 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
MET is a receptor tyrosine kinase known for its pleiotropic effects in tumorigenesis. Dysregulations of MET expression and/or signaling have been reported and determined to be associated with inferior outcomes in breast cancer patients rendering MET a versatile candidate for targeted therapeutic intervention. Crizotinib is a multi-targeted small-molecule kinase inhibitor for MET, ALK, and ROS1 kinases. This study evaluated the anti-proliferative, cytotoxic, anti-migratory, and anti-invasive effects of crizotinib in breast cancer cells in vitro. Cell viability was assessed by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) colorimetric assay. In vitro wound-healing assay was used to examine the effect of crizotinib on breast cancer cell migration. The expressions of Ki-67, MET, and phospho-MET receptors were characterized using immunofluorescence staining. Results showed that crizotinib has significant anti-proliferative activity on all mammary tumor cells with IC50 values of 5.16, 1.5, and 3.85 µM in MDA-MB-231, MCF-7, and SK-BR-3 cells, respectively. Crizotinib induced cytotoxic effects in all breast cancer cells examined. Combined treatment of small dose of crizotinib with paclitaxel or doxorubicin exhibited a highly synergistic inhibition of growth of MDA-MB-231 and MCF-7 cells with combination index values <1 while no significant effect was observed in SK-BR-3 cells compared with individual compounds. Treatment with crizotinib demonstrated a remarkable reduction in the expression of Ki-67 protein in all 3 tested cell lines. Crizotinib inhibited migration and invasion of MDA-MB-231 cells in a dose-dependent fashion. Crizotinib reduced MET receptor activation in MDA-MB-231 cells when treated at effective concentrations. In conclusion, crizotinib suppressed proliferation, migration, and invasion of breast cancer cells in vitro. The results of this study demonstrated that combined treatment of crizotinib with chemotherapeutic agents resulted in a synergistic growth inhibition of specific breast cancer cell lines.
Introduction
Breast cancer is not a single disease but is highly heterogeneous at both the molecular and clinical level.Citation1 Comprehensive gene expression profiling of large sets of tumors has revealed 4 major molecular subtypes of breast cancer: luminal A, luminal B, HER2-enriched, and basal-like breast cancer.Citation2–Citation5 Both luminal A and luminal B breast cancers are hormone receptor-positive and have expression patterns reminiscent of the luminal epithelial component of the breast.Citation6 HER2-enriched tumors are identified with overexpression/amplification of the ErbB2/HER2 gene and are generally hormone receptor-negative.Citation1,Citation2 Basal-like tumors are predominantly triple-negative lacking expression of hormone receptors and HER2.Citation2 These subtypes have been associated with distinct pathological features and clinical outcomes in which patients with luminal A tumors have the best prognosis, while those with basal-like breast cancer have the worst prognosis.Citation1,Citation2 Despite advancements in targeted therapies, cytotoxic chemotherapy remains a cornerstone treatment of breast cancer.Citation7,Citation8
Multiple receptor tyrosine kinases (RTKs) were identified for their oncogenic potential in breast cancer.Citation9,Citation10 Recently, strong evidence has supported the role of the hepatocyte growth factor (HGF) and its receptor, MET, in the development and progression of breast carcinoma.Citation11 Activation of MET induces receptor dimerization and tyrosine autophosphorylation within the catalytic site regulating kinase activity. The phosphorylated tyrosines create a multifunctional docking site for a wide spectrum of transducers and adaptors, including PI3K, viral oncogene homolog (Src), GRB2, Shc, PLC-γ, SHP2 phosphatase, and STAT.Citation12,Citation13 The involvement of such a diverse number of effectors allows the activation of different downstream pathways, including the Akt-NFκB and the RAS-MAPK signaling pathways.Citation14 Ultimately, activation of MET resulted in upregulation of diverse tumor cell functions, including cell proliferation, survival, motility, invasion, angiogenesis, and metastasis.Citation15,Citation16 Clinical studies showed that MET is overexpressed in 20%–30% of breast cancer cases and is a strong, independent predictor of decreased survival which correlated with poor patient outcome.Citation17–Citation20 Breast cancer cells have been shown to express MET and thus could be sensitive to MET inhibitors.Citation21–Citation23
Because of its diverse roles in cellular processes important in oncogenesis and cancer progression, MET is considered to be an important target in anti-cancer therapy. Recently, it has been proposed that inhibition of MET may be a targeted therapy regardless of the type of cancer.Citation24 Several strategies have been developed to suppress MET activity, including monoclonal antibodies directed against MET, inhibitors of MET expression, and small-molecule tyrosine kinase inhibitors.Citation25,Citation26 In this regard, small molecule kinase inhibitors offer the most versatile approach by inhibiting HGF-dependent tumors as well as tumors driven by other MET-dependent mechanisms, such as receptor amplification and activating mutations.Citation27 Crizotinib is an oral small-molecule tyrosine kinase inhibitor of ALK, MET, and ROS1 kinases.Citation28 Crizotinib obtained European and USA Food and Drug Administration (FDA) approval for the treatment of non-small-cell lung cancer (NSCLC) patients having ALK rearrangements.Citation29,Citation30 Crizotinib showed remarkable anti-proliferative activity, anti-angiogenic, and cytotoxic effects in multiple types of cancers.Citation31–Citation33 Despite the availability of this MET inhibitor, limited number of studies in literature had assessed the anti-cancer effects of crizotinib in breast cancer.Citation24,Citation34,Citation35
This study aimed to investigate in vitro activity of crizotinib in different molecular subtypes of breast cancer. In addition, the effect of combined crizotinib treatment with cornerstone chemotherapeutic agents available clinically for management of breast cancer has been examined in this study.
Methodology
Chemicals, reagents, and antibodies
Crizotinib, paclitaxel, and doxorubicin were purchased from Tocris Bioscience Company (Bristol, UK). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was obtained from Sigma Aldrich (St Louis, MO, USA). Primary antibodies for Ki-67, MET, and phospho-MET as well as goat anti-rabbit Alexa Fluor®594 secondary antibody, and Fluoroshield mounting medium with DAPI were purchased from Abcam (Cambridge, MA, USA).
Cell lines and culture conditions
Human breast cancer cell lines MDA-MB-231, MCF-7, and SK-BR-3 were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). MDA-MB-231 breast cancer cell line represents basal-like subtype which is negative for hormone receptors and HER2 expression.Citation36 MCF-7 cells represent luminal A subtype, which are positive for hormone receptors and negative for HER2. SK-BR-3 cancer cells represent HER2-positive subtype, which are negative for hormone receptors and positive for overexpression/amplification of HER2.Citation36 Cells were maintained in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 0.1 mg/mL streptomycin. All cells were maintained at 37°C in an environment of 95% air and 5% CO2 in a humidified incubator. For subculturing, cells were washed with Ca2+- and Mg2+-free phosphate-buffered saline (PBS) and incubated in 0.05% trypsin containing 0.02% EDTA in PBS for 5–15 min at 37°C.
Experimental treatments
Crizotinib was dissolved in dimethyl sulfoxide (DMSO) to provide a final 40 mM stock solution. Paclitaxel and doxorubicin were dissolved in DMSO to form a 10 mM stock solution of each. These stock solutions were used to prepare various concentrations of treatment media. The final concentration of DMSO was maintained the same in all treatment groups within a given experiment and never exceeded 0.1%.
Measurement of viable cell number
Viable cell count was determined using the MTT colorimetric assay.Citation37 Briefly, treatment media were replaced with fresh media containing 0.42 mg/mL MTT and incubated for 3 h at 37°C in a humidified incubator. At the end of incubation period, media were removed and formazan crystals were dissolved in DMSO (100 µL/well for 96-well plates). Optical density was measured at 570 nm on a microplate reader (BioTech, Winooski, VT, USA). Number of cells/well was calculated against a standard curve prepared by plating various concentrations of cells, as determined using a hemocytometer at the start of each experiment.
Cell growth and cytotoxicity studies
To evaluate the effect of crizotinib, chemotherapy, and the combination of both compounds on viability of breast cancer cells, growth studies were performed. Breast cancer cells were initially seeded at 1×104 cells/well (6 replicates/group) in 96-well plates in RPMI-1640 media containing 10% FBS and allowed to attach overnight. Next day, cells were divided into different treatment groups and were exposed to respective control or experimental treatments in mitogen-free media for 48 h. In cytotoxicity studies, cells were plated as described earlier and allowed to grow to a confluency of 70%–80%. Afterward, cells were divided into different treatment groups and fed fresh treatment or control media for 24 h. Viable cell number was determined at the end of the experiments using the MTT assay. Each experiment was repeated at least 3 times.
Immunocytochemical fluorescent staining
Breast cancer cells were seeded on 8-chamber culture slides (Ibidi Company, Martinsried, Germany) at 5×104 cells/chamber (2 replicates/group) in 10% FBS RPMI-1640 media and allowed to attach overnight. Next day, cells were washed with PBS and incubated in serum-free media supplemented with 0.5% FBS containing desired concentrations of crizotinib for 24 h. At the end of treatment, media were removed and cells were rinsed 3 times with pre-cooled PBS. MDA-MB-231 cells were then fixed in methanol pre-cooled to −20°C for 2 min, while MCF-7 and SK-BR-3 cells were fixed with 1:1 vol of methanol:acetone pre-cooled to −20°C for 2 min and permeabilized with 0.2% triton X-100 in PBS for 2 min. Fixed cells were washed with PBS and blocked with 5% goat serum in PBS at room temperature for 1.5 h. Afterward, cells were incubated in specific primary antibodies to Ki-67 (1:250), MET (1:250), and phospho-MET (1:100) overnight at 4°C in 5% goat serum in PBS. Cells were washed in pre-cooled PBS followed by incubation with goat anti-rabbit Alexa Fluor 594-conjugated secondary antibody (1:3,000) in 5% goat serum in PBS for 1 h at room temperature. After final washing, cells were embedded in Fluoroshield mounting medium with DAPI (Abcam). Fluorescent images were captured using Nikon’s Eclipse E600 microscope (Nikon Instruments, Melville, NY, USA) at a magnification of 20×. Percentage of cells expressing Ki-67, MET, or phospho-MET was calculated by counting numbers of positive cell staining for each marker as a proportion of the total number of cells counted (stained with DAPI). Cells were counted manually in 5 photomicrographs captured randomly for every treatment group.
Migration assay
In vitro wound-healing assay was used to assess directional cell motility in 2 dimensions. MDA-MB-231 cells were plated in sterile flat-bottom 12-well plates (3 replicates/group) and incubated in 10% FBS RPMI-1640 media and then allowed to form a subconfluent cell monolayer per well, overnight. A scratch was drawn at the center of each cell monolayer using sterile 200 µL pipette tips. Cells were washed with 1× PBS and incubated in 0.5% FBS RPMI-1640 culture media containing desired concentrations of crizotinib for 24 h. At the end of incubation period, medium was removed, and MDA-MB-231 cells were washed in pre-cooled PBS, fixed in methanol previously cooled to −20°C, and stained with crystal violet solution. Wound healing was visualized at 0 and 24 h by BEL INV-100 LED microscope coupled with BEL EUREKAM 5.0 camera (Biovera, Azienda, Rome, Italy) at a magnification of 4×. Digital images were taken using BEL capturing Software microscope (Biovera). Distance travelled by cells was determined by measuring wound width at 24 h and subtracting it from the wound width at the start of treatment (time zero, t0). Values obtained were then expressed as percentage wound closure, setting the gap width at t0 as 100%. Each experiment was performed in triplicate, and distance migrated was calculated in 5 or more randomly selected fields per treatment group.
Invasion assay
Anti-invasive effect of crizotinib treatment in MDA-MB-231 breast cancer cells was measured using Trevigen Cultrex® BME Cell Invasion Assay (Trevigen Inc., Gaithersburg, MD, USA). About 50 µL of basement membrane extract (BME) coat was added per well of the top chamber. After an overnight incubation at 37°C in a 5% CO2 atmosphere, 5×104 cells per 50 µL of RPMI-1640 medium supplemented with 0.5% FBS were added per well of the top chamber containing the final desired concentration of crizotinib treatment. Next, 150 µL of 10% FBS RPMI-1640 medium was added to the lower chamber in which FBS served as chemoattractant. Cells were then incubated at 37°C under 5% CO2 which allowed for cell invasion from the top to the lower chamber. After 24 h of incubation, the top and bottom chambers were aspirated and washed with washing buffer supplied within the kit. About 100 µL of 1× cell dissociation solution/calcein-AM solution was added to the bottom chamber and incubated at 37°C under 5% CO2 for 1 h. Fluorescence of the samples was determined at λex 485 and λem 520 nm, with GloMax® Discover System (Promega Corporation, Fitchburg, WI, USA). The number of cells that invaded through the BME coat was calculated by a standard curve.
Statistical analysis
Data analysis was performed using IBM SPSS statistical package (IBM Corporation, Version 21.0., Armonk, NY, USA). The results are presented as mean ± SEM. Differences between various treatments groups were determined by one-way analysis of variance (ANOVA) followed by post-hoc Tukey’s honest significant difference (HSD) test. All P-values were 2-sided and differences were considered to be statistically significant at P<0.05.
IC50 values (concentration that induced 50% cell growth inhibition) were determined by applying non-linear regression curve fit analysis using GraphPad® Prism software version 6. Assessment of the effect of combined treatment of crizotinib and chemotherapeutic drugs was done by combination index (CI), dose reduction index (DRI), and isobologram analyses. CI is a quantitative representation of the pharmacological interaction between 2 compounds.Citation38,Citation39 A CI value of 1 represented additive interaction, while CI values <1 or >1 indicate synergistic and antagonistic effects, respectively. CI values for the combinations in this study were calculated by the following equation:Citation38,Citation39
where C and X represent the IC50 values of crizotinib and chemotherapeutic drug when used alone for cell growth studies, while Cc and Xc are the IC50 values of crizotinib and chemotherapeutic drug that also inhibited cell growth by 50% when used in combination. DRI values represent fold decrease in the dose of individual drugs when used in combination, as compared with the dose of a single drug that is required to induce the same effect level.Citation38,Citation39 DRI values of .1 are considered favorable allowing less toxicity while retaining the therapeutic efficacy of individual compounds. DRI values for each of the compounds used in this study were calculated by the following equation:Citation38,Citation39
where X is the IC50 value of the compound when used alone for cell growth studies, and Xc is the IC50 value for the same compound when used in combination.Citation38,Citation39
Isobologram analysis is a graphical method to evaluate the effect of equally effective concentration pairs for a single-effect level.Citation40 It is created on a coordinate system comprised of the individual drug concentrations and shows a straight line which represents additive effects for data points on the line. The straight line in each isobologram figure was constructed by plotting IC50 concentrations of crizotinib and chemotherapeutic drug on y- and x-axes, respectively. Data points located above the line indicate antagonistic interaction, while those falling below the line represent synergistic interaction between 2 compounds when given in combination.Citation40,Citation41
Results
Growth and cytotoxic effects of crizotinib on breast cancer cells
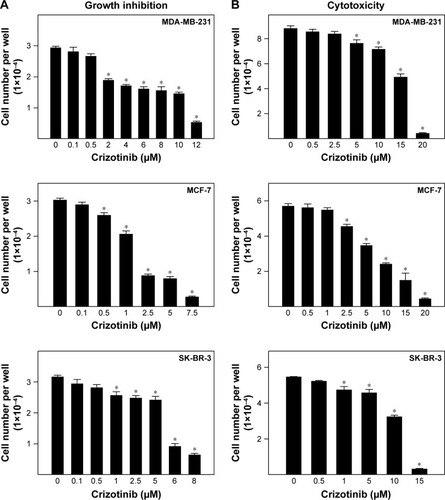
Effects of various doses of crizotinib on in vitro growth of multiple breast cancer cell lines are indicated in . Crizotinib resulted in a dose-dependent reduction in viability of breast cancer cells after 48 h treatment in mitogen-free cell culture media. In MDA-MB-231 cells, treatment with 2–12 µM of crizotinib significantly inhibited growth of MDA-MB-231 cells compared to vehicle-treated controls. In MCF-7 and SK-BR-3 cells, 0.5–7.5 and 1–8 µM crizotinib significantly inhibited growth of both cell lines, respectively. The IC50 values for crizotinib treatment in mitogen-free culture media were 5.16, 1.5, and 3.85 µM in MDA-MB-231, MCF-7, and SK-BR-3 cells, respectively. The cytotoxic effects of acute 24 h treatment exposure to various doses of crizotinib on breast cancer cell viability are shown in . Acute treatment with 5–20 µM crizotinib significantly reduced MDA-MB-231 viable cell number as compared to vehicle-treated controls (). Similarly, acute 24 h treatment with 2.5–20 and 1–15 µM crizotinib resulted in significant cytotoxic effects in MCF-7 and SK-BR-3 cells, respectively.
Figure 1 Growth and cytotoxic effects of crizotinib on breast cancer cells.
Effects of chemotherapeutic agents on growth of breast cancer cells
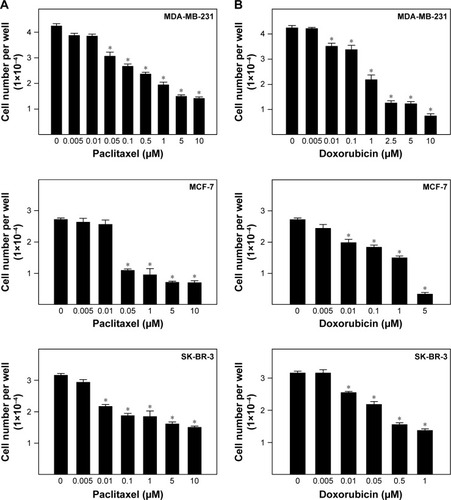
The effects of chemotherapeutic agents of the viability of breast cancer cells are shown in . Exposure to increasing concentrations of the taxane drug, paclitaxel, resulted in a dose-dependent suppression of breast cancer cell growth in vitro (). Paclitaxel treatment with 0.05–10, 0.05–10, and 0.01–10 µM significantly reduced viability of MDA-MB-231, MCF-7, and SK-BR-3 cells, respectively, as compared to vehicle-treated control cells (). The IC50 values for paclitaxel were 1.326, 0.04, and 3.682 µM for MDA-MB-231, MCF-7, and SK-BR-3 cells, respectively. Similarly, the anthracycline drug doxorubicin inhibited growth of breast cancer cells after 48 h treatment in a dose-dependent manner (). Doxorubicin significantly suppressed growth of MDA-MB-231 cells in a concentration range of 0.01–10 µM with IC50 value of 1.3 µM (). Doxorubicin treatment also resulted in a dose-dependent inhibition of MCF-7 and SK-BR-3 cell growth in mitogen-free media with IC50 values of 0.915 and 0.352 µM, respectively ().
Figure 2 Effects of chemotherapeutic agents on growth of breast cancer cells.
Effects of combined treatment of crizotinib and chemotherapeutic agents on growth of breast cancer cells
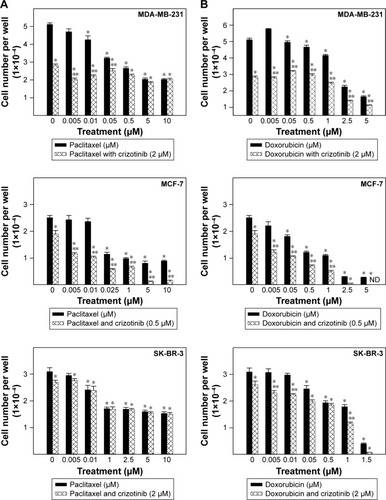
The combination of a single concentration of crizotinib to a dose range of paclitaxel resulted in suppression of breast cancer cell growth in a dose-dependent manner after 48 h of treatment in culture (). Combined treatment of 2 µM crizotinib with 0.005–0.05 µM paclitaxel significantly inhibited MDA-MB-231 cell growth compared to both vehicle-treated control cells and the respective paclitaxel-treated cells (). Similarly, combination of 0.5 µM crizotinib with the taxane significantly suppressed MCF-7 cell growth over a dose range of 0.005–10 µM of paclitaxel compared to respective treatment groups with the taxane alone. In SK-BR-3 cells, addition of crizotinib to a dose range of paclitaxel did not result in a significant suppression of SK-BR-3 cell growth compared to individual compound treatment of the taxane (). The same pattern for the combined treatment was found with the addition of a single concentration of crizotinib to a dose range of doxorubicin in MDA-MB-231, MCF-7, and SK-BR-3 cells (). In both MDA-MB-231 and MCF-7 cells, addition of 2 and 0.5 µM crizotinib to a dose range of doxorubicin significantly reduced viability of MDA-MB-231 and MCF-7 cells compared to their respective individual anthracycline-treated cells (). However, in SK-BR-3 cells, combined treatment of crizotinib and doxorubicin did not result in a significant inhibition of SK-BR-3 cell growth for most of the dose range applied.
Figure 3 Effects of combined treatment of crizotinib and chemotherapeutic agents on growth of breast cancer cells.
Abbreviation: ND, not detectable.
Isobologram analysis for the effect of combination treatment of crizotinib and chemotherapeutic drugs is shown in . Results showed that the growth inhibitory effect of combined treatment of crizotinib with paclitaxel was synergistic in both MDA-MB-231 and MCF-7 cells as indicated by data point in each isobologram located well below the line defining an additive effect (). However, isobologram for the combined treatment of crizotinib and paclitaxel in SK-BR-3 cells indicated antagonism as shown by data point located above the line of additivity (). Isobologram analysis for the effect of combination treatment of crizotinib and doxorubicin showed similar pattern suggestive of synergistic interaction between both compounds for growth inhibition of MDA-MB-231 and MCF-7 cells and antagonistic interaction in SK-BR-3 cells (). The IC50 values calculated for paclitaxel treatment in combination with crizotinib were 0.061 µM for MDA-MB-231, 0.006 µM for MCF-7, and 2.866 µM for SK-BR-3 cells. For doxorubicin, IC50 values calculated for the anthracycline in combination with crizotinib were 0.777, 0.011, and 0.375 µM for each of MDA-MB-231, MCF-7, and SK-BR-3 cells, respectively. CI calculated for growth suppressive effects of combined crizotinib and chemotherapy treatment indicated high level of synergism with values <1 for MDA-MB-231 and MCF-7 breast cancer cells (). In addition, DRI values calculated for combined crizotinib and chemotherapy treatments showed multiple-fold reductions for both compounds when used in combination among MDA-MB-231 and MCF-7 cell lines (). However, antagonism was shown as indicated by CI values of >1 when both therapies were combined in treatment of SK-BR-3 cells ().
Figure 4 Isobolograms for the anti-proliferative effects of combined treatment of crizotinib and chemotherapeutic drugs in breast cancer cells.
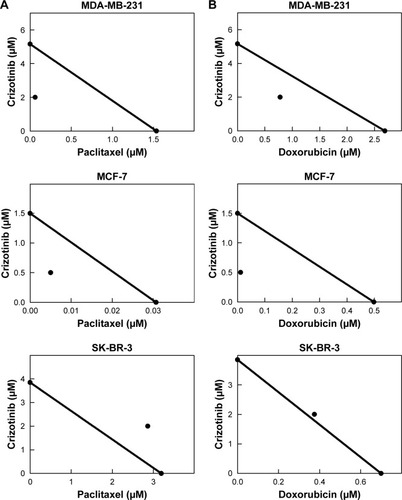
Table 1 CI and DRI values for combined treatment of crizotinib and chemotherapeutic drugs resulting in 50% reduction in growth of multiple breast cancer cell lines
Effect of crizotinib treatment on Ki-67 labeling in breast cancer cells
Positive expression of the nuclear protein Ki-67 is a known marker of cell proliferation.Citation42 In vehicle-treated control cells, Ki-67 expression was observed in 93.3%, 91.8%, and 36.4% of MDA-MB-231, MCF-7, and SK-BR-3 cancer cells, respectively (). In MDA-MB-231 cells, treatment with 2.5–7.5 µM crizotinib resulted in a dose-dependent reduction of Ki-67 expression in treatment groups as compared to control cells after 24 h duration in cell culture (). Similarly, the number of Ki-67 positive cells was significantly reduced in MCF-7 cells treated with 0.1–3 µM crizotinib compared to vehicle-treated control group (). In SK-BR-3 cells, crizotinib treatment resulted in reduced Ki-67 expression at concentrations of 5–7.5 µM compared to control cells ().
Figure 5 Effect of crizotinib treatment on Ki-67 labeling in breast cancer cells.
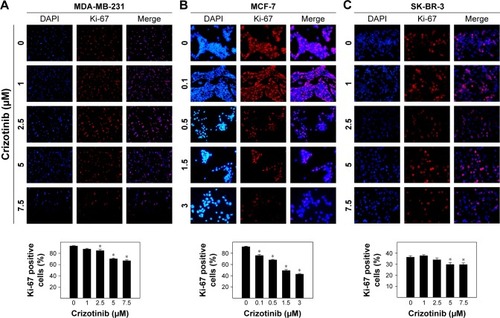
Effect of crizotinib treatment on migration and invasion of breast cancer cells
Effect of various concentrations of crizotinib on migration and invasion of MDA-MB-231 cancer cells is shown in . Almost complete closure of inflicted wounds in MDA-MB-231 cells was observed after 24 h of incubation in media supplemented with 0.5% FBS. Treatment with crizotinib resulted in a dose-dependent inhibition of cell motility after 24 h in culture (). Exposure to crizotinib treatment at the concentration range of 0.5–5 µM significantly suppressed migration of MDA-MB-231 cells as compared to vehicle-treated control group (). Crizotinib treatment in concentration of 7.5–30 µM significantly suppressed invasiveness of MDA-MB-231 cells compared to vehicle-treated control group ().
Figure 6 Effect of crizotinib treatment on migration and invasion of breast cancer cells.
Abbreviation: BME, basement membrane extract.
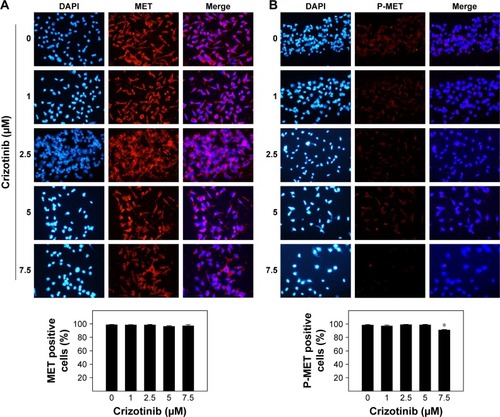
Effect of crizotinib treatment on total levels of MET and phospho-MET in breast cancer cells
Immunofluorescent staining of MET indicated abundant expression of the receptor in MDA-MB-231 cells with cytoplasmic and nuclear localization (). Crizotinib treatment with a dose-range of 1–7.5 µM did not result in a reduction in total levels of MET as compared to vehicle-treated control group (). Immunocytochemical fluorescent staining of phosphorylated MET receptors (phospho-MET) in MDA-MB-231 cells is shown in . The phosphorylated MET corresponds to the phosphorylation of tyrosine moieties Y1230/Y1234/Y1235 of kinase domain corresponding to receptor activation. Phospho-MET receptors are found located both on cell membranes and in cytoplasm of the cells (). Levels of phospho-MET receptors were significantly reduced when cells were treated with 7.5 µM crizotinib compared to respective control group after 24 h treatment ().
Figure 7 Effect of crizotinib treatment on total levels of MET and phospho-MET (P-MET) in breast cancer cells.
Discussion
Historically, RTKs have been shown to correlate with the development and progression of most forms of human malignancies.Citation43 Therefore, targeting RTKs has become an attractive therapeutic option in cancer therapy.Citation43–Citation45 MET is a RTK known to play a significant role in neoplastic transformation, tumor growth, survival, angiogenesis, migration, invasion, metastasis, and resistance to targeted therapies.Citation46,Citation47 Highly expressed levels of MET have been detected in human breast cancer and are associated with high tumor grade and poor prognosis.Citation17,Citation18,Citation48 In addition, multiple clinical studies indicated that overexpression of MET is associated with decreased survival and poor outcomes in terms of increased risk of recurrence and death among breast cancer patients.Citation19,Citation20 In line with this, expression data and tissue microarrays revealed that high MET expression correlates mainly with ER/HER2-negative and the basal subtypes of human breast tumors.Citation13,Citation17,Citation19,Citation20,Citation45 In animal models, studies had shown that activated MET induced a high incidence of mammary tumors with diverse histopathological phenotypes including tumors with basal and luminal characteristics.Citation19,Citation20
The potential implication of HGF/MET axis in the progression of breast carcinomas as well as other cancers has led to a great interest in applying anti-MET therapies clinically.Citation44 Small-molecule tyrosine kinase inhibitors of MET have been developed and evaluated as monotherapy or in combination with other targeted therapies.Citation25 Crizotinib is a multi-targeted RTK inhibitor of MET, ALK, and ROS1.Citation49,Citation50 In this study, crizotinib induced dose-dependent inhibition of growth of multiple breast cancer cell lines in vitro. In addition, acute exposure to crizotinib resulted in cytotoxic activity in the different cell lines investigated. Mammary cancer cell lines provided in these studies represent in vitro models for major molecular subtypes of breast cancer commonly encountered in clinical settings. Earlier findings in literature showed that the human breast cancer cell lines MDA-MB-231, MCF-7, and SK-BR-3 express MET which is also activated in response to ligand exposure.Citation21–Citation23 In this study, the luminal MCF-7 cells were most sensitive to crizotinib anti-proliferative effects, followed by HER2-enriched SK-BR-3 cells and the triple-negative MDA-MB-231 cells. Inhibition of MDA-MB-231 and SK-BR-3 cells was observed at a higher range of micromolar concentrations compared to MCF-7 cells. It is noteworthy to mention that the breast cancer cell lines used in this study have, in addition to different molecular profiles, different doubling times, expression of other growth factor receptors, and the potential cross-talk between different growth factor signaling pathways. Altogether, it is not unexpected that different breast cancer cells would respond to different concentrations of crizotinib. Results in this study revealed that crizotinib treatment reduced the expression of the proliferation marker Ki-67 at concentrations that were effective to inhibit the growth of breast cancer cells. Ki-67 is a nuclear protein detectable in nuclei of proliferating cells in all phases of cell cycle and correlated with growth fraction of any given cell population.Citation51,Citation52 Crizotinib reduced the percentage of Ki-67-positive cells most notably in MCF-7 treated cells compared to both MDA-MB-231 and SK-BR-3 cells. This could explain, in-part, the greater sensitivity of MCF-7 cells to growth inhibition of crizotinib. However, reduced viability of breast cancer cells could also be mediated by the ability of crizotinib to modulate other growth signaling pathways that were not investigated in this study. Megiorni et alCitation53 indicated that crizotinib inhibition of rhabdomyosarcoma cell proliferation and survival was mediated by MET- and ALK-independent mechanisms involving induction of autophagy and accumulation of reactive oxygen radicals.
Chemotherapy clearly remains a cornerstone of breast cancer management in different stages of the disease.Citation8 Among chemotherapeutic agents available for the treatment of breast cancer, anthracycline- and taxane-based regimens proved to be the most powerful systemic treatment.Citation54,Citation55 However, administration of such treatments could be limited by drug toxicity and unfavorable adverse effects. Recently, combination treatment of chemotherapy with novel targeted therapy became a common practice to overcome the limitation of conventional combination regimens in breast cancer.Citation56 Findings from this study indicated that the combination of crizotinib and the taxane paclitaxel or the anthracycline doxorubicin resulted in a synergistic growth inhibition in each of MDA-MB-231 and MCF-7 cells. Interestingly, combined treatment of crizotinib and chemotherapy in SK-BR-3 cells demonstrated only marginal additive effect and was antagonistic for the combination with paclitaxel. Accordingly, these findings suggest that the effect of combined treatment of crizotinib and chemotherapy could be cell-type specific. Despite the cytotoxic effects observed for crizotinib in breast cancer cells, it is possible that more specific molecular mechanisms are mediating the synergism observed in both MDA-MB-231 and MCF-7 cells. Recently, Krytska et alCitation32 showed that crizotinib synergizes with topotecan and cyclophosphamide in human neuroblastoma-derived cell lines with varying ALK statuses. The synergism was explained in terms of higher caspase-dependent apoptosis in combined treatments compared to chemotherapy alone. Interestingly, a recent study investigating the effect of crizotinib in combination with cisplatin in 4 NSCLC cell lines showed remarkable antagonism among the 4 cell lines discouraging crizotinib and cisplatin combination in NSCLC.Citation57 Collectively, the effect of combined crizotinib and chemotherapy in cancer is variable and the level of interaction could be affected by the type of chemotherapeutic agents, type of cancer cells, and possible other factors.
Recent evidence indicated that overexpression of MET is being increasingly related to reducing cancer sensitivity to chemotherapeutic as well as biological treatments and emergence of resistance among different types of tumors.Citation24,Citation58–Citation60 In this regard, the combination between chemotherapy and crizotinib could be appealing in terms of improving sensitivity and reducing resistance to chemotherapeutic drug therapy. Zhou et alCitation35 found crizotinib to reverse resistance and enhance cytotoxicity of chemotherapeutic drugs in multidrug-resistant (MDR) breast cancer cells in vitro and in vivo. These effects were attributed to inhibition of P-gp function in cancer cells, particularly ABCB1.Citation35 Taking into consideration the fact that paclitaxel and doxorubicin are substrates for multiple ABCB1/P-gp transporters potentially expressed by MDA-MB-231 and MCF-7 cells,Citation61–Citation64 it is possible that the synergistic effect for combined treatment is mediated, at least in-part, by inhibiting ABCB1 transporters allowing efficient accumulation of chemotherapy within cancer cells. Moreover, the toxicity profile of MET inhibitors is completely different from that of standard chemotherapy further encouraging evaluation of combined crizotinib and chemotherapeutic treatments.Citation25 Overall, crizotinib had a tolerable adverse effect profile, and most events were mild to moderate in severity in clinical studies.Citation29,Citation65
Activation of MET is known to promote cancer cell scattering, migration, and invasion.Citation66 The basal-like MDA-MB-231 breast cancer cells are known to demonstrate invasive phenotype in vitro.Citation36 Findings from this study showed that crizotinib treatment significantly inhibited migration and invasion of MDA-MB-231 cells in a dose-dependent manner. Immunofluorescent staining for active MET (phospho-MET) was suppressed in MDA-MB-231 cells treated with crizotinib compared to vehicle-control group. Reduced MET activation may explain the anti-proliferative as well as the anti-migratory and anti-invasive effects of crizotinib in triple-negative breast cancer subtype.
To the best of our knowledge, this is the first study to characterize the anti-cancer effects of crizotinib in breast cancer cells. Crizotinib inhibited growth of hormone-dependent, HER2-enriched, and triple-negative breast cancer cells in vitro. The anti-proliferative effects could be explained in terms of reduced expression of Ki-67 in breast cancer cells. Combined treatment of crizotinib and chemotherapy resulted in synergistic growth inhibition in luminal and triple-negative breast cancer subtypes. In triple-negative breast cancer, crizotinib treatment reduced MET receptor activation resulting in inhibition of cell migration and invasion. Collectively, these findings provide insights into the potential activity of crizotinib in tumors not known to have the classical ALK rearrangement. This study encourages further investigations for alternative combination treatments of crizotinib with other chemotherapeutic or targeted therapy in breast cancer analyzing potential molecular targets.
Acknowledgments
This work was supported by the Deanship of Research at Jordan University of Science and Technology (JUST) (grant number 20160127).
Disclosure
The authors report no conflicts of interest in this work.
References
- PolyakKBreast cancer: origins and evolutionJ Clin Invest2007117113155316317975657
- HonJDSinghBSahinABreast cancer molecular subtypes: from TNBC to QNBCAm J Cancer Res2016691864187227725895
- GoldhirschAWoodWCCoatesASPanel MembersStrategies for subtypes – dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011Ann Oncol20112281736174721709140
- SorlieTTibshiraniRParkerJRepeated observation of breast tumor subtypes in independent gene expression data setsProc Natl Acad Sci U S A2003100148418842312829800
- PerouCMSorlieTEisenMBMolecular portraits of human breast tumoursNature2000406679774775210963602
- TranBBedardPLLuminal-B breast cancer and novel therapeutic targetsBreast Cancer Res201113622122217398
- SchmidtMKoelblHAdjuvant chemotherapy in early breast cancerMinerva Ginecol2012641536522334231
- SwainSMChemotherapy: updates and new perspectivesOncologist201015suppl 5817
- AndrechekERMullerWJTyrosine kinase signalling in breast cancer: tyrosine kinase-mediated signal transduction in transgenic mouse models of human breast cancerBreast Cancer Res20002321121611250712
- HynesNETyrosine kinase signalling in breast cancerBreast Cancer Res20002315415711250704
- RhoOKimDJKiguchiKDigiovanniJGrowth factor signaling pathways as targets for prevention of epithelial carcinogenesisMol Carcinog201150426427920648549
- SattlerMSalgiaRc-Met and hepatocyte growth factor: potential as novel targets in cancer therapyCurr Oncol Rep20079210210817288874
- SierraJRTsaoMSc-MET as a potential therapeutic target and biomarker in cancerTher Adv Med Oncol201131 supplS21S3522128285
- CecchiFRabeDCBottaroDPTargeting the HGF/Met signalling pathway in cancerEur J Cancer20104671260127020303741
- GranitoAGuidettiEGramantieriLc-MET receptor tyrosine kinase as a molecular target in advanced hepatocellular carcinomaJ Hepatocell Carcinoma20152293827508192
- AwazuYNakamuraKMizutaniAA novel inhibitor of c-Met and VEGF receptor tyrosine kinases with a broad spectrum of in vivo antitumor activitiesMol Cancer Ther201312691392423548264
- Gonzalez-AnguloAMChenHKaruturiMSFrequency of mesenchymal-epithelial transition factor gene (MET) and the catalytic subunit of phosphoinositide-3-kinase (PIK3CA) copy number elevation and correlation with outcome in patients with early stage breast cancerCancer2013119171522736407
- InancMOzkanMKaracaHCytokeratin 5/6, c-Met expressions, and PTEN loss prognostic indicators in triple-negative breast cancerMed Oncol201431180124326984
- PonzoMGLesurfRPetkiewiczSMet induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancerProc Natl Acad Sci U S A200910631129031290819617568
- GraveelCRDeGrootJDSuYMet induces diverse mammary carcinomas in mice and is associated with human basal breast cancerProc Natl Acad Sci U S A200910631129091291419567831
- ZhangZWangJJiDFunctional genetic approach identifies MET, HER3, IGF1R, INSR pathways as determinants of lapatinib unresponsiveness in HER2-positive gastric cancerClin Cancer Res201420174559457324973425
- MatteucciEBendinelliPDesiderioMANuclear localization of active HGF receptor Met in aggressive MDA-MB231 breast carcinoma cellsCarcinogenesis200930693794519357348
- GotteMKerstingCRadkeIKieselLWulfingPAn expression signature of syndecan-1 (CD138), E-cadherin and c-met is associated with factors of angiogenesis and lymphangiogenesis in ductal breast carcinoma in situBreast Cancer Res200791R817244359
- DuYYamaguchiHWeiYBlocking c-Met-mediated PARP1 phosphorylation enhances anti-tumor effects of PARP inhibitorsNat Med201622219420126779812
- Gozdzik-SpychalskaJSzyszka-BarthKSpychalskiLC-MET inhibitors in the treatment of lung cancerCurr Treat Options Oncol201415467068225266653
- ScagliottiGVNovelloSvon PawelJThe emerging role of MET/HGF inhibitors in oncologyCancer Treat Rev201339779380123453860
- BellonSFKaplan-LefkoPYangYc-Met inhibitors with novel binding mode show activity against several hereditary papillary renal cell carcinoma-related mutationsJ Biol Chem200828352675268318055465
- SolomonBJMokTKimDWPROFILE 1014 InvestigatorsFirst-line crizotinib versus chemotherapy in ALK-positive lung cancerN Engl J Med2014371232167217725470694
- CappuzzoFMoro-SibilotDGautschiOManagement of crizotinib therapy for ALK-rearranged non-small cell lung carcinoma: an expert consensusLung Cancer2015872899525576294
- KazandjianDBlumenthalGMChenHYFDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangementsOncologist20141910e5e1125170012
- DasAChengRRHilbertMLSynergistic effects of crizotinib and temozolomide in experimental FIG-ROS1 fusion-positive glioblastomaCancer Growth Metastasis20158516026648752
- KrytskaKRylesHTSanoRCrizotinib synergizes with chemotherapy in preclinical models of neuroblastomaClin Cancer Res201622494896026438783
- KimHJYoonARyuJYc-MET as a potential therapeutic target in ovarian clear cell carcinomaSci Rep201663850227917934
- ZouHYLiQLeeJHAn orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanismsCancer Res20076794408441717483355
- ZhouWJZhangXChengCCrizotinib (PF-02341066) reverses multidrug resistance in cancer cells by inhibiting the function of P-glycoproteinBr J Pharmacol201216651669168322233293
- HollidayDLSpeirsVChoosing the right cell line for breast cancer researchBreast Cancer Res201113421521884641
- RissTLMoravecRANilesALCell viability assaysSittampalamGSCoussensNPNelsonHAssay Guidance ManualBethesda, MDEli Lilly & Company and the National Center for Advancing Translational Sciences2004143
- ChouTCTheoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studiesPharmacol Rev200658362168116968952
- ChouTCDrug combination studies and their synergy quantification using the Chou-Talalay methodCancer Res201070244044620068163
- TallaridaRJAn overview of drug combination analysis with isobologramsJ Pharmacol Exp Ther200631911716670349
- TallaridaRJCombination analysisAdv Exp Med Biol201067813313720738015
- UrruticoecheaASmithIEDowsettMProliferation marker Ki-67 in early breast cancerJ Clin Oncol200523287212722016192605
- TakeuchiKItoFReceptor tyrosine kinases and targeted cancer therapeuticsBiol Pharm Bull201134121774178022130229
- ZhuKKongXZhaoDLiangZLuoCc-MET kinase inhibitors: a patent review (2011–2013)Expert Opin Ther Pat201424221723024266843
- LinklaterESTovarEAEssenburgCJTargeting MET and EGFR crosstalk signaling in triple-negative breast cancersOncotarget2016743699036991527655711
- BlumenscheinGRJrMillsGBGonzalez-AnguloAMTargeting the hepatocyte growth factor-cMET axis in cancer therapyJ Clin Oncol201230263287329622869872
- BoccaccioCComoglioPMMET, a driver of invasive growth and cancer clonal evolution under therapeutic pressureCurr Opin Cell Biol2014319810525305631
- YanSJiaoXZouHLiKPrognostic significance of c-Met in breast cancer: a meta-analysis of 6010 casesDiagn Pathol2015106226047809
- KwakELBangYJCamidgeDRAnaplastic lymphoma kinase inhibition in non-small-cell lung cancerN Engl J Med2010363181693170320979469
- CuiJJTran-DubeMShenHStructure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK)J Med Chem201154186342636321812414
- GerdesJSchwabULemkeHSteinHProduction of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferationInt J Cancer198331113206339421
- VeroneseSMGambacortaMGottardiOScanziFFerrariMLamperticoPProliferation index as a prognostic marker in breast cancerCancer19937112392639318508358
- MegiorniFMcDowellHPCameroSCrizotinib-induced antitumour activity in human alveolar rhabdomyosarcoma cells is not solely dependent on ALK and MET inhibitionJ Exp Clin Cancer Res20153411226445453
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)PetoRDaviesCComparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trialsLancet2012379981443244422152853
- GogineniKDeMicheleACurrent approaches to the management of Her2-negative metastatic breast cancerBreast Cancer Res201214220522429313
- YardleyDADrug resistance and the role of combination chemotherapy in improving patient outcomesInt J Breast Cancer2013201313741423864953
- Van Der SteenNDebenCDeschoolmeesterVBetter to be alone than in bad company: the antagonistic effect of cisplatin and crizotinib combination therapy in non-small cell lung cancerWorld J Clin Oncol20167642543228008383
- RaghavKPGonzalez-AnguloAMBlumenscheinGRJrRole of HGF/MET axis in resistance of lung cancer to contemporary managementTransl Lung Cancer Res20121317919325806180
- WangJChengJXc-Met inhibition enhances chemosensitivity of human ovarian cancer cellsClin Exp Pharmacol Physiol2017441798727658187
- LiEHuZSunYSmall molecule inhibitor of c-Met (PHA665752) suppresses the growth of ovarian cancer cells and reverses cisplatin resistanceTumour Biol20163767843785226695152
- ChenZShiTZhangLMammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: a review of the past decadeCancer Lett2016370115316426499806
- SaunaZEKimIWAmbudkarSVGenomics and the mechanism of P-glycoprotein (ABCB1)J Bioenerg Biomembr2007395–648148718058211
- van AmerongenRBernsATXR1-mediated thrombospondin repression: a novel mechanism of resistance to taxanes?Genes Dev200620151975198116882973
- LiJLiuJGuoNZhangXReversal of multidrug resistance in breast cancer MCF-7/ADR cells by h-R3-siMDR1-PAMAM complexesInt J Pharm2016511143644527444552
- SahuAPrabhashKNoronhaVJoshiADesaiSCrizotinib: a comprehensive reviewSouth Asian J Cancer201322919724455567
- LawrenceRESalgiaRMET molecular mechanisms and therapies in lung cancerCell Adh Migr20104114615220139696
