Abstract
Aim
We have previously shown that the long noncoding RNA prostate cancer-associated transcript 6 (PCAT6) promoted the proliferation and invasion of lung adenocarcinoma (LUAD) cells. In this study, the diagnostic significance of tissue and serum PCAT6 was evaluated in non-small cell lung cancer (NSCLC).
Materials and methods
Tissue expression of PCAT6 was systematically evaluated in five Gene Expression Omnibus datasets (GSE19804, GSE18842, GSE30219, GSE19188, and GSE27262). Circulating and tissue expressions of PCAT6 were detected by quantitative reverse-transcriptase polymerase chain reaction in NSCLC patients from Union Hospital.
Results
PCAT6 was significantly increased in lung cancer tissues and could be used to distinguish LUAD from adjacent normal tissues with an area under the receiver operating characteristic curve (AUC) of 0.9210 (p<0.0001; sensitivity, 98.82%; specificity, 78.57%) in GSE30219, 0.9333 (p<0.0001; sensitivity, 86.67%; specificity, 90.77%) in GSE19188, 0.9584 (p<0.0001; sensitivity, 92.00%; specificity, 96.00%) in GSE27262, and 0.9574 (p<0.0001; sensitivity, 95.89%; specificity, 87.67%) in patients from Union Hospital. As for lung squamous cell carcinoma (LUSC), the AUC of PCAT6 was 0.9567 (p<0.0001; sensitivity, 100%; specificity, 85.71%) in GSE30219, 0.9795 (p<0.0001; sensitivity, 96.30%; specificity, 92.31%) in GSE19188, and 0.9942 (p<0.0001; sensitivity, 100%; specificity, 98.04%) in patients from Union Hospital. We further noticed that the plasma levels of PCAT6 were significantly increased in 73 LUAD and 51 LUSC patients compared with 39 healthy controls (p<0.0001). The AUC of circulating PCAT6 was 0.9213 (p<0.0001; sensitivity, 87.67%; specificity, 97.44%) in LUAD and 0.9583 (p<0.0001; sensitivity, 94.12%; specificity, 100%) in LUSC.
Conclusion
Together with our previous findings, our results suggest that PCAT6 could be used as a potential diagnostic and prognostic biomarker in NSCLC.
Introduction
Non-small cell lung cancer (NSCLC) has become the main cause of cancer-related deaths in China, and the mortality of this disease has increased for more than four times during the past three decades.Citation1,Citation2 Furthermore, NSCLC could remain a major health problem for at least the next 50 years.Citation3 Although the development of new diagnostic and treatment strategies has promoted the survival of NSCLC patients, the overall 5-year survival rate is still <20%, mainly because it is usually detected at an advanced stage.Citation4 Diagnosis of NSCLC at an early stage is one way that can improve a patient’s survival;Citation5 however, early diagnosis remains a challenge due to the lack of specific biomarkers.
In recent years, methods for lung cancer diagnosis include the use of imaging techniques and blood-fluid tests for tumor markers. For example, the largest lung cancer screening trial showed that screening high-risk group with computerized tomography (CT) or low-dose CT relatively reduced mortality by 20% compared to chest radiography.Citation6 However, it often leads to overdiagnosis and unnecessary surgeries due to increased false-positive results, and the cumulative exposure to radiation resulted by annual examinations also represents a considerable health risk.Citation7,Citation8 Thus, developing blood-fluid tests for the diagnosis of early stage NSCLC is clinically important, as blood samples are easily acquired in a relatively noninvasive manner compared with biopsy or surgery. The most widely used serum biomarkers of lung cancer screening include carcinoembryonic antigen (CEA), cytokeratin 19 fragment (CYFRA21-1), and neuron-specific enolase (NSE). However, the sensitivity and specificity of these biomarkers is commonly ranged between 50% and 90%, and false-negative rate is commonly >50% for the diagnosis of NSCLC at an early stage.Citation9 Given these limitations, identification of novel serum biomarkers with high sensitivity and specificity for the diagnosis of NSCLC is of immediate need.
During the past decade, mounting evidence has confirmed that dysregulation of lncRNAs, acting either as oncogenes or as tumor suppressors, is an important cause of certain cancers.Citation10–Citation12 Increasing evidence have shown that long noncoding RNAs (lncRNAs) has led to the development of a new field of molecular diagnosis of cancer. Plasma level of long intergenic non-protein-coding RNA 152 (LINC00152) is significantly elevated and has the potential to be used as a blood-based biomarker for the diagnosis of gastric cancer in patients.Citation13 lncRNA16 (ENST00000539303) is significantly elevated in plasma samples of lung cancer patients, and GAS5 expression is decreased in NSCLC plasma samples, thus making lncRNAs a potential biomarker of lung cancer diagnosis.Citation5,Citation14
lncRNA prostate cancer-associated transcript 6 (PCAT6) was first identified in keratinocyte-enhanced cellular proliferation and colony formation of prostate cancer cells in an androgen-independent way.Citation15 In lung cancer, PCAT6 was also found to be upregulated using Affymetrix HG-U133 plus 2.0 array with an lncRNA classification pipeline.Citation16 Previously, we have confirmed that PCAT6 is significantly upregulated in cancer tissues compared with adjacent normal tissues and positively correlated with metastasis of lung adenocarcinoma (LUAD) patients.Citation17 In addition, PCAT6 was found to be negatively correlated with overall survival of lung cancer patients with retrospective analysis.Citation17 Therefore, we set out to investigate whether serum PCAT6 was increased in LUAD and lung squamous cell carcinoma (LUSC), two major types of NSCLC, and has the potential to be used as a noninvasive diagnostic biomarker of NSCLC.
Materials and methods
GEO lung cancer gene expression data
To identify PCAT6 expression in lung cancer, we searched relevant Gene Expression Omnibus (GEO) datasets (https://www.ncbi.nlm.nih.gov/geo/). As a result, five panels of lung cancer gene expression datasets, including GSE27262, GSE19804, GSE19188, GSE30219, and GSE18842, were selected to compare PCAT6 expression between lung cancer tissues and normal tissues. summarizes the details of these five datasets.
Table 1 Characteristics of five GEO datasets included in this study
Participants and tissue samples
Seventy-three plasma samples, biopsy specimens of LUAD tissues and adjacent normal tissues, 51 plasma samples and biopsy specimens of LUSC tissues and adjacent normal tissues were collected from the department of thoracic surgery of Union Hospital (Wuhan, People’s Republic of China). Tissue biopsy specimens were collected and immediately snap-frozen in liquid nitrogen and stored at −80°C until to be used. Thirty-nine control plasma samples were collected from healthy donors without cancer. Approximately 4 mL of venous blood was collected from each participant, and plasma was separated within 2 h by centrifugation at 1,200 ×g for 10 min at 4°C to spin down blood cells, followed by centrifugation at 12,000 ×g for 10 min at 4°C to completely remove cellular components or cell debris. The supernatant plasma was then carefully collected and stored at −80°C until to be used. The study was approved by the Ethical Review Board for Research of Union Hospital, affiliated to Tongji Medical College of Huazhong University of Science and Technology. All the participants had signed the written informed consent.
RNA extraction and cDNA synthesis
Total RNA was extracted from tissue specimens using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions, and plasma RNA was extracted using TRIzol LS reagent (Invitrogen). The isolated RNA concentration was calculated and normalized with RNase-free water and then reverse-transcribed into cDNA using PrimeScript™ RT reagent kit with gDNA Eraser (RR047A; Takara, Dalian, People’s Republic of China). Reverse transcription conditions were performed as follows: 42°C for 2 min, and then 37°C for 15 min, 85°C for 5 s, followed by storage at 4°C. All cDNA samples were stored at −80°C until use.
Quantitative reverse-transcriptase polymerase chain reaction
Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) was conducted using SYBR Premix Ex Taq (Takara), according to the manufacturer’s instructions. Briefly, all the reactions were carried out on an ABI7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA), and 2 μL cDNA was used as template. The qRT-PCR amplification was performed as follows: an initial denaturation at 95°C for 5 min, followed by 45 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. The 2−ΔΔCt method was used to quantify the fold change of PCAT6 expression in tumor samples versus normal control samples as we previously described.Citation17 GAPDH was used as an internal control, and all reactions were performed in triplicate. The primer sequences were as follows: glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-GGGAGCCAAAAGGGTCAT-3′ (forward) and 5′-GAGTCCTTCCACGATACCAA-3′ (reverse); PCAT6, 5′-CAGGAACCCCCTCCTTACTC-3′ (forward) and 5′-CTAGGGATGTGTCCGAAGGA-3′ (reverse).Citation15
Statistical analysis
All data are presented as mean ± SD from at least three separate experiments and analyzed by using the GraphPad Prism V.6.00 software (GraphPad Software, La Jolla, CA, USA). Comparison between two groups for statistical significance was performed with two-tailed Student’s t-test. For more groups, one-way ANOVA followed by Newman–Keuls post hoc test was used. Receiver operating characteristic (ROC) curves were established to evaluate the diagnostic value of PCAT6 for differentiating tumors from controls. A p-value of <0.05 was considered to be statistically significant.
Results
Tissue PCAT6 is upregulated and has a significant diagnostic value in LUAD and LUSC.
In a previous study, we have confirmed that PCAT6 expression is increased in patients with LUAD and predicts a poor overall survival.Citation17 Then, we further evaluated PCAT6 expression in lung cancer patients from five GEO datasets (GSE19804,Citation18 GSE18842,Citation19 GSE30219,Citation20 GSE19188,Citation21 and GSE27262Citation22). As shown in , these five GEO datasets include patients with NSCLC, that is, LUAD and LUSC. PCAT6 expression was significantly increased in tissues of LUAD and LUSC compared with normal noncancerous tissues in these five datasets (). Then, we further evaluated the diagnostic value of PCAT6 in these five datasets using ROC analyses. The results showed that the value of the area under the ROC curve (AUC) was 0.9578 (95% CI 0.9209–0.9947) in GSE19804 (), 0.9584 (95% CI 0.8915–1) in GSE27262 (), 0.9210 (95% CI 0.8326–1) in GSE30219 (), and 0.9333 (95% CI 0.8864–9802) in GSE19188 () for LUAD and normal controls. With regard to LUSC, the AUC value was 0.9567 (95% CI 0.8935–1) in GSE30219 () and 0.9795 (95% CI 0.9548–1) in GSE19188 () versus normal controls.
Figure 1 Tissue expression of PCAT6 significantly increased in NSCLC patients from GEO datasets.
Abbreviations: GEO, Gene Expression Omnibus; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; PCAT6, prostate cancer-associated transcript 6.
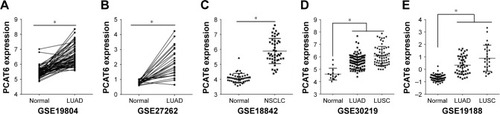
Figure 2 ROC curves of tissue PCAT6 expression for differentiating NSCLC tissue from normal tissue.
Abbreviations: AUC, area under the ROC curve; GEO, Gene Expression Omnibus; NSCLC, non-small cell lung cancer; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; ROC, receiver operating characteristics; PCAT6, prostate cancer-associated transcript 6.
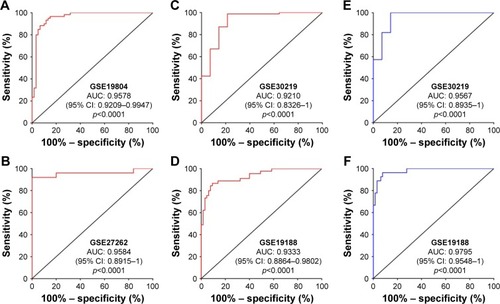
Next, we further validated the expression of PCAT6 in 73 paired LUAD tissues and normal counterparts, and 51 paired LUSC tissues and normal counterparts from our local hospital. As shown in , PCAT6 was significantly increased in LUAD tissues (p<0.0001, ) as well as in LUSC tissues (p<0.0001, ) compared with normal counterparts. The diagnostic value of PCAT6 for LUAD tissues and normal counterparts was 0.9574 (95% CI 0.9232–0.9916; ), and for LUSC tissues, it was 0.9942 (95% CI 0.9825–1; ). The sensitivity, specificity, accuracy, and Yoden index of PCAT6 for distinguishing LUAD and LUSC from normal controls in five GEO datasets and our local hospital are summarized in . The results showed that PCAT6 had a significant higher level of sensitivity and specificity than CEA, which had a 51.1% sensitivity and a 84.8% specificity in NSCLC.Citation14 Together, these results indicate that PCAT6 has a potential significance with respect to sensitivity and specificity in the diagnosis of NSCLC.
Figure 3 The tissue expression and diagnostic value of PCAT6 for NSCLC patients from Union Hospital.
Abbreviations: AUC, area under the receiver operating characteristic curve; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; PCAT6, prostate cancer-associated transcript 6; qRT-PCR, quantitative reverse-transcriptase polymerase chain reaction.
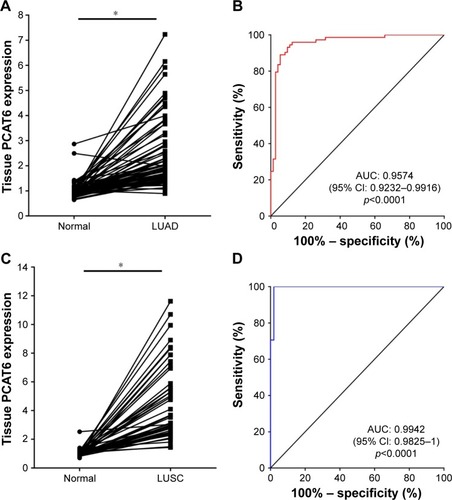
Table 3 Performance of PCAT6 in the differential diagnosis of NSCLC from healthy controls
Plasma PCAT6 has a diagnostic potential significance in the diagnosis of NSCLC
Plasma samples are easily acquired in a relatively noninvasive manner compared with biopsy or surgery. Thus, developing noninvasive techniques for the diagnosis of early stage NSCLC is clinically important. To examine whether plasma PCAT6 had diagnostic potential, plasma from the abovementioned 73 LUAD patients, 51 LUSC patients, and 39 normal healthy donors was collected. Plasma PCAT6 expression was significantly increased in LUAD and LUSC patients compared with normal healthy donors (p<0.0001, ). Then, we further evaluated the correlation between plasma PCAT6 and the patients’ clinicopathological characteristics (). Plasma expression of PCAT6 was positively correlated with TNM stage (p<0.0001) and metastasis status (p<0.0001) of LUAD and LUSC, respectively. But it was not correlated with tumor size and smoking history. The diagnostic value of plasma PCAT6 was also evaluated, and the results showed that PCAT6 had an AUC value of 0.9213 (95% CI 0.8663–0.9763; ) for LUAD and 0.9583 (95% CI 0.9109–1; ) for LUSC. The sensitivity and specificity of PCAT6 for distinguishing LUAD and LUSC from healthy controls was 87.67%/97.44% and 94.12%/100%, respectively (). Together, these results indicate that plasma PCAT6 may be exploited as a promising noninvasive biomarker of NSCLC.
Figure 4 The diagnostic potential of circulating PCAT6 for NSCLC.
Abbreviations: AUC, area under the receiver-operating characteristic curve; HD, healthy donors; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; PCAT6, prostate cancer-associated transcript 6; qRT-PCR, quantitative reverse-transcriptase polymerase chain reaction.
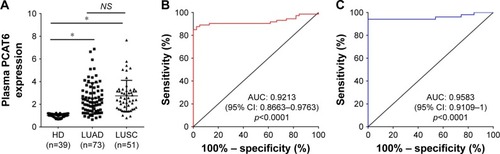
Table 2 Analysis of the relationship between circulating PCAT6 level and clinicopathological characteristics
Discussion
So far, numerous studies have shown that lncRNAs play an important role in tumor occurrence, invasion, and metastasis. With the implementation of next-generation sequencing platforms for molecular diagnostics, an increasing number of lncRNAs, such as MALAT1, colon cancer-associated transcript 2 (CCAT2), LINC01133, and GAS5, have been identified to be correlated with lung cancer, which were reported with the potential to be used as biomarkers for the diagnosis, prognosis, and personalized treatment indicators for NSCLC.Citation5,Citation14,Citation16,Citation23–Citation25 However, none of the currently identified biomarkers are sensitive or specific enough for reliable NSCLC screening in clinical settings.Citation26 Thus, identification of novel lncRNA-based biomarkers for the early diagnosis of NSCLC will have significant clinical benefits.
In a previous study, we have determined the biological role of lncRNA PCAT6 in the progression of LUAD and found that PCAT6 was significantly elevated in lung cancer tissues and positively correlated with tumor size, TNM stage, and lymph node metastasis.Citation17 Therefore, PCAT6 may have the diagnostic potential in NSCLC. In this study, the expression and diagnostic value of PCAT6 in cancer tissues was first examined in 349 NSCLC patients from five GEO datasets (GSE19804,Citation18 GSE18842,Citation19 GSE30219,Citation20 GSE19188,Citation21 and GSE27262Citation22). The expression of tissue PCAT6 was consistently increased and showed a great diagnostic value (AUC >0.9; sensitivity, 86.67%–100%; specificity, 78.57%–96%) in NSCLC patients included in the five GEO datasets. The result was further reconfirmed in samples from our local hospital, including 73 LUAD patients and 51 LUSC patients. Therefore, these results suggest that PCAT6 may have the potential to be used as a diagnostic marker of NSCLC patients.
Determination of blood-based circulating biomarkers is a simple, inexpensive, and noninvasive test that greatly facilitates early diagnosis of cancer, even earlier than CT imaging.Citation27 To date, no study has quantified the expression level and the diagnostic potential of PCAT6 in plasma. Here, we first investigated the diagnostic value of PCAT6 in plasma distinguishing NSCLC patients, including LUAD and LUSC, from healthy controls. The results showed that plasma PCAT6 had an AUC value of 0.9213 (95% CI 0.8663–0.9763) for LUAD and 0.9583 (95% CI 0.9109–1) for LUSC, significantly higher than GAS5 (0.832, 95% CI 0.754–0.893) and CEA (0.700, 95% CI 0.611–0.779) according to a previous report.Citation14 Furthermore, the sensitivity and specificity of PCAT6 for distinguishing LUAD and LUSC from healthy controls were 87.67%/97.44% and 94.12%/100%, respectively, which were significantly higher than the combination of GAS5 and CEA (86.7%/90.9%).Citation14 Thus, these results indicated that plasma PCAT6 may be exploited as a promising noninvasive biomarker of NSCLC, even better than the previously reported biomarkers.
Conclusions
In summary, our study first systematically evaluated the diagnostic potency of PCAT6 in NSCLC patients from five GEO datasets and our local hospital, and identified that circulating PCAT6 is a potential biomarker of NSCLC diagnosis. Furthermore, PCAT6 also predicted a poor overall survival of LUAD patients in our previous study.Citation17 Thus, PCAT6 may play a pivotal role in carcinogenesis and progression of NSCLC. Unfortunately, we only confirmed that PCAT6 regulates p53 and c-myc expressions in an indirect manner, and the exact molecular mechanisms of PCAT6 in NSCLC remain unclear and require further investigation.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant no 81000032) and the Natural Science Foundation of Hubei Province (No 02.07.16040048).
Disclosure
The authors report no conflicts of interest in this work.
References
- ZhouMGuoMHeDA potential signature of eight long non-coding RNAs predicts survival in patients with non-small cell lung cancerJ Transl Med20151323126183581
- SheJYangPHongQBaiCLung cancer in China: challenges and interventionsChest201314341117112623546484
- FossKMSimaCUgoliniDNeriMAllenKEWeissGJmiR-1254 and miR-574–5p: serum-based microRNA biomarkers for early-stage non-small cell lung cancerJ Thorac Oncol20116348248821258252
- HeuversMEHegmansJPStrickerBHAertsJGImproving lung cancer survival; time to move onBMC Pulm Med20121217723234250
- ZhuHZhangLYanSLncRNA16 is a potential biomarker for diagnosis of early-stage lung cancer that promotes cell proliferation by regulating the cell cycleOncotarget2017857867787727999202
- National Lung Screening Trial Research TeamAberleDRAdamsAMReduced lung-cancer mortality with low-dose computed tomographic screeningN Engl J Med2011365539540921714641
- HeuversMEStrickerBHAertsJGGeneralizing lung-cancer screening resultsN Engl J Med2012366219219322236243
- BachPBMirkinJNOliverTKBenefits and harms of CT screening for lung cancer: a systematic reviewJAMA2012307222418242922610500
- HurJLeeHJNamJEAdditional diagnostic value of tumor markers in cytological fluid for diagnosis of non-small-cell lung cancerBMC Cancer201212139222954172
- SuXMaloufGGChenYComprehensive analysis of long non-coding RNAs in human breast cancer clinical subtypesOncotarget20145209864987625296969
- YuanJHYangFWangFA long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinomaCancer Cell201425566668124768205
- LiHYuBLiJOverexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancerOncotarget2014582318232924810858
- LiQShaoYZhangXPlasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancerTumour Biol20153632007201225391424
- HanLMaPLiuSMZhouXCirculating long noncoding RNA GAS5 as a potential biomarker in breast cancer for assessing the surgical effectsTumor Biol201637568476854
- DuZFeiTVerhaakRGIntegrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancerNat Struct Mol Biol201320790891323728290
- YangJLinJLiuTAnalysis of lncRNA expression profiles in non-small cell lung cancers (NSCLC) and their clinical subtypesLung Cancer201485211011524906504
- WanLZhangLFanKChengZXSunQCWangJJKnockdown of long noncoding RNA PCAT6 inhibits proliferation and invasion in lung cancer cellsOncol Res201624316117027458097
- LuTPTsaiMHLeeJMIdentification of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in nonsmoking womenCancer Epidemiol Biomarkers Prev201019102590259720802022
- Sanchez-PalenciaAGomez-MoralesMGomez-CapillaJAGene expression profiling reveals novel biomarkers in nonsmall cell lung cancerInt J Cancer2011129235536420878980
- RousseauxSDebernardiAJacquiauBEctopic activation of germline and placental genes identifies aggressive metastasis-prone lung cancersScience Transl Med20135186186ra166
- HouJAertsJden HamerBGene expression-based classification of non-small cell lung carcinomas and survival predictionPLoS One201054e1031220421987
- WeiTYJuanCCHisaJYProtein arginine methyltransferase 5 is a potential oncoprotein that upregulates G1 cyclins/cyclin-dependent kinases and the phosphoinositide 3-kinase/AKT signaling cascadeCancer Sci201210391640165022726390
- WeberDGJohnenGCasjensSEvaluation of long noncoding RNA MALAT1 as a candidate blood-based biomarker for the diagnosis of non-small cell lung cancerBMC Res Notes20136151824313945
- QiuMXuYYangXCCAT2 is a lung adenocarcinoma-specific long non-coding RNA and promotes invasion of non-small cell lung cancerTumor Biol201435653755380
- SunCLiSZhangFLong non-coding RNA NEAT1 promotes non-small cell lung cancer progression through regulation of miR-377-3p-E2F3 pathwayOncotarget2016732517845181427351135
- HileyCTLe QuesneJSantisGChallenges in molecular testing in non-small-cell lung cancer patients with advanced diseaseLancet3881004810021011
- AbboshCBirkbakNJWilsonGAPhylogenetic ctDNA analysis depicts early stage lung cancer evolutionNature2017545765544645128445469
