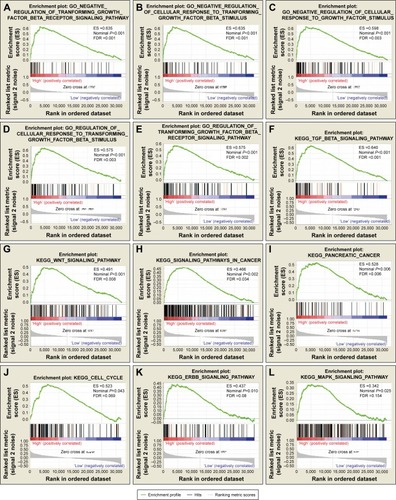
Figure 7 GSEA results of ADH6 expressed in PAAD patients.
Abbreviations: ADH, alcohol dehydrogenase; ES, enrichment score; FDR, false discovery rate; GSEA, gene set enrichment analysis; PAAD, pancreatic adenocarcinoma.
Discussion
Ethanol is first metabolized by ADH isoenzymes and then the resulting product is further metabolized by aldehyde dehydrogenase (ALDH) isoenzymes into acetic acid.Citation16 The disproportion between ADH and ALDH may lead to an increased ability for ethanol oxidation and less capability to remove acetaldehyde, resulting in deleterious alcohol metabolites accumulating in vivo, which may subsequently cause oncogenesis.Citation17 ADH isoenzymes are divided into several classes on the basis of differences in biological characteristics.Citation18 Isoenzymes of class I ADH are encoded by ADH1A, ADH1B, and ADH1C, whereas class II, III, IV, and class V ADH are encoded by ADH4, ADH5, ADH6, and ADH7, respectively.Citation19 Numerous studies have shown that the serum levels of class I ADH are a potential diagnostic marker in multiple cancers, including renal cell,Citation20 brain,Citation21 colorectal,Citation22 endometrial,Citation23 and cervical cancers.Citation24 Similar diagnostic values can also be observed in class III ADH for pancreatic cancer,Citation8 and class IV ADH for esophagealCitation25 and gastric cancers.Citation26 The diagnostic values of ADH isoenzymes in other cancers have not yet been reported, but the difference of ADH isoenzymes between cancer patients and healthy subjects has already been observed. Both the serum and tissue expression level of class I and total ADH were significantly increased in liver,Citation27,Citation28 colorectal,Citation29,Citation30 and brain cancers,Citation31,Citation32 while ALDH levels were not statistically significant different between cancer patients and healthy subjects. The upregulation trend of class I ADH between cancer and healthy tissue can also be found in cervical,Citation33 ovarian,Citation34 endometrial,Citation35 and renal cell cancers,Citation36 whereas the upregulation trend of class I ADH for cancer serum has been reported in bladder and esophageal cancers.Citation37,Citation38 However, the expression of class I ADH in breast cancer was significantly decreased compared to the tissue of healthy subjects, and the expression of ALDH was unchanged.Citation39 An upregulation trend of class III and class IV ADH was also shown in pancreatic and esophageal tumor tissue, respectively.Citation7,Citation40 Besides the use of the differences of ADH isoenzymes expression for cancer diagnosis, the genetic variant of ADH can also change the susceptibility of cancer. Polymorphisms of ADH1C are significantly associated with the risk of colorectal cancer,Citation41 oral squamous cell carcinoma,Citation42,Citation43 bladder cancer,Citation44 upper aerodigestive tract cancers,Citation45,Citation46 head and neck squamous cell carcinoma,Citation47 and gastric cancer.Citation48,Citation49
Despite numerous reports of ADH isoenzyme diagnosis values and cancer susceptibility, the prognostic value of ADH genes has rarely been investigated. A previous study by Wei et al indicated that ADH4 may act as a potential prognostic marker for hepatocellular carcinoma (HCC), and lower expression of ADH4 may be associated with a worse survival.Citation50 Our previous study on hepatitis B virus (HBV)-related HCC also demonstrates that the upregulation of ADH1A, ADH1C, and ADH6 in HCC tumor tissues was associated with favorable prognosis, whereas high ADH1C and ADH5 reduced the risk of tumor recurrence in HBV-related HCC, respectively.Citation51 In the current study, our results indicate that a high expression of ADH1A and ADH6 has a protective effect in PAAD prognosis, while a high expression of ADH5 may increase the risk of death. In addition, the combination of these three ADH genes has prediction values for PAAD prognosis, and the prognosis difference between different combination groups was significant. Our findings imply that individuals of these three ADH genes may serve as a PAAD prognostic marker; however, their combination showed a strong interaction and better predictive value for PAAD prognosis. Once validated, ADH genes may be valuable biomarkers in PAAD diagnosis and prognostic prediction, and later these biomarkers may be used in combination with other clinical diagnosis and prognostic factors for decision-making in PAAD management.
To investigate the potential mechanism of ADH genes in PAAD prognosis, we used a genome-wide RNA sequencing dataset in GSEA and substantiated that both ADH1A and ADH6 were involved in drug metabolism cytochrome P450 and fatty acid metabolism pathways. Human cytochrome P450 (CYP) enzymes are mainly distributed in the smooth endoplasmic reticulum and involved in detoxification through the metabolism of toxic fat-soluble substances to water-soluble substances, which are then excreted.Citation52
P450 enzymes play a key role in cancer formation and cancer treatment, and mediate the metabolic activation of anticancer drugs and precarcinogens, as well as anticancer drug inactivation.Citation53 An in vitro study has substantiated that the expression of CYP2B1 enzymes (retrovirus-mediated transduction) leads to an increased susceptibility to ifosfamide in pancreatic cancer.Citation54 Studies suggest that P450 enzymes have the potential to be used as distinguishing markers in pancreatic pathology and targets of pancreatic cancer gene therapy.Citation55,Citation56 On the basis of GSEA results, we deduced that both ADH1A and a high expression of ADH6 were involved in the P450-related pathway and biological processes that are associated with the progress and treatment of pancreatic cancer, and may play a role in OS of PAAD via P450.
TGF-β regulation-related pathways and biological processes, Wnt, the cell cycle, ErbB, and the MAPK signaling pathway were significantly enriched in the ADH5 high-expression group. TGF-β family members participate in multiple cellular functions such as proliferation, apoptosis, differentiation, and migration. Research by Friess et al substantiated that an enhanced expression of TGF-β isoforms in pancreatic cancer was correlated with a worse survival,Citation57 while a study by Glazer et al demonstrated that patients with early-stage pancreatic cancer have longer median survival with TGFβ1 overexpression.Citation58 In addition, studies have reported that the TGF-β pathway may serve as a potential target for targeted therapyCitation59,Citation60 and the inhibition of the TGF pathway can decrease PAAD growth and invasiveness.Citation61 These findings suggest that high ADH5 expression may influence TGF-β regulation-related pathways and biological processes, and, therefore, may play a role in PAAD prognosis.
The Wnt signaling pathway plays an important role in physiological and pathological processes including the occurrence and development of tumors. Activation of the Wnt/β-catenin signaling pathway may enhance pancreatic cancer developmentCitation62 and increase pancreatic cancer tumorigenicity via miR-744.Citation63 Moreover, Jiang et al indicated that Wnt2 expression in pancreatic cancer tissues was significantly associated with tumor development by activation of the Wnt pathways and serves as a potential candidate for targeted therapy of pancreatic cancer.Citation64 The ErbB signaling pathway functions through ErbB family members (including ErbB-1, ErbB-2, ErbB-3, and ErbB-4) and the MAPK pathway is a common downstream target of all ErbB receptors. Previous studies indicate that ErbB-1 and ErbB-3 play an important role in pancreatic cancer and may serve as a potential candidate for targeted therapy of pancreatic cancer.Citation65–Citation67 Furthermore, Koizumi et al revealed that it is necessary for p38 MAPK signaling activation in gemcitabine-induced cell death in pancreatic cancer, and conclude that p38 MAPK signaling pathways could serve as a novel target for gemcitabine-based therapy.Citation68 In summary, the potential mechanism of high ADH5 expression in PAAD prognosis is probably because it is involved in multiple biological processes and signaling pathways that are related to pancreatic cancer development, the cell cycle, targeted therapy, and survival.
There were some limitations to our study that need to be recognized. First, the clinical information from the TCGA database was not comprehensive, and the information for PAAD patients from the TCGA, such as tumor size, histology, lymphatic invasion, venous invasion, and treatment, was not available on the TCGA website. Therefore, our study evaluated the association between ADH gene expression and OS on the basis of multivariate survival analysis that was only adjusted for alcohol history and tumor stage in a Cox proportional hazards regression model. Second, only four PAAD adjacent normal tissues expression data are available in TCGA, and this resulted in a test with low power. Therefore, further investigations of ADH gene distribution between tumors and adjacent normal tissues are needed. Third, our current study based on the TCGA database to analyze the prognosis prediction of the mRNA expression level of ADH isozymes lacks verification at protein level. Therefore, future research is still needed to address these issues.
Despite these limitations, ours is the first study to investigate the association between individual ADH gene expression and OS in PAAD patients, as well as the joint effects of prognostic values among three ADH genes. We also investigated the potential mechanism of ADH genes in PAAD prognostics using the GSEA approach. These findings provide insight into ADH genes in cancer clinical outcomes and may have clinical utility for prognosis prediction and decision-making in PAAD management.
Conclusion
Our data suggest that ADH1A, ADH5, and ADH6 may be potential prognostic biomarkers of PAAD, and their combination showed a strong interaction and better predictive value for PAAD prognosis. The potential mechanism of ADH1A and ADH6 in PAAD prognosis was that a high expression of ADH1A and ADH6 was involved in the P450-related pathway and biological processes, while high ADH5 expression was involved in the TGF-β regulation-related pathway and biological processes, Wnt, the cell cycle, ErbB, and the MAPK signaling pathway. Functional experiments will be needed for the further validation of these findings in a future study. Due to the small sample size and incomplete clinical information in the current study, further well-designed and larger sample size studies are necessary to validate our results.
Acknowledgments
This work was supported in part by the National Nature Science Foundation of China (No: 81560535, 81072321, 30760243, 30460143 and 30560133), 2009 Program for New Century Excellent Talents in University (NCET), Guangxi Nature Sciences Foundation (No: GuiKeGong 1104003A-7), and Guangxi Health Ministry Medicine Grant (Key-Scientific Research-Grant Z201018). Self-raised Scientific Research Fund of the Health and Family Planning Commission of Guangxi Zhuang Autonomous Region (Z2016318). The authors thank Dr Ketuan Huang, Tingdong Yu, Wei Qin, Chengkun Yang, Guangzhi Zhu, Hao Su, Xiangkun Wang, Zhengtao Liu, and Prof Lequn Li, Xue Qin, Liming Shang, Xinping Ye, Bin Chen, Kaiyin Xiao, Minhao Peng, Zhen Liu, and Sicong Lu for their contribution on manuscript revision. Thanks also go to the contributors of the Cancer Genome Atlas for sharing the PAAD RNA sequencing dataset on open access. In addition, we also would like to acknowledge the helpful comments that our reviewers provided to this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
- KamisawaTWoodLDItoiTTakaoriKPancreatic cancerLancet201638810039738526830752
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- ZengHZhengRGuoYCancer survival in China, 2003–2005: a population-based studyInt J Cancer201513681921193025242378
- HaberPSApteMVApplegateTLMetabolism of ethanol by rat pancreatic acinar cellsJ Lab Clin Med199813242943029794700
- ChrostekLJelskiWSzmitkowskiMPuchalskiZAlcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) activity in the human pancreasDig Dis Sci20034871230123312870777
- JelskiWChrostekLSzmitkowskiMThe activity of class I, II, III, and IV of alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in pancreatic cancerPancreas200735214214617632320
- JelskiWKutylowskaELaniewska-DunajMSzmitkowskiMAlcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) as candidates for tumor markers in patients with pancreatic cancerJ Gastrointestin Liver Dis201120325525921961092
- MohamedAASolimanHIsmailMEvaluation of circulating ADH and MIC-1 as diagnostic markers in Egyptian patients with pancreatic cancerPancreatology2015151343925464937
- AndersSHuberWDifferential expression analysis for sequence count dataGenome Biol20101110R10620979621
- ShaulYDYuanBThiruPMERAV: a tool for comparing gene expression across human tissues and cell typesNucleic Acids Res201644D1D560D56626626150
- SubramanianATamayoPMoothaVKGene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profilesProc Natl Acad Sci U S A200510243155451555016199517
- MoothaVKLindgrenCMErikssonKFPGC-1alpha-responsive genes involved in oxidative phosphorylation is coordinately downregulated in human diabetesNat Genet200334326727312808457
- ReinerAYekutieliDBenjaminiYIdentifying differentially expressed genes using false discovery rate controlling proceduresBioinformatics200319336837512584122
- BenjaminiYHochbergYControlling the false discovery rate: a practical and powerful approach to multiple testingJ R Stat Soc Series B Stat Methodol1995571289300
- KlyosovAAKinetics and specificity of human liver aldehyde dehydrogenases toward aliphatic, aromatic, and fused polycyclic aldehydesBiochemistry19963514445744678605195
- OrywalKSzmitkowskiMAlcohol dehydrogenase and aldehyde dehydrogenase in malignant neoplasmsClin Exp Med201717213113926886278
- HolmesRSAlcohol dehydrogenases: a family of isozymes with differential functionsAlcohol Alcohol Suppl199421271308974326
- JelskiWSzmitkowskiMAlcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in the cancer diseasesClin Chim Acta20083951–21518505683
- OrywalKJelskiWWerelTSzmitkowskiMThe diagnostic significance of serum alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase activity in renal cell cancer patientsExp Mol Pathol2016100341642027086037
- JelskiWLaniewska-DunajMOrywalKKochanowiczJRutkowskiRSzmitkowskiMThe diagnostic value of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) measurement in the sera of patients with brain tumorArch Med Sci201713234635228261287
- JelskiWMroczkoBSzmitkowskiMThe diagnostic value of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) measurement in the sera of colorectal cancer patientsDig Dis Sci201055102953295720069455
- OrywalKJelskiWZdrodowskiMSzmitkowskiMThe diagnostic value of alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase measurement in the sera of patients with endometrial cancerAnticancer Res20133393725373024023302
- OrywalKJelskiWZdrodowskiMSzmitkowskiMThe diagnostic value of alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase measurement sera of cervical cancer patientsAnticancer Res20163652265226927127132
- JelskiWLaniewska-DunajMNiklinskiJKozlowskiMLaudanskiJSzmitkowskiMThe alcohol dehydrogenase isoenzyme (ADH IV) as a candidate tumour marker of esophageal cancerActa Biochim Polonica2013603489493
- JelskiWOrywalKLaniewskaMSzmitkowskiMThe diagnostic value of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) measurement in the sera of gastric cancer patientsClin Exp Med201010421521920454995
- JelskiWZalewskiBSzmitkowskiMAlcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) activity in the sera of patients with liver cancerJ Clin Lab Anal200822320420918484658
- JelskiWZalewskiBSzmitkowskiMThe activity of class I, II, III, and IV alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) in liver cancerDig Dis Sci20085392550255518224440
- JelskiWZalewskiBChrostekLSzmitkowskiMAlcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) activity in the sera of patients with colorectal cancerClin Expl Med200774154157
- JelskiWZalewskiBChrostekLSzmitkowskiMThe activity of class I, II, III, and IV alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in colorectal cancerDig Dis Sci200449697798115309886
- JelskiWLaniewska-DunajMOrywalKKochanowiczJRutkowskiRSzmitkowskiMThe activity of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) in the sera of patients with brain cancerNeurochem Res201439122313231825300996
- Laniewska-DunajMJelskiWOrywalKKochanowiczJRutkowskiRSzmitkowskiMThe activity of class I, II, III and IV of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) in brain cancerNeurochem Res20133871517152123624825
- OrywalKJelskiWZdrodowskiMSzmitkowskiMThe activity of class I, II, III and IV alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in cervical cancerClin Biochem20114414–151231123421784063
- OrywalKJelskiWZdrodowskiMSzmitkowskiMThe activity of class I, II, III and IV alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in ovarian cancer and ovarian cystsAdv Med Sci201358221622024327532
- OrywalKJelskiWZdrodowskiMSzmitkowskiMThe activity of class I, II, III, and IV alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in endometrial cancerJ Clin Lab Anal201024533433920872569
- OrywalKJelskiWWerelTSzmitkowskiMThe activity of class I, II, III and IV alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in renal cell carcinomaExp Mol Pathol201598340340625779850
- OrywalKJelskiWWerelTSzmitkowskiMThe activity of class I, II, III and IV alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in the sera of bladder cancer patientsActa Biochim Polonica20176418184
- JelskiWKozlowskiMLaudanskiJNiklinskiJSzmitkowskiMAlcohol dehydrogenase isoenzymes and aldehyde dehydrogenase activity in the sera of patients with esophageal cancerClin Exp Med20099213113719184326
- JelskiWChrostekLSzmitkowskiMMarkiewiczWThe activity of class I, II, III and IV alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in breast cancerClin Exp Med200662899316820997
- JelskiWKozlowskiMLaudanskiJNiklinskiJSzmitkowskiMThe activity of class I, II, III, and IV alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) in esophageal cancerDig Dis Sci200954472573018688716
- BongaertsBWde GoeijAFWoutersKAAlcohol consumption, alcohol dehydrogenase 1C (ADH1C) genotype, and risk of colorectal cancer in the Netherlands Cohort Study on diet and cancerAlcohol201145321722521163612
- BrocicMSupicGZeljicKGenetic polymorphisms of ADH1C and CYP2E1 and risk of oral squamous cell carcinomaOtolaryngol Head Neck Surg2011145458659321705789
- SolomonPRSelvamGSShanmugamGPolymorphism in ADH and MTHFR genes in oral squamous cell carcinoma of IndiansOral Dis200814763363918266839
- van DijkBvan HouwelingenKPWitjesJASchalkenJAKiemeneyLAAlcohol dehydrogenase type 3 (ADH3) and the risk of bladder cancerEur Urol200140550951411752857
- McKayJDTruongTGaborieauVA genome-wide association study of upper aerodigestive tract cancers conducted within the INHANCE consortiumPLoS Genet201173e100133321437268
- OzeIMatsuoKSuzukiTImpact of multiple alcohol dehydrogenase gene polymorphisms on risk of upper aerodigestive tract cancers in a Japanese populationCancer Epidemiol Biomarkers Prev200918113097310219861527
- JiYBLeeSHKimKRAssociation between ADH1B and ADH1C polymorphisms and the risk of head and neck squamous cell carcinomaTumour Biol20153664387439625874489
- TerryMBGammonMDZhangFFAlcohol dehydrogenase 3 and risk of esophageal and gastric adenocarcinomasCancer Causes Control20071891039104617665311
- HidakaASasazukiSMatsuoKJPHC Study GroupGenetic polymorphisms of ADH1B, ADH1C and ALDH2, alcohol consumption, and the risk of gastric cancer: the Japan Public Health Center-based prospective studyCarcinogenesis201536222323125524923
- WeiRRZhangMYRaoHLPuHYZhangHZWangHYIdentification of ADH4 as a novel and potential prognostic marker in hepatocellular carcinomaMed Oncol20122942737274322147505
- ShangLZhuGSuHIdentification of alcohol dehydrogenase as a potential prognostic marker in HBV-related hepatocellular carcinomaInt J Clin Exp Med201710344574472
- NebertDWRussellDWClinical importance of the cytochromes P450Lancet200236093401155116212387968
- Rodriguez-AntonaCIngelman-SundbergMCytochrome P450 pharmacogenetics and cancerOncogene200625111679169116550168
- HlavatyJPetznekHHolzmullerHEvaluation of a gene-directed enzyme-product therapy (GDEPT) in human pancreatic tumor cells and their use as in vivo models for pancreatic cancerPLoS One201277e4061122815775
- LiuSXXiaZSZhongYQGene therapy in pancreatic cancerWorld journal of gastroenterology20142037133431336825309069
- GandhiAVSaxenaSRellesDDifferential expression of cytochrome P450 omega-hydroxylase isoforms and their association with clinicopathological features in pancreatic ductal adenocarcinomaAnn Surg Oncol201320Suppl 3S636S64323846787
- FriessHYamanakaYBuchlerMEnhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survivalGastroenterology19931056184618568253361
- GlazerESWelshEPimientoJMTeerJKMalafaMPTGFbeta1 overexpression is associated with improved survival and low tumor cell proliferation in patients with early-stage pancreatic ductal adenocarcinomaOncotarget201781999100627895310
- CravenKEGoreJWilsonJLKorcMAngiogenic gene signature in human pancreatic cancer correlates with TGF-beta and inflammatory transcriptomesOncotarget20167132334126586478
- JavleMLiYTanDBiomarkers of TGF-beta signaling pathway and prognosis of pancreatic cancerPLoS One201491e8594224465802
- GasparNJLiLKapounAMInhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasivenessMol Pharmacol200772115216117400764
- SanoMDriscollDRDeJesus-MongeWEActivation of WNT/beta-catenin signaling enhances pancreatic cancer development and the malignant potential via up-regulation of Cyr61Neoplasia2016181278579427889647
- ZhouWLiYGouSMiR-744 increases tumorigenicity of pancreatic cancer by activating Wnt/beta-catenin pathwayOncotarget2015635375573756926485754
- JiangHLiQHeCActivation of the Wnt pathway through Wnt2 promotes metastasis in pancreatic cancerAm J Cancer Res20144553754425232495
- ThomasGChardesTGaboritNHER3 as biomarker and therapeutic target in pancreatic cancer: new insights in pertuzumab therapy in preclinical modelsOncotarget20145167138714825216528
- TroianiTMartinelliECapassoATargeting EGFR in pancreatic cancer treatmentCurr Drug Targets201213680281022458527
- MiyabayashiKIjichiHMohriDErlotinib prolongs survival in pancreatic cancer by blocking gemcitabine-induced MAPK signalsCancer Res20137372221223423378339
- KoizumiKTannoSNakanoYActivation of p38 mitogen-activated protein kinase is necessary for gemcitabine-induced cytotoxicity in human pancreatic cancer cellsAnticancer Res20052553347335316101149
