Abstract
Studies have shown that single-nucleotide polymorphisms in MDM2 gene may play important roles in the development of malignant tumor. The association of del1518 polymorphism (rs3730485) in the MDM2 promoter with cancer susceptibility has been extensively studied; however, the results are contradictory. To quantify the association between this polymorphism and overall cancer risk, we conducted a meta-analysis with 12,905 cases and 10,026 controls from 16 eligible studies retrieved from PubMed, Embase, and Chinese Biomedical (CBM) databases. We assessed the strength of the connection using odds ratios (ORs) and 95% confidence intervals (CIs). In summary, no significant associations were discovered between the del1518 polymorphism and overall cancer risk (Del/Del vs Ins/Ins: OR =1.01, 95% CI =0.90–1.14; Ins/Del vs Ins/Ins: OR =1.03, 95% CI =0.96–1.12; recessive model: OR =0.98, 95% CI =0.90–1.07; dominant model: OR =1.03, 95% CI =0.94–1.12; and Del vs Ins: OR =1.01, 95% CI =0.94–1.07). In the stratified analysis by source of control, quality score, cancer type, and ethnicity, no significant associations were found. Despite some limitations, the current meta-analysis provides solid statistical evidence of lacking association between the MDM2 del1518 polymorphism and cancer risk.
Introduction
Worldwide cancer incidence and mortality continues to increase greatly. More than 14.1 million cancer cases and 8.2 million cancer-associated deaths were reported by the latest GLOBOCAN estimates. The burden of cancer has become a serious global problem, particularly in economically developing countries, on account of aging society, smoking, nutritional status, obesity, and physical inactivity. Despite restriction on tobacco use, advocation on vaccination, early diagnosis, and treatment that can prevent cancer mortality effectively, the causes of cancer are still far from clear.Citation1 According to molecular epidemiological researches, genetic polymorphisms have been implicated in diverse carcinogenesis mechanisms.Citation2,Citation3
p53, a tumor suppressor protein, is implicated in almost half of all human cancer. In response of genotoxic stress and oncogenic signals, p53 actives a transcriptional program to induce several cellular damage responses, including apoptosis, cell cycle halt, and autophagy.Citation4,Citation5 In certain cases, p53 activity is depressed by the overexpression of MDM2, a cellular antagonist. MDM2 acts as a major node in the P53 pathway. The MDM2 is an ubiquitin ligase E3 for p53, which promotes the degradation of P53 by the proteasome. There is a negative feedback loop between P53 and MDM2, in which activating p53 protein increases MDM2 transcription, and the resulting MDM2 protein interacts with p53, thereby causing p53 degradation.Citation6 Previous reports have clearly shown that polymorphisms associate with diseases susceptibility by altering affected proteins structurally and functionally. Human MDM2 gene is located on chromosome 12q14.3–q15.1, which contains two promoters, an upstream constitutive promoter (P1) and an internal promoter (P2).Citation7,Citation8 The genetic variations within either of promoters may alter the expression of MDM2. For instance, MDM2 SNP309 (rs2279744, T>G) within promoter P2 enhances the affinity of promoter with the transcriptional activator SP1 to increase MDM2 transcription, thereby promoting tumor development in the different tissues.Citation9 In addition, a del1518 polymorphism (rs3730485), a 40 bp insertion/deletion in the MDM2 promoter P1 region, could also affect promoter activity.Citation10 Recently, several lines of evidence have indicated that the del1518 del-allele contributes to an increase in cancer risk;Citation11–Citation13 however, opposite results were also reported.Citation14,Citation15 To explore the precise correlation between del1518 polymorphism and cancer risk, we performed this meta-analysis with all eligible publications.
Methods
Publication search
All possible publications related the association between MDM2 del1518 polymorphism and cancer risk were searched for from PubMed and Embase (up to March 29, 2017). The following items were used: “MDM2 or mouse double minute 2 homolog or human homolog of mouse double minute 2”, “del1518 or rs3730485”, “polymorphism or single-nucleotide polymorphism (SNP) or variant”, and “tumor or cancer or carcinoma or neoplasm”. The reference lists of all eligible studies in the initial search were searched manually to retrieve potentially relevant studies. To broaden our search to find the most relevant research, we also retrieved publications from Chinese Biomedical (CBM) database with items of “MDM2” and “cancer” in Chinese.
Inclusion/exclusion criteria
Studies selected had to meet the following inclusion criteria: 1) evaluating the association between MDM2 del1518 polymorphism and cancer risk, 2) case–control studies, 3) supplying detailed genotype distribution data to estimate the odds ratio (OR) with 95% confidence interval (CI), and 4) published in English or Chinese. Only the latest study was selected from duplicate publications. In addition, studies with genotype frequency distribution of controls departed from Hardy–Weinberg equilibrium (HWE) were excluded from the final analysis.
Data extraction
Information was extracted from studies by two authors (WH and AZ) independently. If two authors had disagreement, a third author would join in the discussion. A final decision would be made by voting. The following information was collected from each study: first author’s surname, year of publication, country of origin, ethnicity, cancer type, control source, total number of cases and controls, genotype methods, percent of males, and numbers of cases and controls with the Ins/Ins, Ins/Del, and Del/Del genotypes for del1518 polymorphism. The subgroup analysis was carried out by ethnicity (Asians and Caucasians), source of control (hospital based [HB] and population based [PB]), and cancer type. Cancer type investigated in only one study was classified into the “others” group.
Statistical methods
Consistency with HWE in the control group was evaluated by Pearson’s goodness-of-fit χ2 test for each study (P<0.05 was considered as statistically significant deviation from HWE). OR and 95% CI were used to assess the strength of the association between the del1518 polymorphism and cancer risk. Pooled risk estimates were calculated under the alleles contrast (Del vs Ins), homozygous (Del/Del vs Ins/Ins), heterozygous (Ins/Del vs Ins/Ins), dominant (Ins/Del and Del/Del vs Ins/Ins), and recessive (Del/Del vs Ins/Del and Ins/Ins) model. We used the chi square-based Q-test to determine the heterogeneity among studies. A P-value of <0.1 means significant heterogeneity. Under such circumstances, the random-effects model would be taken to assess the pooled ORs; otherwise, the fixed-effects would be adopted.Citation16–Citation18 The quality assessment was also performed using the quality assessment criteria () as described previously.Citation19 All studies were scored from 0 to 15, and only studies with a score of ≥12 were regarded as high quality. Subgroup analysis was conducted by cancer type, source of control, quality score, and race. Begg’s funnel plots and Egger’s test were used to assess publication bias. The accuracy and reliability of results were verified by sensitivity analysis by sequentially removing one single study at a time to check the influence of deleted study on pooled ORs. All the statistical tests were conducted by STATA Version 11.0 (StataCorp LP, College Station, TX, USA). P<0.05 indicated statistical significance.
Results
Study characteristics
As shown in , a total of 14 articles were retrieved from the initial literature search. After careful examination and assessment, three publications were excluded for the following reasons: one had duplicate data with the previous researchCitation20 and other two were irrelevant with cancer risk.Citation21,Citation22 All studies were in agreement with HWE, except for an article by Jin et al.Citation11 We included this article for the further study because genotype distribution of the TP53 Arg72 Pro polymorphism was in accordance with HWE in the same study.Citation11 Of these 11 publications, two publications involved two cancer typesCitation14,Citation23 and one publication involved four cancer types.Citation24 We divided these articles into different independent studies based on cancer type. And, the controls of these three publications were included into meta-analysis only once. Main characteristics of each study are summarized in . Totally, there were 12,905 cases ranging from 132 to 2,501 and 10,026 controls ranging from 132 to 3,749 included in the present meta-analysis. Three studies were conducted on esophageal squamous cell carcinoma,Citation14,Citation15,Citation25 two studies were conducted on breast,Citation24,Citation26 colorectal,Citation11,Citation24 ovarian cancer,Citation23,Citation27 and lung,Citation24,Citation28 and five studies were conducted on “others”, such as gastric cardiac adenocarcinoma,Citation14 hepatocellular carcinoma,Citation12 uterine leiomyoma,Citation13 prostate cancer,Citation24 and endometrial cancer.Citation23 Twelve studies were PB,Citation11–Citation14,Citation24–Citation26,Citation28 and four studies were HB.Citation15,Citation23,Citation27 Moreover, there were seven studies performed among CaucasiansCitation13,Citation23,Citation24 and nine studies among Asians.Citation11,Citation12,Citation14,Citation15,Citation25–Citation28 In addition, quality scores of seven studies were <12 and scores of the remaining studies were ≥12.
Figure 1 Flowchart of selection of studies included in the current meta-analysis for the correlation between MDM2 del1518 polymorphism and overall cancer susceptibility.
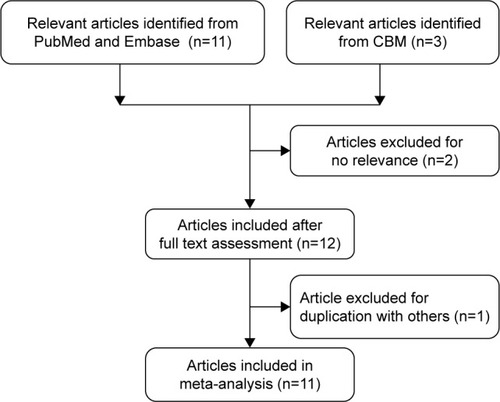
Table 1 Characteristics of studies included in the current meta-analysis
Meta-analysis results
The results for the association between del1518 del/ins polymorphism and cancer risk are shown in and . Overall, the pooled risk estimates suggested that no statistically significant association was found between the polymorphism and cancer risk (Del/Del vs Ins/Ins: OR =1.01, 95% CI =0.90–1.14; Ins/Del vs Ins/Ins: OR =1.03, 95% CI =0.96–1.12; Del/Del vs Ins/Del + Ins/Ins: OR =0.98, 95% CI =0.90–1.07; Ins/Del + Del/Del vs Ins/Ins: OR =1.03, 95% CI =0.94–1.12; Del vs Ins: OR =1.01, 95% CI =0.94–1.07). Moreover, the stratified analysis by source of control, quality score, cancer type, and ethnicity showed no evidence of the association between del1518 polymorphism and overall cancer risk.
Figure 2 Forest plots of effect estimates for MDM2 del1518 polymorphism and overall cancer susceptibility under dominant model (ID + DD vs II).
Abbreviations: CIs, confidence intervals; ORs, odds ratios; ID, Ins/Del; II, Ins/Ins; DD, Del/Del.
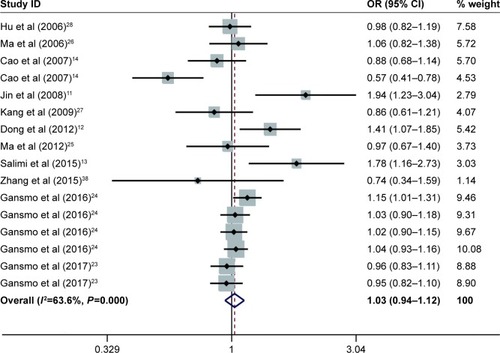
Table 2 Meta-analysis of the association between MDM2 del1518 (rs3730485) polymorphism and overall cancer risk
Heterogeneity and sensitivity analysis
As shown in , significant between-study heterogeneity was observed under the different models (homozygous model: P=0.003; heterozygous model: P=0.009; recessive model: P=0.049; dominant model: P<0.001; and allele comparing: P<0.001); therefore we adopted the random-effects model to generate wider CIs. Sensitivity analysis suggested that ORs were not significantly altered by any single study, indicating that this meta-analysis result was stable and reliable.
Publication bias
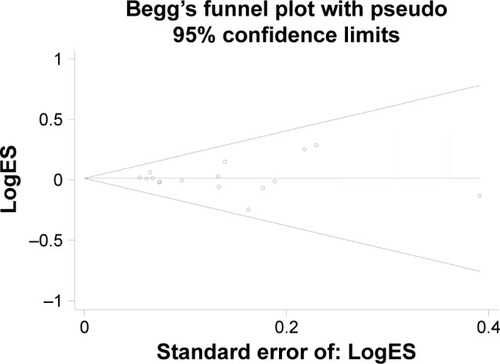
The publication bias was performed by Begg’s funnel plot and Egger’s test. We found no asymmetry of funnel plot (). In addition, the results of Egger’s test did not suggest any evidence of publications bias (homozygous: P=0.919; heterozygous: P=0.921; recessive model: P=0.920; dominant model: P=0.921; and allele comparing model: P=0.990).
Discussion
The tumor suppressor p53, a transcriptional factor, essentially controls the growth and development of normal cells. p53 instability may lead to cell cycle disorder and aberrant cellular apoptosis, thereby strongly contributing to malignant transformation and progression.Citation29,Citation30 The MDM2 gene, a genomic size of 34 kb, contains two promoters, such as a p53-responsive promoter and a p53-independent promoter.Citation8 MDM2, as a major mechanism of genetic toxicity and carcinogenesis, is a principle regulator of the stabilization of p53 in the no-stress condition.Citation31 MDM2, functioning as an E3 ligase, specifically complexes with p53 by the N-terminus domain and induces its degradation through proteasomal pathway.Citation32,Citation33 The expression of MDM2 is elevated by p53-positive regulation. Given a negative autoregulatory loop between MDM2 and p53, the importance of MDM2 has been proved in the central part of p53-assosiated signal pathway.Citation34,Citation35 Genetic variations, including SNPs and other types of polymorphisms, may modify genetic predisposition to diseases.Citation36 Some genetic alterations located in the MDM2 gene, such as SN309,Citation37 have been proved to be associated with cancer risk.Citation16,Citation38,Citation39 As far as we know, there are at least 4,765 polymorphisms found in the MDM2 gene (http://www.ncbi.nlm.nih.gov/projects/SNP). One of the frequently investigated polymorphisms is MDM2 del1518, a common 40 bp Ins/Del polymorphism, located in constitutive promoter with a putative TATA motif.Citation10,Citation26
Due to the special location of del1518 polymorphism, its association with cancer risk has been a hot spot. Salimi et al suggested that women carrying del allele had an increased risk of uterine leiomyoma compared with the women carrying MDM2 40 bp insert allele.Citation13 Jin et al reported that the 40 bp deletion allele had an important role in the oncogenesis of colorectal cancer, specially for rectal cancer.Citation11 In addition, a genetic association study by Dong et al have found that the del1518 was significantly associated with an increased risk of hepatocellular carcinoma.Citation12 While some studies have considered del1518 del-allele as susceptibility loci for the risk of various cancer types,Citation14,Citation15 other studies failed to confirm its contribution to cancer risk.Citation23–Citation28 The discrepant results might be caused by small sample size, and variations among different study populations. Moreover, it is widely believed that malignancies arising from different tissue had completely distinct molecular mechanisms, and even the same cancer type could display significant heterogeneity among different individuals.
In the current meta-analysis, we combined all eligible investigations comprising 12,905 cases and 10,026 controls from 16 studies on MDM2 del1518 polymorphism. We found no significant association between del1518 polymorphism in the overall analysis and stratification analyses by cancer type, ethnicity, source of control, and scores. A latest meta-analysis conducted by Yu et al found that there was no significant difference between del1518 polymorphism and overall squamous cell carcinoma susceptibility with a total of 309 cases and 1,000 controls from three studies, results of which is consistent with the current study.Citation22
To our knowledge, this investigation is the largest and most comprehensive meta-analysis regarding the association between del1518 polymorphism and all cancer type. However, there were still some limitations to be addressed. First, statistical power might be limited to certain degree, especially for stratified analyses, such as cancer type of gastric, hepatocellular, uterine leiomyoma, prostate, and endometrial. Hence, the results of this meta-analysis should be interpreted with caution. Second, because of the stratification analysis of ethnicity included Asians and Caucasians only, we could not take genetic and geographical differences into consideration. Further analysis should contain diverse area and ethnicities. Third, the results of this study were based on unadjusted estimates by the reason of insufficient data of individual such as age, gender, smoking, environment exposure, and lifestyles. Gene–gene and gene–environment interactions could not be explored. Fourth, there was obvious heterogeneity for the meta-analysis, which might owe to differences in cancer types, the populations, geographical area, and study designs. Finally, the included studies were mainly searched for from PubMed, Embase, and CBM; therefore, publication bias might exist in this meta-analysis for the unavailability of the unpublished studies with negative results.
Conclusion
Our present meta-analysis suggested no association between MDM2 gene del1518 polymorphism and overall cancer susceptibility. Further well-designed study with large sample sizes, different ethnicities, cancer types, and gene–environment interactions is needed to confirm our findings.
Acknowledgments
This study was supported by grants from the introduction of talent scientific research start-up fund of Guangdong Second Provincial General Hospital (YY2016-004), Scientific Research Foundation of Wenzhou (Y20100175), Zhejiang Provincial Medical and Health Science and Technology plan (2013RCA035), Project of Science and Technology Department of Zhejiang Province (2014C37003), and Zhejiang Provincial Natural Science Foundation of China (LY16H160054).
Supplementary material
Table S1 Score of quality assessment
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- PharoahPDDunningAMPonderBAEastonDFAssociation studies for finding cancer-susceptibility genetic variantsNat Rev Cancer200441185086015516958
- XuCZhuJFuWMDM4 rs4245739 A > C polymorphism correlates with reduced overall cancer risk in a meta-analysis of 69477 subjectsOncotarget2016744717187172627687591
- LevineAJp53, the cellular gatekeeper for growth and divisionCell19978833233319039259
- LevineAJFinlayCAHindsPWP53 is a tumor suppressor geneCell2004116suppl 2S67S7015055586
- MichaelDOrenMThe p53 and Mdm2 families in cancerCurr Opin Genet Dev2002121535911790555
- BarakYGottliebEJuvengershonTOrenMRegulation of mdm2 expression by p53: alternative promoters produce transcripts with non-identical translation potentialGenes Dev1994815173917497958853
- ZaubermanAFlusbergDHauptYBarakYOrenMA functional p53-responsive intronic promoter is contained within the human mdm2 geneNucleic Acids Res1995231425847651818
- BondGLHuWBondEEA single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humansCell2004119559160215550242
- LalondeMEOuimetMLariviereMKritikouEASinnettDIdentification of functional DNA variants in the constitutive promoter region of MDM2Hum Genomics2012611523244604
- JinMJZhangSSZhangYJPolymorphisms in P53 pathway genes and colorectal cancer riskChin J Dig20082810703705
- DongDGaoXZhuZYuQBianSGaoYA 40-bp insertion/deletion polymorphism in the constitutive promoter of MDM2 confers risk for hepatocellular carcinoma in a Chinese populationGene20124971667022285926
- SalimiSHajizadehAKhodamianMPejmanAFazeliKYaghmaeiMAge-dependent association of MDM2 promoter polymorphisms and uterine leiomyoma in South-East Iran: a preliminary reportJ Obstet Gynaecol Res201541572973425511444
- CaoYYZhangXFGuoWWangRGeHZhangJHAssociation of the MDM2 polymorphisms with susceptibility of esophageal squamous cell carcinoma and that of gastric cardiac adenocarcinomaTumor2007278628632
- ZhangLZhuZWuHWangKAssociation between SNP309 and del1518 polymorphism in MDM2 homologue and esophageal squamous cell carcinoma risk in Chinese population of shandong provinceAnn Clin Lab Sci201545443343726275695
- HeJShiTYZhuMLWangMYLiQXWeiQYAssociations of Lys939Gln and Ala499Val polymorphisms of the XPC gene with cancer susceptibility: a meta-analysisInt J Cancer201313381765177523400628
- HeJXiBRuiterRAssociation of LEP G2548A and LEPR Q223R polymorphisms with cancer susceptibility: evidence from a meta-analysisPLoS One2013810e7513524146750
- HeJWangFZhuJHChenWCuiZJiaWHNo association between MTR rs1805087 A > G polymorphism and non-Hodgkin lymphoma susceptibility: evidence from 11 486 subjectsLeuk Lymphoma201556376376724956146
- HeJLiaoXYZhuJHAssociation of MTHFR C677T and A1298C polymorphisms with non-Hodgkin lymphoma susceptibility: evidence from a meta-analysisSci Rep20144615925146845
- WangDJLiYZhouRMAssociation of p73 and MDM2 polymorphisms with the risk of epithelial ovarian cancerTumor2009194892897
- SunPZhangZWanJZhaoNJinXXiaZAssociation of genetic polymorphisms in GADD45A, MDM2, and p14 ARF with the risk of chronic benzene poisoning in a Chinese occupational populationToxicol Appl Pharmacol20092401667219596022
- YuHLiHZhangJLiuGInfluence of MDM2 polymorphisms on squamous cell carcinoma susceptibility: a meta-analysisOnco Targets Ther201696211622427785069
- GansmoLBBjornslettMHalleMKMDM2 promoter polymorphism del1518 (rs3730485) and its impact on endometrial and ovarian cancer riskBMC Cancer20171719728158999
- GansmoLBVattenLRomundstadPAssociations between the MDM2 promoter P1 polymorphism del1518 (rs3730485) and incidence of cancer of the breast, lung, colon and prostateOncotarget2016719286372864627081698
- MaJZhangJNingTChenZXuCAssociation of genetic polymorphisms in MDM2, PTEN and P53 with risk of esophageal squamous cell carcinomaJ Hum Genet201257426126422336889
- MaHHuZZhaiXPolymorphisms in the MDM2 promoter and risk of breast cancer: a case-control analysis in a Chinese populationCancer Lett2006240226126716288830
- KangSWangDJLiWSAssociation of p73 and MDM2 polymorphisms with the risk of epithelial ovarian cancer in Chinese womenInt J Gynecol Cancer200919457257719509552
- HuZMaHLuDGenetic variants in the MDM2 promoter and lung cancer risk in a Chinese populationInt J Cancer200611851275127816152608
- VousdenKHPrivesCBlinded by the light: the growing complexity of p53Cell2009137341319410540
- AshcroftMVousdenKHRegulation of p53 stabilityOncogene199918537637764310618703
- MidgleyCALaneDPp53 protein stability in tumour cells is not determined by mutation but is dependent on MDM2 bindingOncogene1997151011799294611
- LainSLaneDImproving cancer therapy by non-genotoxic activation of p53Eur J Cancer20033981053106012736103
- LukashchukNVousdenKHUbiquitination and degradation of mutant p53Mol Cell Biol200727238284829517908790
- NagSQinJSrivenugopalKSWangMZhangRThe MDM2-p53 pathway revisitedJ Biomed Res201327425427123885265
- JinSLevineAJThe p53 functional circuitJ Cell Sci2001114pt 234139414011739646
- EngleLJSimpsonCLLandersJEUsing high-throughput SNP technologies to study cancerOncogene200625111594160116550159
- WangHGWuQYZhouHThe MDM2 SNP309T>G polymorphism increases bladder cancer risk among Caucasians: a meta-analysisAsian Pac J Cancer Prev201415135277528125040988
- ZhangYYangDZhuJHChenMBShenWXHeJThe association between NQO1 Pro187Ser polymorphism and urinary system cancer susceptibility: a meta-analysis of 22 studiesCancer Invest2015332394025608636
- ZhangTXieSZhuJHLiQWHeJZengAPAssociation of IL10 -819C>T and -592C>A polymorphisms with non-Hodgkin lymphoma susceptibility: evidence from published studiesJ Cancer20156870971626185532