Abstract
Background
The cytotoxic effects of microtubule-targeting agents (MTAs) are often attributed to targeted effects on mitotic cells. In clinical practice, MTAs are combined with DNA-damaging agents such as ionizing radiation (IR) with the rationale that mitotic cells are highly sensitive to DNA damage. In contrast, recent studies suggest that MTAs synergize with IR by interfering with the trafficking of DNA damage response (DDR) proteins during interphase. These studies, however, have yet to demonstrate the functional consequences of interfering with interphase microtubules in the presence of IR. To address this, we combined IR with an established MTA, mebendazole (MBZ), to treat glioma cells exclusively during interphase.
Materials and methods
To test whether MTAs can sensitize interphase cells to IR, we treated GL261 and GBM14 glioma cells with MBZ during 3–9 hours post IR (when the mitotic index was 0%). Cell viability was measured using a WST-1 assay, and radiosensitization was quantified using the dose enhancement factor (DEF). The effect of MBZ on the DDR was studied via Western blot analysis of H2AX phosphorylation. To examine the effects of MTAs on intracellular transport of DDR proteins, Nbs1 and Chk2, cytoplasmic and nuclear fractionation studies were conducted following treatment of glioma cells with MBZ.
Results
Treatment with MBZ sensitized interphase cells to the effects of IR, with a maximal DEF of 1.34 in GL261 cells and 1.69 in GBM14 cells. Treatment of interphase cells with MBZ led to more sustained γH2AX levels post IR, indicating a delay in the DDR. Exposure of glioma cells to MBZ resulted in a dose-dependent sequestration of Chk2 and Nbs1 in the cytoplasm.
Conclusion
This study demonstrates that MBZ can sensitize cancer cells to IR independently of the induction of mitotic arrest. In addition, evidence is provided supporting the hypothesis that MTA-induced radiosensitization is mediated by inhibiting DDR protein accumulation into the nucleus.
Introduction
Microtubule-targeting agents (MTAs) have been used for a long time against a wide range of malignancies. It is generally believed that MTAs kill cancer cells by causing cell cycle arrest in M-phase, followed by activation of apoptotic pathways and cell death.Citation1–Citation4 Many studies have used this rationale to explain the potent radiosensitization effects exerted by MTAs,Citation5,Citation6 ie, by inducing mitotic arrest, MTAs increase the proportion of tumor cells in a phase of the cell cycle that is very susceptible to DNA damage.Citation7,Citation8 Currently, chemoradiotherapy regimens including MTAs have been proven effective for the treatment of breast cancer, esophageal cancer and a variety of other neoplasms.Citation9,Citation10
Support for a mitosis-based mechanism for the therapeutic effects of MTAs has been derived from the characteristic side effects of these drugs, which include hair loss, neutropenia and gastrointestinal upset. These deleterious effects demonstrate the profound sensitivity of rapidly dividing tissues to MTAs. However, the action of MTAs on mitotic cells fails to explain their clinical efficacy against many slow-growing solid tumors with exceptionally low mitotic indices.Citation11 Most human tumors have a doubling time of 30–60 days or longer, making it unlikely that mitotic arrest serves as a critical mechanism of MTA-induced therapeutic benefit.Citation12 A prime example of this “proliferation rate paradox” is the significant activity of MTAs against adenocarcinoma of the prostate, a highly indolent cancer.Citation13,Citation14 Thus, a number of interphase-based mechanisms for the efficacy of MTAs in cancer therapy have been proposed, although not without controversy.Citation15,Citation16
A recent study has shown that the MTAs, vincristine (VCR) and paclitaxel, can delay DNA damage repair.Citation17 These MTAs were also shown to interfere with the trafficking of DNA damage response (DDR) proteins, including ATM, ATR and p53, from the cytosol to the nucleus, strongly suggesting that MTAs can sensitize cells to radiation by blocking microtubule-based transport of DDR proteins into the nucleus during interphase.
It is challenging to physically separate mitotic from interphase cells in the presence of an MTA, as this results in a steady accumulation of new mitotic cells as long as the MTA is present. Thus, the question remains as to what extent the role of MTAs in radiosensitization is caused by interference with microtubule-facilitated nuclear import. To address this question, we took advantage of the fact that ionizing radiation (IR) treatment induces G2–M cell cycle arrest, thereby transiently eliminating the mitotic cell population and strongly enriching for interphase cells. Using glioblastoma cells and the MTA, mebendazole (MBZ), as a model system, we show that the effect of MBZ in interphase is responsible for the majority of the radiosensitization effect of this MTA.
Materials and methods
Cell lines and reagents
GL261 (glioma) cells were obtained from the National Cancer Institute (Frederick, MD, USA). Cells were cultured in macrophage serum-free medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum, 1% penicillin/streptomycin and 1 mM L-glutamine. GBM14 cells have been described previously,Citation18 and they were obtained by Dr J Sarkaria at Mayo Clinic (Rochester, MN, USA) and cultured in StemPro media (Thermo Fisher Scientific), as directed by the manufacturer.
Drug treatment
For each experiment, GL261 and GBM14 cells were cultured without drug or treated with MBZ (Sigma-Aldrich Co., St Louis, MO, USA). MBZ stocks were prepared by dissolving the drug in dimethyl sulfoxide (DMSO; Sigma-Aldrich Co.). About 24 hours after plating, glioma cells were treated with MBZ, while control cells were treated with 0.01% DMSO.
Irradiation procedure
For all experiments requiring radiation, cells were irradiated using a biological irradiator (RS2000; Rad Source Technologies, Buford, GA, USA).
Antibodies
Phospho-MPM2 was obtained from EMD Millipore (Billerica, MA, USA), and γH2AX, H2AX, Chk2, Nbs1 and GAPDH were purchased from Cell Signaling (Danvers, MA, USA).
Immunofluorescence
GL261 cells were seeded at a density of 30,000 cells/well in 24-well plates containing cover slips coated with laminin (Sigma-Aldrich Co.). The following day, cells were exposed to 6 Gy of IR. At designated time points post IR, cells were washed in PBS and fixed in 4% formaldehyde in PBS at room temperature. Once the cells were fixed, immunofluorescence was performed using an MPM2 antibody (EMD Millipore) and counterstained with 4,6-Diamidino-2-phenylindole, dihydrochloride (DAPI). For each condition, a total of 10 fluorescence micrograph images were taken with a Zeiss Axiovert 200M inverted microscope (Thornwood, NY, USA), running on Axiovision software. For each image field, the total number of cells and the number of MPM2-positive cells were quantified. The mitotic index was calculated at each time point using the number of MPM2-positive cells as a percentage of the total number of cells counted in all 10 fields.
Cell viability assays
GL261 cells were seeded in 96-well plates at a density of 1,000 cells/well. GBM14 cells were seeded at a density of 10,000 cells/well. About 24 hours after seeding, cells were treated with 25–150 nM MBZ and irradiated with 3, 6 or 9 Gy. Drug treatment was applied for a 6-hour time window beginning either 6 hours pre IR or 3 hours post IR. Following the 6-hour window, the drug was washed out and replaced with fresh medium. Control cells were either left nonirradiated or irradiated with 3, 6 or 9 Gy of IR. Cell viability was examined 72 hours post IR using a WST-1 cell viability assay (Abcam, Cambridge, MA, USA) at an absorbance of 450 nm. For any given radiation dose–response, data were normalized by the fraction of viable cells treated with a given dose of drug in the absence of radiation. The radiosensitizing effect of MBZ and VCR was quantified using the dose enhancement factor (DEF) at the point of 50% (DEF50) cell viability. The DEF was calculated for each MBZ concentration using the following formula: (surviving fraction with radiation alone)/(surviving fraction with radiation + MBZ).
Assessment of the DDR
GL261 cells were seeded at a density of 800,000 cells/well in 6-cm dishes. About 24 hours after seeding, cells were irradiated with 6 Gy of IR, followed by treatment with 150 nM MBZ during 3–9 hours post IR. Control cells were either left nonirradiated or irradiated with 6 Gy. All cells were harvested in lysis buffer at 0.5, 3, 6 and 9 hours post IR. The lysis buffer was composed of 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 10% Nonidet P-40, 1× protease inhibitor (Thermo Fisher Scientific), phosphatase inhibitor (Hoffman-La Roche Ltd., Basel, Switzerland) and 0.4 U/mL Benzonase (EMD Millipore). For each condition, 60 μg of cell lysate was diluted in 1× NuPAGE Sample Reducing Agent (Thermo Fisher Scientific) and 1× NuPAGE LDS Sample Buffer (Thermo Fisher Scientific). All samples were loaded onto a NuPAGE, 4%–12% Bis-Tris Protein Gel (Thermo Fisher Scientific) for Western blot analysis. Histone H2AX and γH2AX were detected by Western blot using anti-histone H2AX or anti-γ-H2AX (Ser139) monoclonal antibodies (Cell Signaling).
Nuclear and cytoplasmic fractionation
GL261 cells were seeded at a density of 500,000 cells in 6-cm dishes. The following day, cells were treated with 3 Gy. MBZ treatment (25–250 nM) was performed for a 6-hour time window beginning 3 hours post IR. Irradiated control cells were treated with 3 Gy followed by 0.01% DMSO in medium during the same time period. Nonirradiated control cells were treated with 0.01% DMSO in medium for a total of 6 hours. After the 6-hour period of MBZ treatment, cells were harvested for protein analysis in cytoplasmic (C) and nuclear (N) fractions. The C and N fractions were collected according to the protocol of the NE-PER Nuclear and Cytoplasmic Extraction Reagents Kit (Thermo Fisher Scientific). For each sample, 20% of the final volume of the C or N fractions was diluted in 1× NuPAGE Sample Reducing Agent and 1× NuPAGE LDS Sample Buffer. All samples were loaded onto a NuPAGE, 4%–12% Bis-Tris Protein Gel for Western blot analysis. Blots were incubated in antibodies: Nbs1, Chk2, GAPDH and H2AX monoclonal antibodies (all from Cell signaling). Cytoplasmic and nuclear fractions were normalized by GAPDH and H2AX levels, respectively. The percentage of cytoplasmic retention of DDR proteins was calculated by the following formula: [C/(C + N)] × 100%.
Statistical analysis
All analyses were performed using Graphpad Prism 7 software. Radiosensitization experiments were analyzed using the two-way ANOVA method to compare the mean cell viability between treatment groups. The interaction between the MBZ treatment group and radiation dose was also examined. One-way ANOVA was used to compare the concentration of a drug that gives half-maximal response (EC50) values for each effect as outlined in . If a significant difference between means was found by ANOVA, then multiple comparisons between treatment groups were conducted. The Tukey–Kramer method was used to adjust for multiple comparisons. Studies examining the effect of MBZ on intracellular transport of DDR proteins were analyzed with unpaired two-tailed Student’s t-test for direct comparisons of means. The same method was used to compare means in the analysis of H2AX phosphorylation following exposure to IR. For all studies, results were considered statistically significant for values of P<0.05.
Table 1 DEF50 for MBZ-mediated radiosensitization, cytoplasmic sequestration of DDR proteins and induction of mitotic arrest
Results
IR transiently eliminates the mitotic cell population
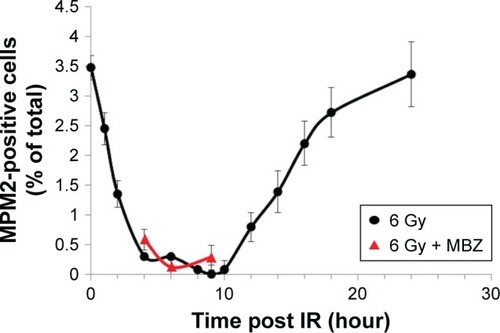
To select for a population composed entirely of interphase cells, we took advantage of the fact that IR treatment induces G2–M cell cycle arrest and transiently eliminates the mitotic cell population. Glioma cells were treated with 6 Gy of IR. To monitor the mitotic index, we quantified the proportion of MPM2-positive cells by immunofluorescence at several time points post IR. MPM2 is an antibody that recognizes a phosphorylated serine/threonine epitope found in proteins that are phosphorylated at the onset of mitosis.Citation19 MPM2 is recognized as a reliable mitotic marker in the literature and has been used to assess the mitotic index in cells exposed to radiation and a number of chemotherapeutic agents.Citation20–Citation22 The baseline mitotic index of the glioma cell population was 3.5% and was strongly inhibited during a period of 3–10 hours after exposure to 6 Gy of IR. Recovery of the mitotic cell population begins after 10 hours post IR and completes by 24 hours post IR. To confirm that the mitotic index remained strongly inhibited even in the presence of MBZ, we applied MBZ during 3–9 hours post IR. In the presence of MBZ, the mitotic index remained suppressed at 4 hours (mitotic index =0.58%), 6 hours (mitotic index =0.12%) and 9 hours (mitotic index =0.28%) post IR. Thus, the treatment of GL261 glioma cells with 6 Gy of IR eliminated the mitotic cell population for a time period of ~6 hours ().
Figure 1 IR temporarily eliminates the mitotic cell population.
Abbreviations: IR, ionizing radiation; SEM, standard error of the mean.
MTAs sensitize interphase cells to IR
In the following experiments, we compared the radiosensitizing effect of MTAs when applied during different time periods with respect to IR, using both murine GL261 cells and primary patient-derived GBM14 cells. A simplified schematic of all three treatment conditions is shown in . To examine whether MTAs can sensitize glioma cells to IR, we treated GL261 cells with MBZ ( and ) or VCR (). In order to test, whether MTAs can sensitize interphase cells to IR, we treated glioma cells with 25–150 nM MBZ during 3–9 hours post IR ( and ). The use of MBZ after exposure to IR made it possible to study the impact of MBZ independent of its effect on mitotic cells. Cells were exposed to MBZ after different doses of IR. After this 6-hour time frame, the drug was washed out and replaced with a drug-free medium. The radiosensitizing effect of MBZ on interphase cells was compared with the effect of MBZ on a cell population composed of both interphase and mitotic cells. Thus, cells were treated with MBZ for a 6-hour time window immediately prior to irradiation, and subsequently the drug was either washed out and replaced with drug-free medium ( and ) or left in the original medium until the end of the assay, 72 hours post IR ( and ). Treatment with MBZ post IR sensitized GL261 cells to IR with a maximal DEF at 50% viability (DEF50) of 1.34 (). Treatment for a 6-hour time window pre IR, which increased the mitotic index to 9.2% from a baseline value of 2.8%,Citation23 sensitized glioma cells to IR with a maximal DEF50 of 1.2 in GL261 cells () and 1.33 in GBM14 cells (). In the GL261 cell line, maximal radiosensitization was observed when the drug was applied pre IR and left in the medium for 72 hours, DEF50 =1.41 (). In the GBM14 cell line, the application of the drug pre IR followed by 72-hour incubation sensitized cells to IR with a DEF50 =1.60 (). Maximal radiosensitization in the GBM14 cell line was achieved when MBZ was applied post IR, DEF50 =1.69 (). These observations show a maximal or near-maximal radiosensitization effect of MBZ when applied to cells during interphase.
Figure 2 Timing of MBZ application with respect to IR.
Abbreviations: IR, ionizing radiation; MBZ, mebendazole.
Figure 3 MBZ sensitizes GL261 cells to IR.
Abbreviations: DEF, dose enhancement factor; IR, ionizing radiation; MBZ, mebendazole; SEM, standard error of the mean.
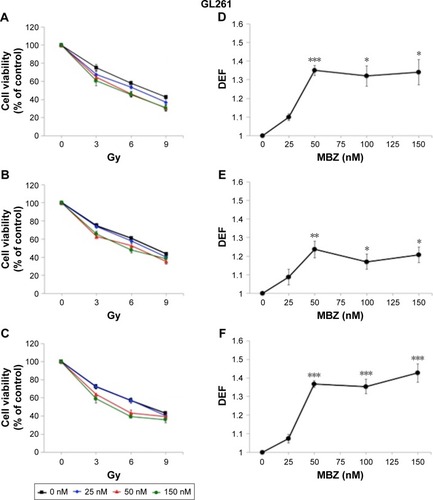
Figure 4 MBZ sensitizes GBM14 cells to IR.
Abbreviations: DEF, dose enhancement factor; IR, ionizing radiation; MBZ, mebendazole; SEM, standard error of the mean.
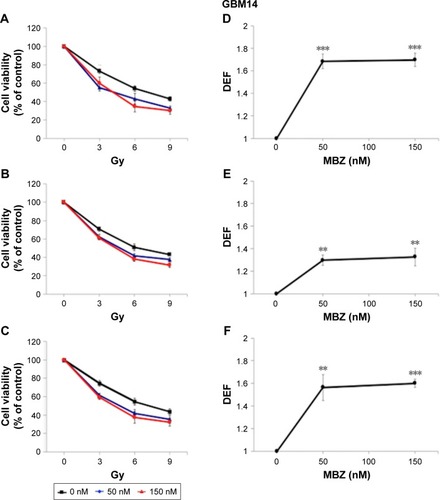
Figure 5 VCR sensitizes GL261 cells to IR.
Abbreviations: DEF, dose enhancement factor; IR, ionizing radiation; MBZ, mebendazole; SEM, standard error of the mean; VCR, vincristine.
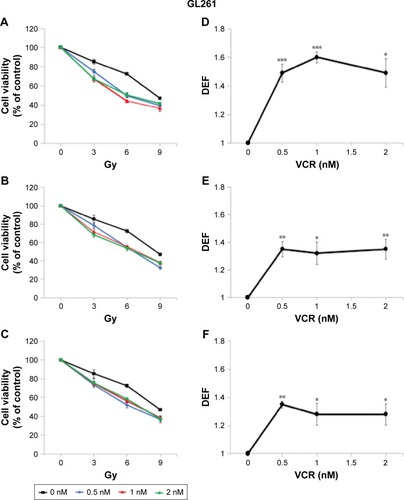
To investigate whether these findings can be generalized to other MTAs, we performed an identical set of experiments in GL261 cells using VCR, an established MTA that is frequently used in clinical practice. The radiosensitizing effect of VCR was greatest when applied post IR, DEF50 =1.53 (). The application of VCR for a 6-hour period pre IR radiosensitized GL261 cells with a DEF50 of 1.34 (). When VCR was applied pre IR and left in the medium for the remaining 72 hours, the magnitude of the radiosensitizing effect was quite similar, DEF50 =1.30 (). Thus, it appears that the radiosensitizing effect of both MBZ and VCR is largely determined by the impact of these agents on interphase cells.
MBZ treatment prolongs DDR after irradiation
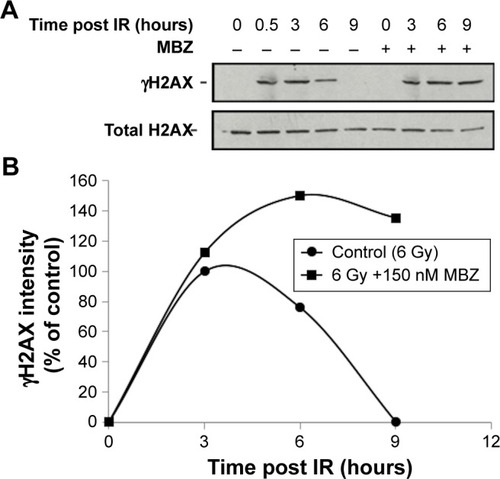
To confirm that MTAs sensitize interphase cells to IR by interfering with the DDR, we examined the level of IR-induced phosphorylated H2AX (γH2AX), which is a highly sensitive marker of DNA damage.Citation24,Citation25 We exposed glioma cells to 6 Gy of IR, followed by treatment with 150 nM MBZ during 3–9 hours post IR. Cells were harvested at different time points post IR, and γH2AX was quantified by Western blot analysis. As shown in , treatment with MBZ led to more sustained γH2AX levels in response to IR, indicating a delay in the DDR. This finding demonstrated that MTAs sensitize interphase cells to IR by interfering with the DDR.
Figure 6 MBZ prolongs the DDR after exposure to IR.
Abbreviations: DDR, DNA damage response; IR, ionizing radiation; MBZ, mebendazole; SEM, standard error of the mean.
MBZ interferes with the trafficking of DDR proteins
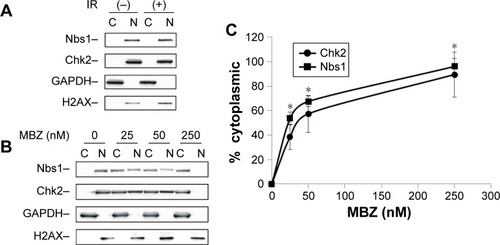
To investigate whether MTAs synergize with IR by disrupting intracellular transport of DDR proteins, we performed cytoplasmic and nuclear (C/N) fractionation studies with glioma cells that had been exposed to IR followed by treatment with MBZ. Similar to the cell viability studies, MBZ was applied during 3–9 hours post IR (only when the mitotic cell population was absent). Glioma cells were treated with different concentrations of MBZ followed by C/N fractionation. Each fraction was analyzed by Western blot. We selected two DDR proteins, Chk2 and Nbs1, to serve as potential targets of MTA-mediated toxicity. Chk2 is a key protein kinase involved in the DDR that is responsible for cell cycle checkpoint activation and DNA repair following DNA damage induced by IR.Citation26 Nbs1 is a crucial component of an enzymatic complex that repairs double strand breaks (DSBs) following irradiation or heat shock.Citation27
In control cells, which had not been treated with MBZ, Chk2 and Nbs1 were localized entirely in the nucleus (). Radiation treatment did not significantly alter the intracellular distribution of DDR proteins (). Treatment of glioma cells with MBZ sequestered Chk2 and Nbs1 in the cytoplasm in a dose-dependent manner (). These results demonstrate that MBZ, like other MTAs,Citation17 inhibits the trafficking of DDR proteins from the cytoplasm to the nucleus.
Figure 7 MBZ interferes with the trafficking of DDR proteins from the cytoplasm to the nucleus.
Abbreviations: C, cytoplasmic; DDR, DNA damage response; IR, ionizing radiation; MBZ, mebendazole; N, nuclear; SE, standard error.
MBZ sensitizes glioma cells to IR by interfering with the trafficking of DDR proteins
To more closely examine the relationship between the effects of MBZ on intracellular trafficking of DDR proteins and IR sensitization, we compared the EC50 values for radiosensitization (DEF), cytoplasmic sequestration of DDR proteins and induction of mitotic arrest by MBZ () in GL261 cells. The EC50 was defined as the concentration of MBZ required to achieve a half-maximal effect. The EC50 of cytoplasmic sequestration for Chk2 (31 nM) and Nbs1 (25 nM) was significantly lower than the EC50 of mitotic arrest (192 nM). Most notably, the EC50 of radiosensitization (35 nM) was very similar to the EC50 for cytoplasmic sequestration of DDR proteins, but significantly lower than the EC50 of mitotic arrest (P<0.01). Similarly, the EC50 for radiosensitization by VCR (<0.5 nM) is significantly lower than the EC50 for mitotic arrest in GL261 cells (2.5 nM).Citation23 These findings support the hypothesis that MBZ indeed radiosensitizes GL261 cells in large part by interfering with intracellular trafficking of DDR proteins during interphase.
Discussion
Studying the mechanisms of action of MTAs in cell toxicity, and in particular whether MTAs target cells in mitotic phase or interphase, has been hampered by the difficulty in separating these two cell populations. In this study, we transiently eliminated the mitotic cell population by irradiating glioblastoma cells, which allowed us to examine the extent to which the MTA, MBZ, can radiosensitize these cells in a cell population that is essentially made up of interphase cells only. Our results show that in this system, the effect of MBZ in interphase is responsible for the majority of the radiosensitization effect caused by this MTA.
For a long time, MTAs have been believed to inhibit tumor growth primarily by targeting mitotic cells, but this hypothesis has come under considerable scrutiny.Citation11,Citation12,Citation15,Citation16 We examined the role of interphase microtubules by determining the effect of MBZ as a radiosensitizer, when present during a time window in which mitosis is prevented by G2–M cell cycle arrest. We showed that this regimen is better at radiosensitization of tumor cells than when MBZ is present before IR administration, leading to an increase in the number of mitotic cells, and is very similar to that caused by chronic treatment with MBZ, strongly indicating that interphase microtubules are indeed the targets of MBZ. In addition, the EC50 of MBZ for radiosensitization is much lower than that for inducing mitotic arrest, further supporting the notion that the radiosensitizing effect of MBZ is independent of its effect on mitosis. We found essentially the same results for VCR. Thus, most likely this conclusion holds for a wide range of MTAs.
We also observed that MBZ, even when applied for only 6 hours, leads to a strong delay in the DDR. Thus, our results strongly support the hypothesis that MTAs prolong DNA damage repair by interfering with the trafficking of DDR proteins from the cytosol to the nucleus.Citation17
Interestingly, the EC50 for radiosensitization by MBZ (35 nM) is very similar to that of cytoplasmic sequestration of DDR proteins by MBZ (25 nM), which is much lower than the EC50 of MBZ for the induction of mitotic arrest (184 nM), further supporting the notion that MTAs radiosensitize by blocking trafficking of DDR proteins to the nucleus. The low EC50 of MBZ for the inhibition of DDR protein trafficking is surprising, because it is also much lower than the EC50 for microtubule depolymerization (132 nM) that we determined recently.Citation23 This EC50 largely reflects that of the depolymerization of interphase microtubules, as most of the cells are in interphase.
Although the mechanistic basis for the relatively low EC50 of MBZ for the inhibition of DDR protein trafficking remains to be determined, it will be of great interest to examine whether our findings with MBZ extend to other MTAs. Our observation that radiosensitization can be accomplished at a concentration of MBZ that is significantly lower than the concentration needed for cell killing on its own also has important clinical implications, as it suggests the possibility to utilize a dose of MBZ that minimizes toxicity. Importantly, our results also have implications for the optimal timing of administration of MTAs when used as radiosensitizers. Indeed, we show that optimal radiosensitization is obtained when inhibition of microtubule formation is achieved during the post-IR DNA repair period.
This study also underlines the critical role of microtubule-based transport in the response to DNA damage. Thus, further elucidation of these mechanisms may lead to the identification of novel therapeutic targets for radiosensitization.
Conclusion
We have shown that MBZ sensitizes cancer cells to IR in a manner that is largely independent of the induction of mitotic arrest by this microtubule inhibitor. We also provide evidence that MBZ-induced radiosensitization is mediated by inhibiting DDR protein accumulation into the nucleus. Thus, this study strongly supports a critical role for DDR protein trafficking in the response to radiation, suggesting that elements of the protein trafficking machinery can be mined for additional radiosensitization targets.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
GBM14 cells were kindly provided by Dr J Sarkaria, Mayo Clinic, Rochester, MN, USA. All data generated and analyzed during this study are included in this publication. This study was supported by grants to MS from the Swim Across America Foundation and the Project To Cure Foundation and to RR from the Zankel Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
- WangTWangHSoongYPaclitaxel-induced cell deathCancer200088112619262810861441
- BhallaKMicrotubule-targeted anticancer agents and apoptosisOncogene2003229075908614663486
- TuYChengSZhangSSunHXuZVincristine induces cell cycle arrest and apoptosis in SH-SY5Y human neuroblastoma cellsInt J Mol Med201331111311923129065
- JordanMAThrowerDWilsonLEffects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. Implications for the role of microtubule dynamics in mitosisJ Cell Sci19921024014061506423
- GabikianPTylerBZhangILiKWBremHWalterKRadiosensitization of malignant gliomas following intracranial delivery of paclitaxel biodegradable polymer microspheresJ Neurosurg201412051078108524605841
- TishlerRGeardCHallESchiffPTaxol sensitizes human astrocytoma cells to radiationCancer Res199252349534971350755
- SinclairWKCyclic X-ray responses in mammalian cells in vitro. 1968Radiat Res20121782AV112AV12422870963
- ElkindMSuttonHRadiation response of mammalian cells grown in culture: I. Repair of X-Ray damage in surviving Chinese hamster cellsRadiat Res196013455659313726391
- NeunerGPatelASuntharalingamMChemoradiotherapy for esophageal cancerGastrointest Cancer Res20093576519461907
- MandilarasVBouganimNSpayneJConcurrent chemoradiotherapy for locally advanced breast cancer-time for a new paradigm?Curr Oncol201522253225684986
- MitchisonTProliferation rate paradox in antimitotic chemotherapyMol Biol Cell2012231622210845
- Komlodi-PasztorESackettDWilkersonJFojoTMitosis is not a key target of microtubule agents in patient tumorsNat Rev Clin Oncol2011824425021283127
- MancusoAOudardSSternbergCEffective chemotherapy for hormone-refractory prostate cancer (HRPC): present status and perspectives with taxane-based treatmentsCrit Rev Oncol Hematol20076117618517074501
- TannockIde WitRBerryWDocetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancerN Engl J Med20043511502151215470213
- OgdenAPadmashreeRReidMAnejaRInterphase microtubules: chief casualties in the war on cancerDrug Discov Today201419782482924201225
- FieldJKanakkantharaAMillerJMicrotubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule functionBioorg Med Chem201422185050505924650703
- PoruchynskyMKomlodi-PasztorETrostelSMicrotubule-targeting agents augment the toxicity of DNA-damaging agents by disrupting intracellular trafficking of DNA repair proteinsProc Natl Acad Sci U S A201511251571157625605897
- CarlsonBGroganPMladekARadiosensitizing effects of temozolomide observed in vivo only in a subset of O6-methylguanine-DNA methyltransferase methylated glioblastoma multiforme xenograftsInt J Radiat Oncol Biol Phys200975121221919695438
- TapiaCKutznerHMentzelTSavicSBaumhoerDGlatzKTwo mitosis-specific antibodies, MPM-2 and Phospho-Histone H3 (Ser28), allow rapid and precise determination of mitotic activityAm J Surg Pathol2006301838916330946
- TaoYLeteurCCalderaroJThe Aurora B kinase inhibitor AZ1152 sensitizes cancer cells to fractionated irradiation and induces mitotic catastropheCell Cycle20098193172318119755861
- AkihitoAChanoTFutamiKRECQL1 and WRN proteins are potential therapeutic targets in head and neck squamous cell carcinomaCancer Res201171134598460721571861
- LindgrenTStigbrandTJohanssonLRiklundKErikssonDAlterations in gene expression during radiation-induced mitotic catastrophe in HeLa Hep2 cellsAnticancer Res20143483875388025075008
- De WittMGambleAHansonDRepurposing mebendazole as a replacement for vincristine for the treatment of brain tumorsMol Med Epub20174510.2119/molmed.2017.00011
- MahLEl-OstaAKaragiannisTγH2AX: a sensitive molecular marker of DNA damage and repairLeukemia20102467968620130602
- SharmaASinghKAlmasanAHistone H2AX phosphorylation: a marker for DNA damageMethods Mol Biol201292061362622941631
- ZanniniLDeliaDBuscemiGChk2 kinase in the DNA damage response and beyondJ Mol Cell Biol20146644245725404613
- LamarcheBOrazioNWeitzmanMThe MRN complex in double-strand break repair and telomere maintenanceFEBS Lett20105841726822695
