Abstract
Background
Many studies have provided increasing evidence to demonstrate that HSP27 has been involved in the development of gastric cancer; however, they all include few patients and the results remain controversial. Hence, we conducted a meta-analysis to evaluate correlations between HSP27 and the clinicopathological characteristics of gastric cancer.
Methods
An electronic search for relevant articles was conducted in PubMed, Cochrane Library, Web of Science, EMBASE database, Chinese CNKI, and Wan Fang. Data on the relationship between HSP27 expression and lymph node metastasis, serosal invasion, gender, tumor size, differentiation, and TNM stage were extracted. Pooled odds ratios and 95% confidence intervals were estimated by forest plot.
Results
The pooled analyses suggested that HSP27 expression was significantly associated with the incidence of gastric cancer. However, HSP27 expression had no significant relationship with lymph node metastasis, serosal invasion, gender, tumor size, differentiation, and TNM stage.
Conclusion
Our meta-analysis demonstrated that HSP27 may play vital roles in tumorigenesis and deterioration of gastric cancer. However, further high-quality studies are needed to provide more reliable evidence.
Introduction
Gastric cancer is one of the leading causes of malignancy-related death around the world. It has been reported that gastric cancer is the fifth most common cancer worldwide, and the fatality rate is 75%, accounting for 8.8% of the total deaths due to cancer.Citation1 Although there is a trend of decreasing mortality rates of stomach cancer attributed to cancer screening, reduced prevalence of risk factors, and improved treatments, the prognosis of gastric patients remains poor.Citation2,Citation3 Heat shock proteins (HSPs) are ubiquitous, highly conserved proteins, which are induced by heat shock and environmental and physiopathological stresses.Citation4 Recent evidence suggested that HSPs may have a close relationship with gastric neoplasia.Citation5–Citation7 Besides, it has been reported that the expression of HSPs also correlated with clinical characteristics in gastric cancer.Citation8–Citation10
HSP27 is a member of the small HSPs and is a major molecular chaperone with a protective effect on cells, which has the function of regulating normal cell physiology and the cellular stress response.Citation11–Citation13 HSP27 is expressed at low levels in normal cells.Citation14 However, its aberrant expression has been reported in various malignant tumors, such as gastric,Citation15 colon,Citation16 hepatocellular,Citation17 lung,Citation18 cervical,Citation19 ovarian,Citation20 and breast cancer.Citation21 Moreover, these reports have also indicated the oncogenic potential of HSP27 and its utility as a cancer biomarker. A previous meta-analysis reported that overexpression of HSP27 was closely related to clinicopathological features, including the differentiation degree, lymphatic metastasis, clinical stage, squamous cell carcinoma, and tumor size in non-small-cell lung cancer.Citation22 Although some studies have explored the role of HSP27 overexpression in stomach cancer,Citation8,Citation15,Citation23 there is still no summary evidence for the association between HSP27 and gastric cancer. Therefore, we conducted this meta-analysis to explore the relationship between the HSP27 expression and clinicopathological characteristics of gastric cancer patients.
Methods
Identification and eligibility of relevant studies
We searched PubMed, Cochrane Library, Web of Science, EMBASE database, Chinese CNKI, and Wan Fang to identify studies that assessed the clinicopathological factors and prognostic value of HSP27 expression in gastric cancer patients using immunohistochemistry (IHC). The search ended in July 1, 2017. Search words were “heat shock protein 27”, “HSP27”, “gastric cancer”, “gastric carcinoma”, and “stomach neoplasm”. The reference list of included studies was also checked to find out other qualified studies.
Studies were included if the following criteria were met: 1) the study was published in English or Chinese with full text being available; 2) patients in studies were clearly diagnosed with gastric cancer; 3) the definition of HSP27-positive was tested by IHC method; 4) studies included were case–control studies that evaluated the correlation between HSP27 expression and clinicopathological features or prognosis in stomach cancer; 5) studies included at least one primary outcome of interest; and 6) letters, reviews, conference abstracts, and duplicated studies were excluded.
Data extraction
Two reviewers independently screened all literature to determine whether the relevant articles meet the included criteria. Extracted data including the first author’s name, publication year, sample size, country, and clinicopathological parameters (expression, lymph node metastasis, serosal invasion, gender, tumor size, differentiation, and TNM stage). Disagreements were resolved by reextraction or third-party adjudication.
Quality assessment
The quality of each included studies was assessed by the Newcastle–Ottawa Scale (NOS) criteria, and we regarded studies with NOS score of ≥6 as a good quality, while studies with NOS score ≤5 were considered as poor quality.
Statistical analysis
STATA 12.0 was used for statistical calculations. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to evaluate the association between positive HSP27 expression and clinicopathological features (lymph node metastasis [yes vs no], serosal invasion [yes vs no], gender [male vs female], tumor size [≤5 cm vs >5 cm], differentiation type [low vs high/moderate], TNM stage [I/II vs III/IV]), meanwhile the expression of HSP27 between cancer tissues and control tissues was also evaluated. The heterogeneity between the studies was evaluated by I2 test. Fixed effects model was chosen when there was no significant heterogeneity (I2<50%, P-Het >0.1). If heterogeneity was significant, the random effects model would be used. Potential causes of statistical heterogeneity were explored by subgroup analysis. Publication bias was examined by the Begg’s funnel plot test.
Results
Eligible studies
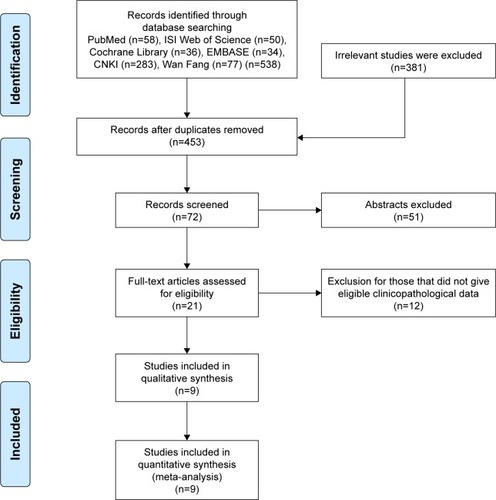
As shown in , we identified 538 relevant articles through combined manual and computerized retrieval from PubMed, Cochrane Library, Web of Science, EMBASE database, Chinese CNKI, and Wan Fang. Of these, 517 were excluded after reviewing the titles and abstracts because these articles were duplicated, non-HSP27-related, or did not involve testing of tumor tissues. A total of 21 studies were assessed by reading the full text, and then 12 studies were excluded due to insufficient information and/or lack of cut-off value of HSP27 expression. Finally, 9 eligible articles were included in this meta-analysis.Citation15,Citation24–Citation31
The characteristics of the included studies are summarized in . These studies were published from 2002 to 2015, and a total of 624 gastric cancer patients were enrolled. Sample sizes ranged from 34 to 118 patients. Four of these studies enrolled ≤60 patients and 5 studies included >60 patients. Eight of these studies evaluated patients from China and one from Greece. All of these studies scored ≥6 in methodological assessment, which implied they were of high quality.
Table 1 Characteristics of studies included in the meta-analysis
Meta-analysis
In this meta-analysis, we assessed the correlation between HSP27 expression and clinicopathological features of gastric carcinoma. As shown in and , overexpression of HSP27 was associated with the incidence of gastric cancer (OR =4.73, 95% CI =2.22–10.05, P=0.000). However, our result showed that overexpression of HSP27 was not significantly associated with lymph node metastasis (OR =1.32, 95% CI =0.64–2.72, P=0.453), serosal invasion (OR =1.31, 95% CI =0.81–2.12, P=0.267), gender (OR =0.73, 95% CI =0.50–1.08, P=0.120), tumor size (OR =0.56, 95% CI =0.16–1.91, P=0.354), differentiation (OR =1.47, 95% CI =0.51–4.22, P=0.473), and TNM stage (OR =0.70, 95% CI =0.46–1.08, P=0.111).
Figure 2 Forest plot of studies evaluating the relationship between HSP27 expression and clinicopathological features: (A) expression; (B) lymph node metastasis; (C) serosal invasion; (D) gender; (E) tumor size; (F) differentiation; (G) TNM stage.
Abbreviations: OR, odds ratio; CI, confidence interval.
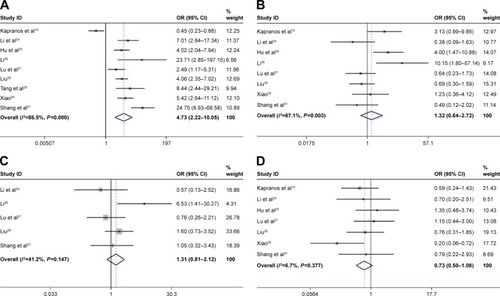
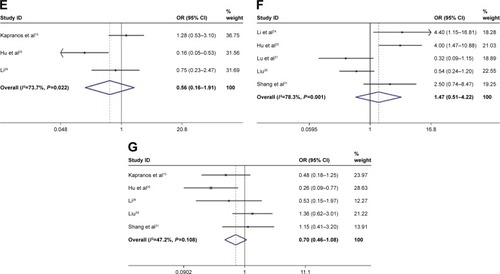
Table 2 HSP27 clinicopathological features for gastric carcinoma
Publication bias and sensitivity analysis
As shown in , Begg’s test suggested that publication bias existed for expression (P=0.048). However, as shown in , there was no publication bias for lymph node metastasis (P=0.902), serosal invasion (P=1.000), gender (P=1.000), tumor size (P=0.296), differentiation (P=0.806), and TNM stage (P=0.462). Hence, the sensitivity analysis was conducted to evaluate whether individual studies influenced pooled OR, and the result indicated that no study substantially influenced pooled OR, which indicates that more studies need to be included for further research.
Subgroup analysis
Subgroup analysis was mainly performed on sample size to explore the potential sources of heterogeneity. As seen in , while in both subgroups divided by sample size, HSP27 expression was correlated to tumor incidence (n≤60: OR =12.15, 95% CI =6.12–24.13, P=0.000; n.60: OR =2.51, 95% CI =1.05–6.01, P=0.039). However, the heterogeneity of HSP27 expression mainly existed in the bigger sample size subgroup (n>60) (I2=88.2%). Sample size did not influence the relationship between HSP27 and lymph node metastasis and also tumor size, and there was great heterogeneity in subgroups (lymph node metastasis: n≤60: I2=79.2%; n.60: I2=64.6%; tumor size: n.60: I2=86.8%). When divided by sample size, the subgroup analysis showed that in small sample size group (n≤60), upregulated HSP27 was associated with poor differentiation (OR =3.23, 95% CI =1.31–7.96, P=0.011). While in bigger sample size group (n.60), there was no relationship between differentiation and HSP27 expression (OR =0.90, 95% CI =0.21–3.91, P=0.892). Additionally, the heterogeneity of HSP27 expression mainly existed in the bigger sample size subgroup (n>60) (I2=84.2%). With these results, the heterogeneity of HSP27 expression and differentiation was most likely caused by the sample size.
Table 3 Subgroup analysis of expression, lymph node metastasis, tumor size, and differentiation
Discussion
HSPs have been reported to be overexpressed in a wide range of human tumors, the expression of HSPs was associated with tumor cell growth, differentiation, resistance to apoptosis, and poor prognosis.Citation32,Citation33 HSP27, an ATP-independent molecular chaperone, is a member of the small HSPs,Citation34 and it has a protective effect on stress conditions, such as oxidative stress and chemical stress.Citation13,Citation35 Overexpression of HSP27 is associated with carcinogenesis, such as suppression of apoptosis, increased cytoprotection, and multidrug resistance.Citation14 Cytoplasmic HSP27 may bind to cytochrome c released from the mitochondria to the cytosol and prevents cytochrome-c-mediated caspase-dependent cell death.Citation36 HSP27 also inhibits apoptosis by regulating other signaling pathways, including Fas receptor pathway,Citation37 protein kinase AKT,Citation38 signal transducer and activator of transcription-3,Citation39 and the NF-kB signaling pathway.Citation40 As for cancer therapy resistance, HSP27 can confer cytoprotection by repairing the damaged proteins and DNAs from cytotoxic drug with higher efficiency, protecting the microvasculature inside tumors.Citation32
High levels of HSP27 can be found in multiple human malignancies, and the association of HSP27 with gastric cancer and clinicopathologic features has been explored for several years. However, the available data have not yet been fully analyzed. In this paper, we conducted a meta-analysis to investigate the association between HSP27 expression of stomach carcinoma patients and clinicopathologic features.
Combining the outcomes of 624 patients from 9 studies, our analysis revealed that positive HSP27 expression significantly correlated with the incidence of gastric cancer. In addition, statistically significant correlations were not observed between HSP27 expression and clinicopathological features including lymph node metastasis, serosal invasion, gender, tumor size, differentiation, and TNM stage.
In this analysis, fiveCitation15,Citation25,Citation26,Citation28,Citation30 of eight, fourCitation25,Citation26,Citation28,Citation31 of five, and threeCitation25,Citation27,Citation31 of four studies suggest that the expression of HSP27 correlated with the lymph node metastasis, TNM stage, and differentiation. However, the overall result showed no statistically significant association between HSP27 expression and these parameters. A previous meta-analysisCitation22 reported that HSP27 expression was related to differentiation degree, lymphatic metastasis, and clinical stage in non-small-cell lung cancer. Unexpectedly, our results were the opposite. A possible reason is that the results are associated with different kinds of cancer. Without doubt, further high-quality studies are needed to provide more reliable evidence.
In our study, there is a significant heterogeneity in the analysis of HSP27 and several clinicopathological features. Although we conducted subgroup analysis and sensitivity analysis, the source of heterogeneity has not been fully explained. However, the heterogeneity may be produced in the following aspects. First, all the included studies tested the expression of HSP27 by IHC method, the quality of paraffin section, the antigen retrieval methods, and the use of primary antibody, and the dilutions of the antibodies were different, leading to a potential bias. Second, the cut-off defining a section with positive HSP27 expression is arbitrary, and the calculation error of positive results is unavoidable, which might also result in heterogeneity. In addition, all our findings, except for HSP27 expression, exhibited no publication bias. As eligible articles for meta-analysis were limited, and our review included only fully published studies, in order to overcome the publication bias, more studies will be needed for further research.
Limitations
In this meta-analysis, several limitations should be considered: 1) the number of included studies was limited, therefore, the association of HSP27 expression with clinicopathological features still needs to be studied in a larger number of samples; 2) the sample sizes are relatively small; 3) most of the included studies were based on the population from China, so this may not generalize global populations.
Conclusion
The available evidence suggests that the incidence of gastric cancer is strongly dependent on the overexpression of HSP27. Clearly, further high-quality research studies with larger sample size are still needed to provide a more representative and convincing statistical analysis in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
- FockKMReview article: the epidemiology and prevention of gastric cancerAliment Pharmacol Ther201440325026024912650
- MalvezziMBonifaziMBertuccioPAn age-period-cohort analysis of gastric cancer mortality from 1950 to 2007 in EuropeAnn Epidemiol20102089890521074104
- BertuccioPChatenoudLLeviFRecent patterns in gastric cancer: a global overviewInt J Cancer2009125366667319382179
- HendrickJPHartlFUMolecular chaperone functions of heat shock proteinsAnnu Rev Biochem1993623493848102520
- IsomotoHOkaMYanoYExpression of heat shock protein (Hsp) 70 and Hsp 40 in gastric cancerCancer Lett2003198221922812957361
- PartidaROTorresJFloresLLPolymorphisms in TNF and HSP-70 show a significant association with gastric cancer and duodenal ulcerInt J Cancer201012681861186819626584
- LiuXYeLWangJFanDExpression of heat shock protein 90 beta in human gastric cancer tissue and SGC7901/VCR of MDR-type gene gastric cancer cell lineChin Med J (Engl)1999121121133113711721455
- GiaginisCDaskalopoulouSSVgenopoulouSHeat shock protein-27, -60 and -90 expression in gastric cancer: association with clinicopathological variables and patient survivalBMC Gastroenterol200991419203381
- ZuoDSDaiJBoAHSignificance of expression of heat shock protein 90 alpha in human gastric cancerWorld J Gastroenterol20039112616261814606110
- LiXSXuQFuXYLuoWSHeat shock protein 22 overexpression is associated with the progression and prognosis in gastric cancerJ Cancer Res Clin Oncol201414081305131324804817
- LindquistSCraigEAThe heat-shock proteinsAnnu Rev Genet1988226316772853609
- GethingMJSambrookJProtein folding in the cellNature1992355635533451731198
- CarverJARekasAThornDCSmall heat-shock proteins and clusterin: intra- and extracellular molecular chaperones with a common mechanism of action and function?IUBMB Life2003551266166814769002
- CioccaDROesterreichSChamnessGCBiological and clinical implications of heat shock protein 27: a reviewJ Natl Cancer Inst19938519155815708411230
- KapranosNKomineaAKonstantinopoulosPAExpression of the 27-kDa heat shock protein (HSP27) in gastric carcinomas and adjacent normal, metaplastic, and dysplastic gastric mucosa, and its prognostic significanceJ Cancer Res Clin Oncol2002128842643212200599
- YuZZhiJPengXClinical significance of HSP27 expression in colorectal cancerMol Med Rep20103695395821472339
- JooMChiJGLeeHExpressions of HSP70 and HSP27 in hepatocellular carcinomaJ Korean Med Sci200520582983416224158
- MałuseckaEZborekAKrzyzowska-GrucaSExpression of heat shock proteins HSP70 and HSP27 in primary non-small cell lung carcinomas. An immunohistochemical studyAnticancer Res2001212A1015102111396134
- LomnytskaMIBeckerSBodinIDifferential expression of ANXA6, HSP27, PRDX2, NCF2, and TPM4 during uterine cervix carcinogenesis: diagnostic and prognostic valueBr J Cancer2011104111011921119665
- LangdonSPRabiaszGJHirstGLExpression of the heat shock protein HSP27 in human ovarian cancerClin Cancer Res1995112160316099815962
- IoachimETsanouEBriasoulisEClinicopathological study of the expression of hsp27, pS2, cathepsin D and metallothionein in primary invasive breast cancerBreast200312211111914659340
- LiSZhangWFanJClinicopathological and prognostic significance of heat shock protein 27 (HSP27) expression in non-small cell lung cancer: a systematic review and meta-analysisSpringerplus201651116527512624
- HuangQYeJHuangQHeat shock protein 27 is over-expressed in tumor tissues and increased in sera of patients with gastric adenocarcinomaClin Chem Lab Med201048226326919961396
- LiGXLiPYangZMExpression and clinical significance of HSP27 and Survivin in gastric carcinomaShaanxi Medical Journal20103919396 Chinese
- HuFLHeQYLiXPExpression and clinical significance of P-cadherin and HSP27 proteins in gastric carcinomaActa Universitatis Medicinalis Nanjing201512630 Chinese
- LiGQExpression and significance of heat shock protein27 and p53 in gastric carcinomaChin J Clin Gastroenterol20154216218 Chinese
- LuAQQinZSLeiSYExpression and significance of heat shock protein 27 in gastric carcinomaJ Snake20072108110 Chinese
- LiuBThe role and potential mechanism of heat shock protein-27 in the metastasis and invasion of gastric cancer]ThesisThe Fourth Military Medical University2014 Chinese
- TangDTangSWLuoZWThe expression of heat shock protein27 in gastric cancerTumor20093272275 Chinese
- XiaoYCXCR1 relationship with clinical pathological features of gastric cancer and its related protein and the differences in protein HSP27 functional verificationThesisCentral South University2014 Chinese
- ShangJCQuYKLiuWXThe expression and clinical significance of HSP27, 70 and 90 in gastric cancerWorld Health Digest Medical Periodical2012924445448 Chinese
- CioccaDRCalderwoodSKHeat shock proteins in cancers: diagnostic, prognostic, predictive, and treatment implicationsCell Stress Chaperones20051028610316038406
- CreaghEMSheehanDCotterTGHeat shock proteins-modulators of apoptosis in tumour cellsLeukemia20001471161117310914538
- KimKKKimRKimSHCrystal structure of a small heat-shock proteinNature199839466935955999707123
- StetlerRAGaoYSignoreAPHSP27: mechanisms of cellular protection against neuronal injuryCurr Mol Med20099786387219860665
- BrueyJMDucasseCBonniaudPHsp27 negatively regulates cell death by interacting with cytochrome cNat Cell Biol20002964565210980706
- CharetteSJLavoieJNLambertHInhibition of daxx-mediated apoptosis by heat shock protein 27Mol Cell Biol200020207602761211003656
- KonishiHMatsuzakiHTanakaMActivation of protein kinase B (akt/rac-protein kinase) by cellular stress and its association with heat shock protein Hsp27FEBS Lett19974102–34934989237690
- SongHEthierSPDziubinskiMLStat3 modulates heat shock 27kDa protein expression in breast epithelial cellsBiochem Biophys Res Commun2004314114315014715258
- GuoKKangNXLiYRegulation of HSP27 on NF-kappaB pathway activation may be involved in metastatic hepatocellular carcinoma cell apoptosisBMC Cancer2009910019331697